Abstract
Glioblastoma multiforme (GBM) is the most malignant brain tumor in adults with a median survival of 14.6 mo under the best available treatment. New treatment strategies are therefore urgently required, for which a profound understanding of tumor biology is necessary. Much effort has been devoted to tumor-specific aberrant signaling processes. Recently it was discovered that the transcription factor Gli1, which is activated by hedgehog signaling, is a highly predictive marker in GBM, as determined by immunohistochemistry. To determine whether GBM cells have transcriptionally active Gli1, we performed experiments with reporter genes with cells isolated from surgically removed human tumors and cell lines. We also determined whether the hedgehog signaling inhibitor cyclopamine influences reporter gene expression and cell viability, and we determined the expression of Gli1, SHH and Patched1 by quantitative real-time RT-PCR. Reporter gene analysis of nine cultures and four cell lines demonstrated a significantly enhanced transcriptional activity in six tumor cell cultures and all cell lines. Analysis of cell viability in the presence of cyclopamine revealed a response of all cell cultures with the exception of one primary culture and one cell line, but only one cell line responded to cyclopamine with reduced hedgehog signaling activity. This indicates that the toxicity of cyclopamine toward GBM cells is independent from hedgehog signaling. Since no correlation between hedgehog activity and SHH, Gli1 and Patched1 mRNA levels was observed we conclude that other mechanisms aside from transcriptional regulation of these factors are responsible for hedgehog activity in tumor cells derived from GBM.
Keywords: :
Introduction
Glioblastoma multiforme (GBM) is the most common primary tumor of the adult brain. According to the classification of the world health organization (WHO), GBM is the most malignant type of glioma and classified as WHO Grad IV. In addition to radiotherapy, standard therapy includes medication with nitrosourea compounds such as carmustine or lomustin. These alkylating agents are also used in the treatment of other brain cancers such as medulloblastoma and astrocytoma, and for the treatment of multiple myeloma and lymphoma. The alkylating substance temozolomide has attracted much attention for treatment of GBM, since it was demonstrated that temozolomide medication combined with conventional therapy increased median survival of patients to 14.6 mo, compared with 12.1 mo in patients treated solely with radiotherapy.Citation1 Unfortunately, survival is still poor and the benefit from temozolomide is restricted to patients with a low or missing activity of the DNA-repair enzyme O6-methylguanine-DNA methyltransferase (MGMT).Citation2 Several alternative approaches have been recently considered to treat GBM. These strategies either target various receptors, such as the epidermal growth factor receptor (EGFR), the vascular endothelial growth factor receptor (VEGFR), the platelet derived growth factor receptor (PDGFR), or their downstream second-messengers. Other targets are the mammalian target of rapamycin (mTOR), protein kinase C (PKC), farnesyl transferase or histone deacetylase (HDAC). Some of these strategies have already been evaluated in clinical trials. Unfortunately, response to drugs is heterogeneous among patients and no ultimate treatment strategy is in sight.
More recently, the hedgehog (HH) signaling pathway has been considered as a potential target for the treatment of GBM. Originally described in Drosophila melanogaster,Citation3 this pathway is important for embryonic development in vertebrates (for a review see ref. Citation4). Like other pathways involved in embryogenesis, aberrant HH activation has been associated with different forms of cancer. In medulloblastoma, basal cell carcinoma and rhabdomyosarcoma dysregulation of hedgehog signaling is important for tumor initiation and maintenance. In addition, HH signaling has been detected in breast carcinoma, esophageal carcinoma, hepatic carcinoma, lung carcinoma, lymphoma, ovarian carcinoma and prostate carcinoma, although its role is still uncertain (for a recent review see ref. Citation5).
In vertebrates, under physiological conditions, HH signaling is initiated by binding of one of three HH ligands, designated Sonic hedgehog (SHH), Indian hedgehog (IHH) and Dessert hedgehog (DHH). Binding of a ligand to the receptor Patched (Ptch), alleviates Ptch-mediated suppression of Smoothened (Smo), a distant relative of G-protein-coupled receptors (GPCRs). This triggers a series of intracellular events, including suppressor of fused (SuFu) and protein kinase A (PKA), that finally lead to the activation of transcription factors of the Gli family (for reviews see refs. Citation6–Citation9). At least three different Gli transcription factors have been identified, designated Gli1, Gli2 and Gli3. Gli1 and Gli2 are transcriptional activators. Gli3 mostly functions as repressor of transcription but can also act as a weak activator (for a review see ref. Citation10). Among the targets of Gli transcription factors are Cyclins D1 and D2, IGFBP-6 and Bcl-2. In addition, other transcription factors such as HNF-3β, FOXM1 or Jun are regulated by Gli transcription fators. In tumors, aberrant activation of these genes may lead to enhanced cell survival and proliferation.
