Abstract
The objective of the ONCODEATH consortium [EU Research Consortium “ONCODEATH” (2006–2010)] was to achieve sensitization of solid tumor cells to death receptor related therapies using rational mechanism-based drug combinations of targeted therapies. In this collaborative effort, during a period of 42 mo, cell and animal model systems of defined oncogenes were generated. Exploitation of generated knowledge and tools enabled the consortium to achieve the following research objectives: (1) elucidation of tumor components which confer sensitivity or resistance to TRAIL-induced cell death; (2) providing detailed knowledge on how small molecule Hsp90, Aurora, Choline kinase, BRAF inhibitors, DNA damaging agents, HDAC and DNMT inhibitors affect the intrinsic apoptotic amplification and execution machineries; (3) optimization of combined action of TRAIL with these therapeutics for optimum effects with minimum concentrations and toxicity in vivo. These findings provide mechanistic basis for a pharmacogenomic approach, which could be exploited further therapeutically, in order to reach novel personalized therapies for cancer patients.
Introduction
Solid tumors commonly respond to current anti-tumor therapies slowly and less impressively than hematopoietic malignancies, with cell death characterized not only by apoptosis but also by necrosis, autophagy or mitotic catastrophe. It is likely that the resistance of solid tumors to treatment might be associated with defects in, or deregulation of, different steps of the cell death pathways. A number of attempts have been undertaken to use the knowledge of these defects to design new drugs, which might either activate or re-activate the cell death machinery of tumor cells. Some of the agents arising from this research, in particular therapeutics exploring TRAIL-mediated pro-death signaling, may turn out to be valuable additions to the future therapeutic arsenal, which will most probably include a combination of conventional cytotoxic drugs, molecular target-based pro-apoptotic drugs as well as cancer targeting vehicles.Citation1 Agonistic antibodies against the tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors and a soluble, truncated TRAIL ligand are in phase I/II clinical trials for the treatment of cancer.
Colorectal cancer (CRC) is the third most common malignancy and the fourth most frequent cause of cancer deaths worldwide, with 945,000 estimated new cases and 492,000 deaths per year in industrialized countries, where the lifetime incidence of the disease is 5%.Citation2 Despite of significant progress in novel anti-cancer drug development, surgery still remains the prime treatment with curative potential for both primary and metastatic CRC.Citation2 In the metastatic setting, the implementation of more recent chemotherapeutic agents (oxaliplatin and irinotecan) has further improved the survival advantage provided by 5FU-based regimens. Lately, the clinical evidence of therapeutic efficacy achieved with agents targeting tumor-specific molecular features has generated enormous scientific and public interest because molecular biology insights have been translated into a survival advantage for the patients. CRC develops through a multistep process of mutations and clonal expansion. In particular, activating somatic mutations of the KRAS, BRAF and PIK3CA oncogenes have been closely associated with colorectal carcinogenesis as an early event in about 50%, 10–20% and 10% of CRC patients, respectively. Of great interest is the autonomous nature of these mutations in the RAS-RAF-MEK-ERK-MAP kinase signaling pathway, where mutation of the KRAS is not required and hence not observed in tumors carrying the BRAF V600E mutation.Citation3 A number of BRAF and MEK inhibitors are now in clinical development.Citation4 Also important is that the PI3K pathway, which for a long time has been associated with cell survival in many systems, has now attracted considerable interest for targeted therapies and again several PI3K and AKT inhibitors are in clinical trials.Citation5 PI3K and heat shock protein 90 (Hsp90) are important proteins for cell survival as they control the activation of key molecules and several anti-cancer drugs have been developed to target them. Hsp90 is an ATP-dependent molecular chaperone that is exploited by malignant cells to support activated oncoproteins, including many cancer-associated kinases and transcription factors, and it is essential for oncogenic transformation.Citation6 We hypothesized that the addition of inhibitors of Hsp90 or PI3K to a TRAIL treatment regime might reverse resistance to TRAIL and induce apoptosis in vitro and in vivo. Additional pathways of interest to be examined in this project are those of choline kinaseCitation7,Citation8 and aurora kinase pathwaysCitation9 shown to have an important contribution in tumor progression. Choline kinase α (ChoKα), has been shown to be overexpressed in different tumor-derived cell lines as well as in different human tumors including breast, lung, prostate, colorectal and ovarian cancers. Increased ChoKα enzymatic activity has been observed in human tumors such as breast and colon cancer. Therefore, ChoKα inhibition constitutes an efficient antitumor strategy with demonstrated antiproliferative activity in vitro and antitumor activity in vivo.