Besides influencing tumor cell proliferation, cell survival and migration, HH signaling may also influence the outcome of the therapeutic treatment of GBM, at least in the case of treatment with temozolomide. This anti-cancer alkylator exerts its effect by increasing O-6-alkylguanine DNA lesions that are normally repaired by O-6-methylguanine-DNA methyltransferse (MGMT). Unfortunately, MGMT expression is linked to Gli1 activity due to a putative Gli1-binding site in the MGMT gene promoter.Citation11 This potential link between HH activity and therapeutic resistance to temozolomide was further consolidated in recent experiments with CD133+ glioma stem cells.Citation12
The first member of the family of Gli transcription factors, designated Gli1, was originally identified as an amplified gene in a human malignant glioma,Citation13 which suggested a link between aberrant HH signaling and GBM development. Although HH activity is clearly deregulated in a subset of human medulloblastomas, there is some controversy in the literature with regard to its role in human GBM, as will be outlined in the discussion section (see below). Briefly, most experiments have been performed with cell lines and/or were based on immunofluorescenceCitation14,Citation15 or quantitative real-time RT-PCR detection of Gli expression,Citation16 and there was no unequivocal and direct proof that Gli-dependent transcription was active in primary cells derived from GBM. Therefore, we have constructed highly sensitive reporter genes, based on the secreted luciferase from Gaussia princes, and analyzed their activity in primary cultured cells from GBM in the absence and presence of the HH antagonist cyclopamine. Since the new vectors have the advantage that cells do not need to be lysed to measure luciferase activity, the cells were used for further experiments to determine the expression of mRNA encoding Gli1, SHH and Patched1 by qRT-PCR.
Results
Reporter gene activity in co-transfection experiments
Co-transfection experiments were performed to evaluate the functionality of the reporter genes developed to detect active Gli transcription factors. Cells from the line T98G were transfected either with the reporter gene “pT81_Gli” carrying the Gli-responsive elements in combination with the reduced promoter from the HSV-tk promoter or with the corresponding control plasmid with mutated Gli-binding sites (“pT81_Gli_Mut”). The reporter genes were co-transfected either with an expression plasmid for Gli1 or an expression plasmid for Gli3 (kindly provided by Vogelstein and SasakiCitation17,Citation18). Luciferase activity from Gaussia luciferase secreted to the medium was determined 24 h after transfection and the expression of the Gli-reporter plasmid was compared with the expression of the corresponding control reporter gene. As shown in , the reporter gene exhibited a nearly 40-fold stronger level of expression than the corresponding control plasmid when co-transfected with Gli1. In the presence of Gli3, the reporter gene still exhibited a 1.5-fold enhancement. It should be noted that cells from the line T98G exhibited an endogenous 3-fold activation of reporter gene activity (). It is obvious that this enhancement is not completely silenced by exogenous expression of Gli3. Most importantly, the data demonstrates that the reporter gene is able to detect enhancement of expression, as it would be expected when Gli1 is present. It should also be noted that all plasmids used as reporter genes were prepared using endotoxin-free protocols to prevent unspecific effects, possibly caused by NF-κB activation.Citation19 In fact, experiments performed with conventionally prepared plasmids revealed such background activities (data not shown).
Figure 1. Luciferase activity of the Gli-reporter gene pT81 co-transfected with expression plasmids for Gli1 and Gli3. Cells from the line T98G were transfected with the pT81 Gli reporter gene together with expression plasmids for Gli1 (hGli1) or Gli3 (hGli3), respectively. Luciferase activity from the Gli reporter plasmid was compared with the activity of the corresponding control plasmid in the presence of the same expression plasmid set to 100%. Error bars indicate standard deviations. For the determination of mean and standard deviation six transfection experiments were performed. **p < 0.01.