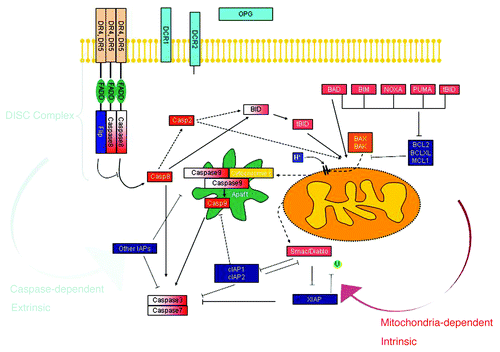
Apoptosis, or programmed cell death, is regulated by tightly controlled intracellular signaling events in response to pathological cytotoxic stimuli, mainly via activating both the intrinsic, mitochondria-based apoptotic signaling pathway and the extrinsic apoptotic signaling pathway directed by the pro-apoptotic ‘death ligands,’ including TNF-α, TNF-related apoptosis-inducing ligand (TRAIL), and Fas.Citation10 Death-inducing signaling complexes (DISCs) of TRAIL-R1 (DR4) or -R2 (DR5) are composed of these receptors, adaptor protein FADD and procaspase-8 or -10. A unique feature of TRAIL-induced apoptotic signaling is its high specificity toward malignant cells while sparing normal unstressed cells. At least in mouse system TRAIL-mediated apoptosis seems to play an important role in elimination of metastatic cells as TRAIL-deficient mice suffer from several times larger number of metastases as their wild-type counterparts.Citation11 Recombinant TRAIL and anti-TRAIL receptors agonistic monoclonal antibodies received significant attention as a potential anti-tumor agent with high specificity and low side-toxicity.Citation12 A number of reports have documented the high potential of these candidate drugs in the killing of tumor cells of various origins. Especially remarkable appears to be the synergism of TRAIL with currently used anti-tumor drugs in inducing the apoptosis of malignant cells. At present, preclinical studies are in progress with recombinant TRAIL or anti-TRAIL receptors agonistic monoclonal antibodies.Citation13
Importantly, not all cancer cells are sensitive to the cytotoxic effects of TRAIL. Notably and in contrast to established cell lines, primary tumors are in many cases resistant to TRAIL or agonistic antibodies.Citation14-Citation16 Moreover, abnormalities of various components of death receptor pathways have been identified in human cancer cells, including deletion, loss or mutation of TRAIL receptors or the initiator caspase-8 as well as overexpression of the activated caspase 8/10 inhibitor FLIP.Citation17 These changes can determine the resistance or sensitivity of a particular cancer cell type to TRAIL-induced apoptosis. Caspase inhibitors from the IAP family (particularly XIAP) and Bcl2 family proteins are also important regulators of mitochondria-dependent apoptosis, participating in blocking or amplification of Death receptor-triggered apoptotic signaling operated in type II cells, in which the intrinsic (mitochondrial) pathway of apoptosis is triggered.Citation18 Importantly, resistance mechanisms operating in tumor cells can be overcome in many cases by the combination of TRAIL treatment with various agents.Citation19 The presence of specific activated oncogenes, like c-myc, RAS through modulation of DR4 and/or DR5 expression and the efficacy of DISC formation and apoptotic signal transduction was shown to provide an explanation for the sensitivity or resistance of tumor cells to TRAIL-induced apoptosis in several systems.Citation20-Citation22 Therefore, it is essential that careful therapeutic strategy selection should be made, as the combination of molecules inhibiting, for example, oncogene pathway components that can potentially play the role of TRAIL agonists may not give the desired synergistic effect.
The ONCODEATH Project
The objective of the ONCODEATH consortium (http://www.eie.gr/nhrf/institutes/ibrb/eu-projects/oncodeath/index-en.html) was to achieve sensitization of solid tumor cells to death receptor related therapies using rational mechanism-based drug combinations of molecularly targeted therapies. The contribution of mutated oncogenes in altering cell death molecules and pathways was examined as a key priority.
The consortium has produced a large number of research tools, such as expression vectors and antibodies. Cell lines with overexpressed or silenced oncogenes, as well as those modulated with respect to factors involved in the signaling and apoptotic pathways in colon cancer have also been generated. Quality assessment of these tools in different applications has been completed. New transgenic mouse models have been generated. Recombinant TRAIL protein has been expressed and utilized. A panel of new small molecule kinase inhibitors have been synthesized and selected for treatment. In addition, improved methods for inducing cell apoptosis and in vivo experiments have been developed and used for the analysis of apoptotic complexes as well as a method for in vivo assessment of tumorigenicity. In addition, rational combinations of the recombinant TRAIL with cell signaling inhibitors have been evaluated. Substantial progress has been made in deciphering the interaction networks operating between oncogenic and apoptotic signaling pathways and protein trafficking at the mitochondria level. The Consortium has identified determinants for inhibitors in sensitizing to apoptosis and has provided detailed knowledge of the effect of the inhibitors on the intrinsic apoptotic amplification and execution machinery related to caspases and Bcl-2 family members during TRAIL-induced apoptosis. We have assessed sensitivity of tumors, both those induced by activated oncogenes in transgenic mice and also molecularly-defined human tumor xenografts in nude mice, to recombinant TRAIL combinations with other molecularly targeted agents.
The current limitations on the effectiveness of current therapeutics in solid tumors, as described above, clearly indicates that the important issue is to further investigate how tumor cells become resistant to apoptosis and based on this fundamental understanding to develop rational mechanism-based strategies to sensitize them to cell death using intelligent drug combinations. Potentially exploitable oncogenic pathways were studied by coordinated use of genomic technologies, imaging technologies, molecular and biochemical analysis, tumor biology approaches and molecular pharmacology studies. The effects of novel molecularly targeted therapeutic agents designed to act on specific oncogenic targets and signaling pathways were tested in cell and animal models. ONCODEATH Members are listed in . The activities presented in and are described below, with the most interesting results generated during the period of 42 mo project duration.
Table 1. The ONCODEATH Consortium
Figure 1. The central objective of this proposal was the investigation of the role of defective apoptotic pathways in human colon tumor cells in order to exploit them in developing novel treatment protocols. A multilateral course of action was planned, which involved genetic, cell biology and biochemical approaches. The Work plan was broken down in activities that partially or totally depended on each other and further dissected to work-packages, which corresponded to the major subdivisions of the program. The structure of the project reflected the complimentarity of the approaches and of the expertise of the participants. Thus, each participant has been contributing to several work-packages. The integration of the single contributions has been fundamental to successfully execute the tasks. A list of developed and/or characterized reagents is presented in .
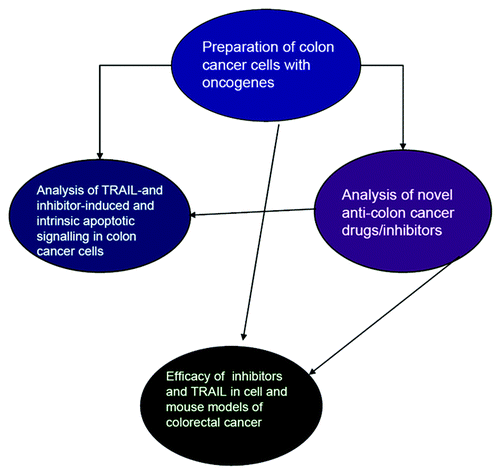
Table 2. Reagents generated and/or characterized by the ONCODEATH consortium
Research tools and approaches produced during the project
For dissecting mechanisms of cross-talk between oncogenic and apoptotic pathways, research tools were generated and exploited during the project. These include: epitope-tagged expression vectors of recombinant TRAIL, recombinant retroviruses/ lentiviruses expressing activated oncogenes and antibodies for DR4 and DR5. In addition, we have generated cell lines with oncogenes including RAS, BRAF, Myc,ChoK, β-catenin stably and/or inducibly overexpressed or silenced by shRNA. Other cell lines with modulation of specific factors like RhoA, Rac1, Cdc42 and apoptotic pathways like Drp1 have been generated. We have isolated and characterized tumor initiating cells and primary tumor cells.Citation16 Quality assessment of these tools in different applications has been completed. New transgenic mouse models have been generated: we have generated triple transgenic mice allowing concomitant and inducible expression of the K-RasV12G oncogene, APC and deletion of both p53 alleles specifically in the intestinal epithelium (ref. Citation23 and manuscript in preparation).