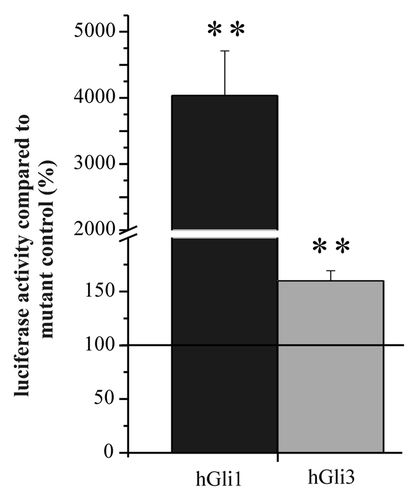
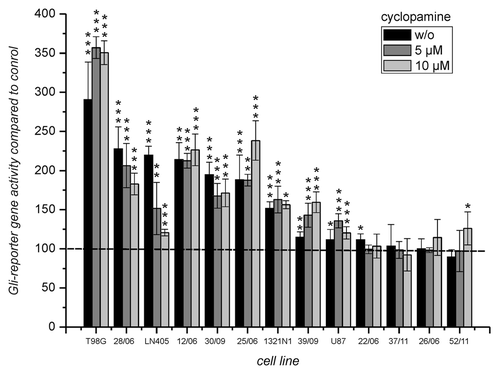
Figure 2. Luciferase expression from the Gli reporter gene after transfection into cells from GBM incubated without and in the presence of 5 and 10 µM cyclopamine. Luciferase activity of cells transfected with the reporter gene “pT81_Gli” was compared with the activity of cells transfected with the corresponding control plasmid “pT81_Gli-Mut” set to 100%. Below the bars the cultures are indicated. Error bars indicate standard deviations. The level of significance for enhanced transcription compared with the control plasmid as determined by student’s t-test is indicated by asterisks. *p < 0.05; **p < 0.005; ***p < 0.0005. For the determination of mean and standard deviations six transfection experiments were performed.
Gli activity and cell viability in primary cultured cells from human glioblastoma in the absence and presence of cyclopamine
In order to investigate whether members of the Gli family of transcription factors are active in cells derived from GBM, reporter gene experiments were performed. The reporter gene “pT81_Gli” and the control plasmid “pT81_Gli_Mut” were transfected into nine primary cell cultures derived from patients with glioblastoma multiforme and into cells from four cell lines. Luciferase activity was determined 24 h after transfection and reporter gene activity from “pT81_Gli” was compared with control activity from “pT81_Gli_Mut.” In addition, cells were incubated during the 24 h period in the absence or presence of 5 and 10 µM cyclopamine. The result of the experiment is presented in . As can be seen, three out of four cell lines and four out of nine primary cultures demonstrated a more than 1.5 times enhanced reporter gene activity. A less pronounced enhancement that was still statistically significant was seen in the fourth cell line (U87) and in two additional primary cultures, indicating an active intracellular hedgehog signaling pathway in all cell lines and in six primary cell cultures. Interestingly, with the exception of LN405 no significant reduction of relative reporter gene activity was observed in the presence of 5 or even 10 µM cyclopamine.
Parallel to the reporter gene experiment, the response to different cyclopamine concentrations in the cultures with regard to viability was analyzed in order to assess whether the concentrations of cyclopamine used in reporter gene experiments were already toxic to the cells. For this experiment we used the cyclopamine concentrations 0, 0.1, 0.5, 1, 2.5, 5, 7.5, 10, 15 and 20 µM and determined viability after 24 h incubation. The measured values were used for a sigmoidal fit using the Boltzman equation. All data fitted well to a sigmoidal response with R-Square values above 0.97 aside from cultures 25/06 (R2 = 0,93) and 22/06 [although R-Square was low in this case only the response to 0 and 0.1 µM were outside the fitting curve that otherwise indicates almost no response to cyclopamine ()]. In all fitting curves are presented. As can be seen, the cell lines behave differently. In all cells with the exception of cells from the line T98G viability at a concentration of 5 µM cyclopamine is close to 90% or above 90% compared with untreated control. At higher concentrations of cyclopamine only cells from the primary culture 22/06 and from the line U87showed almost no response to cyclopamine. The analysis of a possible relation between reporter gene activity in the different cells and their response to cyclopamine is presented in . As can be seen, there is only a very week correlation between these two parameters when the cells T98G, 28/06, LN405, 12/06, 30/09 and 25/06 are compared. This correlation is lost when data from 1321N1 is included although these cells responded significantly with enhanced reporter gene activity. At this point it is interesting to note that an extrapolation of the fitting curves in above the highest concentration used in the experiments (20 µM) indicates that aside from cells T98G and 28/06 cell death does not reach values of 100% death at a theoretical concentration of 1 mM cyclopamine ().
Figure 3. Effect of cyclopamine on the viability of cells derived from GBM. The cells from the experiment in were incubated for 24 h in the presence of different concentrations of cyclopamine (0, 0.1, 0.5, 1, 2.5, 5, 7.5, 10, 15 and 20 µM). Viability was determined by the CellTiterGlo Assay and data was fitted using the Boltzman equation. For comparison viability of cells from untreated control was set to 100%.