Recombinant forms of TRAIL, and new small molecule kinase inhibitors were selected for treatment
In addition, improved methods for the analysis of TRAIL receptors complexes were developed (site-specific biotin labeling of the recombinant TRAIL) for detecting the initial stages of TRAIL-triggered apoptotic signaling. Biotin-labeled TRAIL was then used for the evaluation of a role of endocytosis of the activated receptors complexes or of an effect of activated and overexpressed oncogenes on TRAIL receptors DISC formation. Using an array of various detergents we also optimized protocol for cell lysis and follow-up DISC precipitation.
As one of the major achievements, we have established a high-throughput method for apoptosis measurement for cell lines using the INCell Analyzer, a high-content imaging instrument which is able to measure and analyze many morphological parameters automatically. Direct orthotopic cell microinjection (OCMI) of human colorectal cancer cell lines in animals has been worked out.Citation24 Development and preparation of new forms of tagged (including biotin labeled) recombinant TRAIL, was completed. Improved protocols for detection of TRAIL receptors DISC complex detection have been produced. Newly synthesized PI3K,Citation25 Hsp90,Citation26-Citation28 AuroraCitation29 inhibitors have been studied; choline kinase inhibitors have been characterized further.
Rational combinations of TRAIL with inhibitors have been evaluated
Activated kinase pathways are frequently conferring resistance to TRAIL-induced apoptosis in cancer cells. Therefore, we decided to combine inhibitors of selected pathways with TRAIL, to make tumor cells more sensitive to death. Importantly, sensitization has been observed with combinatorial treatment of TRAIL combined with BRAF or Hsp90 inhibitors that is dependent on the tumor mutation profile. Quercetin is a polyphenol, which has been shown to inhibit cell signaling pathways, such as PI3K pathway. We have shown that quercetin causes colon cancer cells to exhibit autophagic properties,Citation30 and also that it synergizes with TRAIL to induce cell death in colon cancer cells;Citation31 BRAF inhibitors cooperate with TRAIL to overcome oncogenic PIK3CA resistance.Citation32 Significant sensitivity to TRAIL induced apoptosis exhibited by the BRAFV600E transformed cells both at low dose and short incubation time was directly related to the rapid formation and activation of DISC complex in comparison to the control and sensitive to TRAIL, DLD-1 cells. Sensitivity of these cells to TRAIL was also related to overexpression of pro-apoptotic molecules such as DR4, DR5 and BID and the simultaneous downregulation of anti-apoptotic Bcl-2. In summary, for cell lines bearing endogenous BRAFV600E and ectopically expressed BRAFV600E, TRAIL receptors DR4 and DR5 are overexpressed and this can account at least partially for the observed sensitivity of these tumor cells to TRAIL. On the other hand, co-treatment of BRAF specific inhibitor with TRAIL increases apoptosis only in some colon cell lines bearing endogenous BRAFV600E, which can be further increased by co-treatment of PI3K, as a third agent.Citation32 It seems that the mechanism in this latter case depends on the tumor cell line and is currently under investigation.
Data suggest that driver mutations not only in BRAF but also in PIK3CA may confer sensitivity to TRAIL. Co-treatment of BRAF inhibitors with PI3K inhibitors and/or TRAIL can overcome PI3K resistance, depending on the mutation status of the tumor.
Genetic interference of choline kinase by siRNA, as well as pharmacologic inhibition by ChoK inhibitors (ChoKi), induce cell death in selected human cancer cells;Citation33-Citation35 protocols in which selected ChoKi cooperate with TRAIL in inducing colon cancer cell apoptosis have been generated (Álvarez-Miranda et al., submitted). To get an insight in the cellular mechanisms underlying ChoKi potentiating of TRAIL-induced apoptosis, the effect on TRAIL receptors (DR4 and DR5) gene expression were also investigated by quantitative RT-PCR after treatments. No significant changes were seen in DR4 message levels. However, DR5 expression was specifically increased, in a dose-dependent manner, in both DLD-1 and SW620 ChoKi-treated cells and this increased expression was functional (Álvarez-Miranda et al., submitted). As a proof of functional activation, the effect of ChoKi treatment on the expression of DR4 and DR5 in both cell lines on the cell surface was also analyzed by flow cytometry. Selected ChoKi increased DR5 cell surface expression in both SW620 and DLD-1 cell lines. However, no changes in DR4 cell surface expression were observed. Two new enhancers of TRAIL-induced apoptosis of TRAIL-resistant colorectal cancer cells, homoharringtonine (a plant alkaloid derived from the Cephalotuxus fortuneii) and manumycin A, have been identified (Beranova et al. and Borovickova et al., in preparation). Manumycin A, a fanesyltransferase inhibitor, has been shown to cause autophagy in cancer cells. Therefore, we decided to test the hypothesis of its potential synergy with TRAIL. Manumycin A-mediated sensitizing effect was unrelated to TRAIL receptors expression but was partly dependent on ROS production and activation of Mst1/Hippo kinase. Manumycin A did not affect FLIP or XIAP expression in analyzed cell lines but suppressed Mcl-1 expression. Homoharringtonine (HHT) is a quite potent inhibitor of protein synthesis and it is also currently used for alternative treatment of chronic leukemias (X2).Citation36 We tested the hypothesis that HHT could synergize with TRAIL, since they affect different and potentially synergistic pathways for cell death. We found that at the effective 50 nM combinatory concentration, HHT efficiently sensitized TRAIL-resistant RKO and HT-29 cells to TRAIL-mediated elimination of these cells. HHT pro-apoptotic effect is apparently related to downregulation of Mcl-1 expression and activation of JNK kinase. Efficient protocols for treatment of cancer cells with TRAIL alone or in combination with PI3K inhibitors have been established.Citation32 The sensitization mechanism is under investigation, but it may relate to the established role of PI3K pathway as a cell survival mechanism. We identified novel Aurora kinase inhibitor which synergize with TRAIL by increased cleavage of caspase 7 and PARP1. The detailed mechanism of how Aurora kinase inhibitors induce apoptosis is still under investigation. Small cell lung carcinoma cells (SCLC) are resistant to TRAIL. The majority of human SCLC cells have been shown to be resistant to death receptor (DR)-mediated apoptosis because of defects in key signaling molecules, such as DRs and/or initiator caspases. Results suggest that the combination of TRAIL and DNA damaging anticancer agents might be beneficial for elimination of caspase-8-containing SCLC cells.Citation37 We found that inhibition of the main enzymes involved in DNA methylation, namely DNMT1, 3a and 3b, is not sufficient to restore caspase-8 expression (or TRAIL sensitivity) in a panel of SCLC cell lines, suggesting that some other mechanisms might be involved in caspase-8 silencing. More detailed investigation revealed that a combination of the DNMT inhibitor decitabine with an HDAC inhibitor, either valproic acid (VPA) or CI-994, efficiently restored expression of caspase-8 in SCLC cell lines and reduced the levels of cIAP-1 and survivin. Moreover, the combination of these two epigenetic drugs sensitized SCLC cells to TRAIL.Citation38 The most efficient combinatorial treatments are summarized in .
Table 3. Summary of evaluated combinatorial treatments in pre-clinical studies in 2D, 3D cell cultures and animal models
Deciphering the interaction networks of oncogenic and apoptotic pathways and trafficking at the mitochondrial level
From a mechanistic point of view, the Consortium has identified determinants for signaling inhibitors and DNA damaging agents in sensitizing to apoptosis, as well as providing detailed knowledge of the effects of these small molecules on the intrinsic apoptotic amplification and execution machinery related to caspases and Bcl-2 family members during TRAIL-induced apoptosis.
Major achievements include identifying new mobilizing components of Death receptorsCitation39 and assessing the effect of endosomal signaling on the efficacy of caspase-8 processing (Horova et al., in preparation). TRAIL receptor overexpression has been detected in KRAS/BRAF mutant colorectal tumor samples.Citation40 Expression of choline kinase predicts survival of lung cancer patients.Citation41 p73 and p73 fragments affect TRAIL-induced apoptosis.Citation42 We have discovered that Aurora-A regulates nuclear factor-kappaB signaling by phosphorylation of IkappaB α.Citation43 We discovered that Bax activation and stress-induced apoptosis is modulated by the fluidity of the outer mitochondrial membrane.Citation44,Citation45 Increased cholesterol levels in this membrane decrease the ability of Bax to oligomerize upon tBid-induced membrane recruitment and insertion. Moreover, we have shown that membrane hemifusion or hemifission induced by the dynamin related protein Drp1 stimulates tBid induced Bax oligomerization.Citation46 A role of caspase-2 as initiator caspase was also observed in apoptosis triggered by other chemotherapeutic drugs. The presented model for caspases-2 activation includes PIDDosome and DISC complexes formation.Citation47 Mechanisms of regulation of the molecular chaperone Hsp90, of which numerous client proteins are oncogenic, have been analyzed in detail,Citation48,Citation49 providing further information for more efficient drug targeting. In summary, dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Simultaneously reducing the expression of both of these isoforms induces proteasome-dependent degradation of HSP90 client proteins, G1 cell-cycle arrest and extensive tumor-specific apoptosis. Molecules/pathways of the intrinsic apoptotic machinery were identified that are affected by choline kinase inhibitors and can potentially synergize with TRAIL in inducing apoptosis of colon cancer cells.