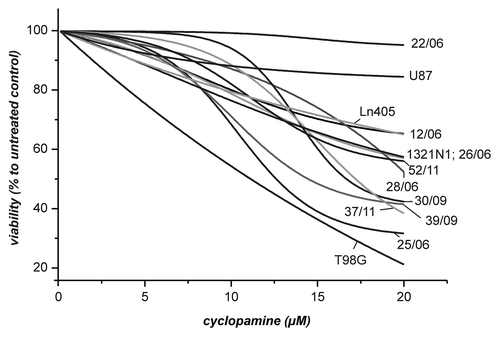
Figure 4. Correlation between reporter gene activity and viability in the presence of 5 and 10 µM cyclopamine. Viability of cells as determined in was compared with the reporter gene activity from (untreated cells). The comparison only includes cells which exhibited at least a reporter gene enhancement above 1.5 x.
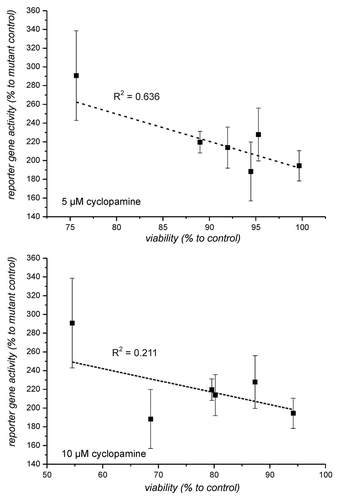
Table 1. Viability of cells in the presence of 1 mM cyclopamine as determined by extrapolation of the fitting curves in
Correlation between SHH, Gli1 and Patched1 mRNA expression and reporter gene activity
We then considered whether the different response of the reporter genes in different cell lines correlates to the expression of SHH, Gli1 and Patched1 mRNA. Cells from four cell lines and seven primary cultures were transfected and cultivated as described and reporter gene activity determined in the supernatants. The same cells were also used for the isolation of mRNA and after reverse transcription, mRNA expression of Gli1, SHH and Patched1 was determined and normalized to the expression of β-actin. As shown in no significant correlation was observed between expression of Gli1 mRNA and enhanced reporter gene expression (). Interestingly, Gli1 mRNA expression was very high in culture 22/06 which had only a weakly enhanced reporter gene activity and exhibited no reduced viability in the presence of cyclopamine. This discrepancy confirms that high Gli1 mRNA expression is not necessarily associated with high hedgehog activity, most probably because mRNA expression is not associated with enhanced expression of Gli1 protein or because of a posttranslational inhibition of unknown nature. Patched1 mRNA expression did not show the extreme differences between cell cultures (Fig. Five B) as did Gli1 mRNA expression. The overall expression of SHH mRNA was very low and in some cases outside of the standard curve (less than 100 copies; open squares in ). Therefore, it is difficult to properly estimate the correlation between expression of SHH mRNA and Gli-reporter gene activity.
Figure 5. Correlation between expression of Gli1, SHH and Patched1 mRNA and reporter gene enhancement. The relative amount of mRNA was determined by normalization of each mRNA amount of Gli1 (A), Patched1 (B) and SHH (C) and to the corresponding amount of β-actin in each sample. Open squares in part C indicate that the amount of SHH mRNA in these samples were below the used standard curve (less than 100 copies).
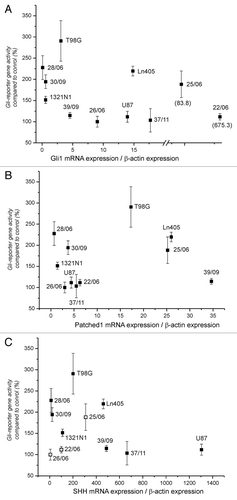
Table 3. Primary cell cultures
Gli1, sonic hedgehog and patched1 expression in tissues and cell cultures determined by qRT-PCR
For a direct comparison of the tumor tissue with the culture derived from it, 12 samples were investigated. Using qRT-PCR we determined mRNA expression of SHH, Gli1, Patched1 and β-actin for normalization. The results are summarized in . In many cell cultures and in four out of 12 tissues the concentration of SHH mRNA was too low for an exact determination. Therefore, a comparison between its expression in tissue and the corresponding cell culture was not possible, although expression in tissues was more frequently within the range of the standard curve (above 100 copies). Although this may point toward a higher hedgehog activity in tissues than in cultures, this possibility is in contrast to the observation that Gli1 mRNA was more strongly expressed in 8 cell cultures than in tumor tissues. Gli1 mRNA expression was equal between culture and tissue in four cases. The concentration of Patched1 mRNA was comparable between tissues and cell cultures in six cases, slightly higher (3 to 5 times) in four tissues and ten times higher in one tissue. One sample exhibited a 7 times higher expression in cell culture compared with tissue, however.