The decreased expression or activation of key survival molecules together with PI3K or HSP90 inhibitor plus TRAIL gave a strong apoptotic response in CRC providing not only a biological mechanistic explanation but also an indication for molecular biomarkers that could be used in future clinical trials
Sensitivity of tumors in transgenic and nude mice to inhibitors alone or in combination with TRAIL
TRAIL has emerged as a promising targeted cancer therapy based on its selectivity toward tumor cells without causing toxicity in normal cells.Citation50-Citation53 TRAIL resistance has been associated with defective ceramide signaling, since combination of C6-ceramide with TRAIL overcomes resistance of SW620 cells to TRAIL-induced apoptosisCitation54 and increased CerS6 expression overcomes TRAIL resistance in SW620 cells.Citation55 As ceramide levels increase specifically in tumor cells after treatment with choline kinase inhibitors,Citation56 we speculated that a synergism could be observed when combining choline kinase inhibitors with TRAIL, especially in tumor cells resistant to TRAIL. Major achievements include demonstration of choline kinase inhibitor-mediated enhancement of TRAIL antitumor activity in colon cancer patient xenograft/SCID mouse models in vivo. Determination of the sensitivity to choline kinase inhibitors of tumors implanted in immunosuppressed mice was performed and assessment of their optimal dosing and in vivo synergism with the recombinant human TRAIL was performed
Final Remarks
Until recently, the approaches to identifying new cancer treatments have been relatively unsophisticated, relying on non-specific strategies that could kill both tumor cells and normal cells.Citation57 New anticancer drugs were identified by measuring effects on cancer cell proliferation or inhibition of tumor growth in experimental animals. However, a convergence of scientific advances in cancer genetics and cell and molecular biology has facilitated the identification of unique molecular targets specific to cancer cells. Alongside genetic methods for target validation, especially RNAi, the use of small molecule tools, or chemical probes, has proved to be extremely useful.Citation58 With an increased understanding of the biological basis for cancer onset and progression, the molecular changes that distinguish malignant cells from normal cells are becoming increasingly apparent, offering a growing range of potential drug targets in the form of altered genes, proteins or corrupted pathways. The increased selectivity offered by these unique targets offers drug developers the opportunity to implement more efficacious and less toxic molecularly targeted treatments. These new opportunities have not gone unnoticed by academic drug discovery groups, biotech and the big Pharma corporations, resulting in promising clinical results with prototypal drugs.Citation59
Already many publications document sensitizing effects of a number of drugs, including DNA damaging agents, proteasome or histone deacetylases inhibitors various kinase inhibitors on TRAIL-induced apoptosis, but it is likely that only fraction of them will be eventually used for a TRAIL-based combinatorial cancer therapies. Our findings, summarized in , that inhibitors of choline kinase, PI3K, BRAF, Aurora kinase and the molecular chaperone Hsp90, as well as quercetin, manumycin A and homoharringtonine can each sensitize cancer cells to TRAIL-induced apoptosis indicates considerable potential for combination treatments. In selected combinatorial treatments, synergistic effect has been observed, in the pre-clinical models. In other cases, synergy was observed only under specific conditions, as indicated in (refs. Citation30-Citation32,Citation37,Citation38 and submitted manuscripts). Rational development of these promising combinatorial treatments, including the use of biomarker-led clinical trials, may save time and money, increase therapeutic efficacy and reduce side effects, thus enhancing patients response and life expectancy.Citation60