Table 2. Comparison of mRNA expression between tissue and the derived cell culture
In addition, we also analyzed the expression of Patched1, Gli1 and SHH in the four commercially available glioma cell lines LN405, U87, T98G and 1321N1. Highest expression of SHH and Gli1 was determined in the lines LN405 and U87. Lowest expression of Gli1 and SHH was observed in cells from the line 1321N1. Expression of Patched1 was high in cells from the line LN405 and slightly lower in cells from T98G. It was low in U87 and very low in 1321N1.
Discussion
Although Gli1 was originally identified as an amplified gene in a human GBM cell lineCitation13 a number of early studies performed in the late 1980s and early 1990s found only a low incidence of Gli1 amplification in human GBM.Citation20,Citation21 In contrast, a more recent study, based on genome-wide copy number analysis, identified 22.6% of 31 GBM samples possessed an amplified Gli1 locusCitation22 and Wang et al. reported that 68% of 44 GBMs exhibited Gli1 immunoreactivity.Citation15 In a very recent publication by Rossi et al.,Citation14 GBM sections from 80 patients (out of 106 patients) stained positive for Gli1. Most interestingly, by correlation analysis, Rossi et al. also demonstrated that Gli1 overexpression was a highly predictive marker of poor prognosis.Citation14 It is difficult to assess whether the discrepancies between the first and the more recent determinations were due to different protocols or the antibodies employed.
A first indication that HH signaling may be relevant for glioma cell proliferation was the observation of Wang et al. that the K-67 labeling index was significantly higher in Gli1-positive gliomas (including the lines U87, SHG-44 and U251) than in Gli1-negative gliomas (and in line A172)Citation23 and Takezaki et al. demonstrated that two cell lines, one established from GBM and another established from anaplastic oligodendroglioma, required HH signaling for cell proliferation and tumorigenesis.Citation24
Although many experiments indicated a possible role of HH signaling in GBM the functional activity of Gli- dependent transcription was not demonstrated.
The experiments presented in our study demonstrate for the first time that Gli-dependent transcriptional activity is present in cells derived from patients with GBM. Enhanced reporter gene activity was detected in all four cell lines and in six out of nine cell cultures analyzed. Comparison ofGli1 mRNA expression between tissue and culture suggests the possibility that HH signaling may be more prominent in the cultures than in the tissues from which they were derived (), but this does not necessarily rule out the likelihood that hedgehog signaling was absent in the tissues. In this context, it is also important to note that expression of mRNA does not necessarily correlate with the expression of the encoded protein. This may also explain the low correlation between mRNA of SHH, Gli1 and Patched1 with reporter gene activity (), especially when the differences between the cell cultures are small. One exception, however, may be the high mRNA expression of Gli1 in the culture 22/06 that did show only a very small reporter gene response. Whether this discrepancy was caused by posttranslational events needs to be addressed by further experiments. In general, the correlation between expression of mRNA for Gli1 or SHH and the intensity of HH signaling appears to be weak as also exemplified by the reporter gene activity in the line T98G that has only small amounts of both mRNAs. In order to unequivocally prove whether HH signaling is present in GBM and contributes to malignancy detailed analysis of HH target gene expression in freshly isolated tumors will be required. If these experiments reveal that tumor relevant HH target genes such as anti-apoptotic genes or genes encoding angiogenic factors and cyclins are activated, then therapeutical strategies should be developed that specifically target HH signaling in GBM. The first idea would be to consider cyclopamine as a potential GBM drug. Cylopamine was initially discovered as the teratogenic agent from Veratrum californicum. When Wang et al. investigated the correlation between HH/Gli1 signaling and cell migration and invasion in glioblastoma cells using the cell lines U87 and A172 they found that only the HH-positive line U87 responded to cyclopamine with decreased migration.Citation15
In our experiments a negative effect of cyclopamine on enhanced reporter gene activity was only observed in one cell line (LN405; ), indicating that reporter gene activity in our cells was independent of extracellular hedgehog ligands. This may also explain the low amount of SHH mRNA determined by qRT-PCR and the complete absence of desert hedgehog or indian hedgehog (data not shown), although we observed a significant effect of cyclopamine on cell viability in almost all cells. Whether this indicates that the effect of cyclopamine is independent of HH signaling has to be addressed in further experiments. One approach would be to compare the effect of cyclopamine with that of the steroidal alkaloid tomatidine which is structurally similar to cyclopamine but does not inhibit HH signaling. An answer to the most important question, whether cyclopamine is a useful drug for some GBMs, has to await further experiments with additional cell cultures and especially non-tumorous neuronal tissue.
Materials and methods
Reagents
If not stated otherwise all chemicals were purchased from Sigma-Aldrich or from Merck.
Patients and tumors
Tumor samples were obtained from freshly isolated tumors. Patients provided written informed consent according to the German laws as confirmed by the local committee. Surgery was performed between 2005 and 2011 at the Clinic for Neurosurgery at the University Hospital in Leipzig, Germany. All samples were diagnosed histological as glioblastoma multiforme. The cell lines used in the study are listed with reference to tumor location, gender and the age of the patient in .
Cell lines and primary cells
All cells were cultivated in DMEM (4.5 g/L glucose, without pyruvate; Gibco) supplemented with 10% fetal calf serum (FCS gold, PAA), 2 mM Glutamax (PAA), 50 µg/ml streptomycin and 30 µg/ml penicillin at 37°C, 5% CO2 in humidified air.
The human GBM cell line T98G and U87 were obtained from the ATCC, the line 1321N1 was originally obtained from the ECACC and the line LN405 was from the German collection of microorganisms and cell cultures (DSMZ, Braunschweig). Primary cell cultures from tumors that were histopathologically confirmed as glioblastoma multiforme were established as described.Citation25
Reporter genes
The reporter gene with Gli-binding sites and its mutant control were constructed as follows. The two oligonucleotides “BHI-Gli-f-BglII” (5′-GAT CCA GAC ATG GGA GGT CTC A-3′) and “BglII-Gli-rev-BHI” (5′-GAT CTG AGA CCT CCC ATG TCT G-3′) and the two oligonucleotides “BHI-Gli_Mut-f-BglII” (5′-GAT CCA GAC ATC AGC TGT CTC A-3′) and “BglII-Gli_Mut-rev-BH1” (5′-GAT CTG AGA CAG CTG ATG TCT G-3′) were hybridized to each other. In this way two double-stranded oligonucleotides were obtained, one with a single Gli-binding site and one with a mutated version of this site. In order to obtain DNA sequences with multiple binding sites each of the double-stranded oligonucleotides was used in a ligation reaction. After digestion with BglII and BamHI fragments containing four repeats of the oligonucleotide were cloned into the BamHI site of the luciferase vector “pT81_Gau.” This vector was constructed by exchanging the Photinus pyralis luciferase from “pT81”Citation26 by the luciferase from Gaussia princeps. Finally, the two reporter genes “pT81_Gli” and “pT81_Gli_Mut,” were obtained. It is important to note, that the reporter genes used for transfection experiments were isolated from bacteria using an endotoxin-free purification kit (Qiagen).
Viability assay
For the determination of viability the CellTiterGlo Assay (Promega) was employed as described previouslyCitation27,Citation25. Luminescence was measured with an integration time of 0.5 sec using a Multilabel reader (Spectra Max M5, Molecular Devices).
Cells were initially cultivated in the wells of 96-well plates (white, clear bottom; µClear, Greiner Bio One) at a density of 5,000 cells/well with 200 µl of medium. After overnight incubation medium was withdrawn and replaced with 100 µl of fresh medium, with or without cyclopamine. Twenty-four hours later, viability was determined in sextuplicate. Just before the assay, medium was exchanged with medium without cyclopamine or vehicle in order to perform the assays under the same conditions.
Transfection
Transient transfection was performed using Turbofect (Fermentas) according to manufacturer’s instructions using 2 µg DNA/4 µl transfection reagent. The cells were cultivated in 6-well plates at different densities. Different densities were chosen to adjust for the different sizes of the cells in order to perform all experiments with a comparable density. Initial densities are indicated in . In general, cells were seeded into wells 24 h before transfection. Four hours before transfection, medium was replaced with fresh medium. At transfection, each well received an equivalent of 0.3 µg of DNA. Four hours later, medium with and without cyclopamine was refreshed and cells were cultivated until determination of Gaussia luciferase activity.
Table 4. Primary cell cultures ad cell lines used for transfection experiments
Determination of luciferase activity
For the determination of Gaussia luciferase activity Gaussia luciferase assay reagent (Gau-LAR) was used (20 mM MOPS (3-(N-morpholino)propanesulfonic acid); 0.75 M KBr; 5 mM MgCl2; 5 mM CaCl2; 1 mM EDTA; 10 µM Coelenterazine; pH of 7.8). The measurement of luciferase activity was performed in white 96-well plates (Greiner bio-one) using an Orion-II microplate luminometer (kindly provided by Dr. A. Berthold, Berthold Detection Systems). In general, luciferase activity was determined in a 1 sec interval after the injection of 50 µl of luciferase assay reagent to 10 µl of cell supernatant using a delay of 0.5s between injection and measurement.
RNA isolation, cDNA preparation and quantitative real-time RT-PCR
RNA from cultured cells and tissue was isolated using an RNeasy Plus Mini Kit (Qiagen) and cDNA was generated using the ImProm-II Reverse Transcription system (Promega). For quantification of Gli1, Patched1 and β-actin mRNA standards were produced from amplification products obtained from PCR reactions with the same primers that were used for quantitative real-time RT-PCR. These products were cloned into the vector pCR2.1-TOPO (Invitrogen). The standard for Sonic hedgehog was obtained by hybridization of the two oligonucleotides “SHH_full_forward”: 5′-CCG GCT TCG ACT GGG TGT ACT ACG AGT CCA AGG CAC ATA TCC ACT GCT CGG TGA AAG CAG AGA ACT CGG TGG CGA A-3′ and “SHH_full_reverse”: 5′-TCG CCA CCG AGT TCT CTG CTT TCA CCG AGC AGT GGA TAT GTG CCT TGG ACT CGT AGT ACA CCC AGT CGA AGC CGG A-3′ generating a double stranded DNA that was also cloned into the vector pCR2.1-TOPO. Prior to use, the standards were linearized by restriction digestion.
Quantitative real-time RT-PCR (qRT-PCR) was performed on a Rotorgene 3000 (Qiagen) using the Sybr-Green method and employing a Maxima SYBR Green qPCR Master Mix Kit (Fermentas). Serial dilutions of linearized plasmids containing the sequence of interested were used for mRNA quantification. Normalization was performed using the determined concentration of β-actin mRNA in each sample.
The following primers were used for qRT-PCR: “Gli1-forward”: 5′-CCA ACT CCA CAG GCA TAC AGG AT-3′; “Gli1-reverse”: 5′-CAC AGA TTC AGG CTC ACG CTT C-3′; “Patched1-forward”: 5′-CCC CTG TAC GAA GTG GAC ACT CTC-3′; “Patched1-reverse”: 5′-AAG GAA GAT CAC CAC TAC CTT GGC T-3′; “SHH-forward”: 5′-CCG GCT TCG ACT GGG TGT ACT A-3′; “SHH-reverse”: 5′-CGC CAC CGA GTT CTC TGC TTT-3′; “beta-actin-forward”: 5′-CCG GGA CCT GAC TGA CTA CCT-3′; “beta-actin-reverse”: 5′-CCT AGA AGC ATT TGC GGT GGA-3′. The primers for Gli1 and Patched1 have already been described by Laurendeau et al.Citation28
Statistical analysis
Statistical analysis employed the Students t-test algorithms implemented in the Excel software (Microsoft Corporation).
Acknowledgments
We would like to thank Dr. A. Berthold (Berthold Detection Systems) for the kind disposal of an Orion II luminometer. In addition, we thank Mr. P. Krauser (PJK GmbH) for the kind supply of coelenterazine and Dr. B. Bryan (Prolume, Nanoligh Inc.) for the kind gift of the Gaussia pCMV-hGLuc vector.
We also have to thank Dr. Hiroshi Sasaki from the RIKEN Center for Developmental Biology, Japan and Dr. Bert Vogelstein from the John Hopkins University, Baltimore, USA, for the kind supply of the expression vectors for Gli1 and Gli3.
SB, HO and AM performed the experiments with technical assistance from RB. AH constructed the reporter genes. CR and JM did the surgery of tumors. JM, RG, JT and ARH supported the project and revised the manuscript. FG designed and coordinated the experiments, contributed conceptually and wrote the manuscript. This work was funded by budget finance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352:987 - 96; http://dx.doi.org/10.1056/NEJMoa043330; PMID: 15758009
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005; 352:997 - 1003; http://dx.doi.org/10.1056/NEJMoa043331; PMID: 15758010
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287:795 - 801; http://dx.doi.org/10.1038/287795a0; PMID: 6776413
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001; 15:3059 - 87; http://dx.doi.org/10.1101/gad.938601; PMID: 11731473
- Barakat MT, Humke EW, Scott MP. Learning from Jekyll to control Hyde: Hedgehog signaling in development and cancer. Trends Mol Med 2010; 16:337 - 48; http://dx.doi.org/10.1016/j.molmed.2010.05.003; PMID: 20696410
- Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer 2003; 3:903 - 11; http://dx.doi.org/10.1038/nrc1229; PMID: 14737121
- Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer 2006; 42:437 - 45; http://dx.doi.org/10.1016/j.ejca.2005.08.039; PMID: 16406505
- Rohatgi R, Scott MP. Patching the gaps in Hedgehog signalling. Nat Cell Biol 2007; 9:1005 - 9; http://dx.doi.org/10.1038/ncb435; PMID: 17762891
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov 2006; 5:1026 - 33; http://dx.doi.org/10.1038/nrd2086; PMID: 17139287
- Stecca B, Ruiz I Altaba A. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J Mol Cell Biol 2010; 2:84 - 95; http://dx.doi.org/10.1093/jmcb/mjp052; PMID: 20083481
- Yoon JW, Gilbertson R, Iannaccone S, Iannaccone P, Walterhouse D. Defining a role for Sonic hedgehog pathway activation in desmoplastic medulloblastoma by identifying GLI1 target genes. Int J Cancer 2009; 124:109 - 19; http://dx.doi.org/10.1002/ijc.23929; PMID: 18924150
- Ulasov IV, Nandi S, Dey M, Sonabend AM, Lesniak MS. Inhibition of Sonic hedgehog and Notch pathways enhances sensitivity of CD133(+) glioma stem cells to temozolomide therapy. Mol Med 2011; 17:103 - 12; http://dx.doi.org/10.2119/molmed.2010.00062; PMID: 20957337
- Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, et al. Identification of an amplified, highly expressed gene in a human glioma. Science 1987; 236:70 - 3; http://dx.doi.org/10.1126/science.3563490; PMID: 3563490
- Rossi M, Magnoni L, Miracco C, Mori E, Tosi P, Pirtoli L, et al. β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol Ther 2011; 11:753 - 61; http://dx.doi.org/10.4161/cbt.11.8.14894; PMID: 21321483
- Wang K, Pan L, Che XM, Cui DM, Li C. Sonic Hedgehog/GLI₁ signaling pathway inhibition restricts cell migration and invasion in human gliomas. Neurol Res 2010; 32:975 - 80; http://dx.doi.org/10.1179/016164110X12681290831360; PMID: 20444323
- Shahi MH, Lorente A, Castresana JS. Hedgehog signalling in medulloblastoma, glioblastoma and neuroblastoma. Oncol Rep 2008; 19:681 - 8; PMID: 18288402
- Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development 1997; 124:1313 - 22; PMID: 9118802
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 1999; 126:3915 - 24; PMID: 10433919
- Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J 2009; 23:21 - 33; http://dx.doi.org/10.1096/fj.08-111096; PMID: 18772349
- Bigner SH, Wong AJ, Mark J, Muhlbaier LH, Kinzler KW, Vogelstein B, et al. Relationship between gene amplification and chromosomal deviations in malignant human gliomas. Cancer Genet Cytogenet 1987; 29:165 - 70; http://dx.doi.org/10.1016/0165-4608(87)90045-8; PMID: 3478127
- Forus A, Flørenes VA, Maelandsmo GM, Meltzer PS, Fodstad O, Myklebost O. Mapping of amplification units in the q13-14 region of chromosome 12 in human sarcomas: some amplica do not include MDM2. Cell Growth Differ 1993; 4:1065 - 70; PMID: 8117620
- Rao SK, Edwards J, Joshi AD, Siu IM, Riggins GJ. A survey of glioblastoma genomic amplifications and deletions. J Neurooncol 2010; 96:169 - 79; http://dx.doi.org/10.1007/s11060-009-9959-4; PMID: 19609742
- Wang K, Pan L, Che X, Cui D, Li C. Gli1 inhibition induces cell-cycle arrest and enhanced apoptosis in brain glioma cell lines. J Neurooncol 2010; 98:319 - 27; http://dx.doi.org/10.1007/s11060-009-0082-3; PMID: 20024601
- Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu J, Kondo T. Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci 2011; 102:1306 - 12; http://dx.doi.org/10.1111/j.1349-7006.2011.01943.x; PMID: 21453386
- Renner C, Seyffarth A, de Arriba SG, Meixensberger J, Gebhardt R, Gaunitz F. Carnosine inhibits growth of cells isolated from human glioblastoma multiforme. Int J Pept Res Ther 2008; 14:127 - 35; http://dx.doi.org/10.1007/s10989-007-9121-0
- Nordeen SK. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques 1988; 6:454 - 8; PMID: 2908509
- Gaunitz F, Heise K. HTS compatible assay for antioxidative agents using primary cultured hepatocytes. Assay Drug Dev Technol 2003; 1:469 - 77; http://dx.doi.org/10.1089/154065803322163786; PMID: 15090184
- Laurendeau I, Ferrer M, Garrido D, D’Haene N, Ciavarelli P, Basso A, et al. Gene expression profiling of the hedgehog signaling pathway in human meningiomas. Mol Med 2010; 16:262 - 70; http://dx.doi.org/10.2119/molmed.2010.00005; PMID: 20386868