Conventional cancer treatments have been based mostly on the ‘one size fits all’ approach. Patients are prescribed cancer drugs in standard doses that are later adjusted by a ‘trial and error’ process. Patients may suffer severe side effects, and while some respond well to the treatment, others show no improvement. Innovations in the fields of genetics and molecular cancer therapeutics have led to a new, personalized treatment approach in which understanding the genetic basis of the individual patient and his or her tumor can help to determine the patient's eligibility for a proposed treatment and predict its success. The advantages of this approach to patients are self-evident. ONCODEATH has used the approach of identifying new cancer drugs under development such as TRAIL and choline kinase, PI3K, BRAF, Aurora and Hsp90 inhibitors as a basis for the construction of novel combinatorial strategies. Our findings, for example, that simultaneous combinatorial treatment of kinase inhibitors and TRAIL shows a strong synergistic effect can provide an alternative treatment approach to those that are currently used in the clinic today. TRAIL and TRAIL-related reagents (currently agonistic anti-TRAIL receptors antibodies, and potentially also small molecules and peptides that activate the apoptotic signaling) are now being recognized and tested as an important and potentially very effective, additional weapons in our ongoing war against malignant transformation and progression. As with all weapons, their effective and safe use needs to be linked to a detailed understanding of the target and its context, i.e., characterization and regulation of TRAIL-induced signaling in cancer cells. Thus, our characterization of the “mode of action” of selected colorectal cancer-related oncogenes in the regard to TRAIL-induced apoptosis could be used for better understanding and implementation of future TRAIL-related treatment strategies of cancer patients.Citation60
In conclusion, we have made considerable progress toward our objective of achieving sensitization of solid tumor cells to death receptor related therapies using rational mechanism-based drug combinations. The targeted drug combinations presented have identified in many cases the mechanism of their action, and have been frequently selected based on the expression and mutation status of the target in the particular tumor. The approaches identified have considerable potential for the treatment of CRC and other malignancies.
NOTE TO AUTHOR: Please cite in the text.
Acknowledgments
This work is supported by the EU grant LSHC-CT-2006–037278 “ONCODEATH.”
Conflict of Interest
P. Workman and P.A. Clarke are employees of The Institute of Cancer Research, which has a commercial interest in the development of PI3K and HSP90 inhibitors and operates a rewards-to-inventors scheme. P. Workman and his team have been involved in a commercial collaboration with Yamanouchi (now Astellas Pharma) and with Piramed Pharma, and intellectual property on PI3K inhibitors arising from the program has been licensed to Genentech. P. Workman was a founder of, consultant to, Scientific Advisory Board member of, and stockholder in Piramed Pharma, which was acquired by Roche. P. Workman and his team have also been involved in a commercial collaboration with Vernalis and intellectual property on HSP90 inhibitors arising from the program has been licensed to Novartis. P. Workman has been a consultant to Novartis. Juan Carlos Lacal is a government employee of CSIC, an Agency of the Spanish Department of Economy and Competitiveness, that has a commercial interest in the development of choline kinase inhibitors as therapeutic drugs. JC Lacal is a founder and stockholder of TCD Pharma, a company which has been licensed for the commercial exploitation of choline kinase inhibitors from CSIC.
References
- Viktorsson K, Lewensohn R, Zhivotovsky B. Apoptotic pathways and therapy resistance in human malignancies. Adv Cancer Res 2005; 94:143 - 96; http://dx.doi.org/10.1016/S0065-230X(05)94004-9; PMID: 16096001
- Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet 2005; 365:153 - 65; http://dx.doi.org/10.1016/S0140-6736(05)17706-X; PMID: 15639298
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949 - 54; http://dx.doi.org/10.1038/nature00766; PMID: 12068308
- Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res 2010; 16:3329 - 34; http://dx.doi.org/10.1158/1078-0432.CCR-09-3064; PMID: 20472680
- Workman P, Clarke PA, Raynaud FI, van Montfort RL. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res 2010; 70:2146 - 57; http://dx.doi.org/10.1158/0008-5472.CAN-09-4355; PMID: 20179189
- Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet?. Clin Cancer Res 2012; 18:64 - 76; http://dx.doi.org/10.1158/1078-0432.CCR-11-1000; PMID: 22215907
- Hernando E, Sarmentero-Estrada J, Koppie T, Belda-Iniesta C, Ramírez de Molina V, Cejas P, et al. A critical role for choline kinase-alpha in the aggressiveness of bladder carcinomas. Oncogene 2009; 28:2425 - 35; http://dx.doi.org/10.1038/onc.2009.91; PMID: 19448670
- Ramírez de Molina A, Gallego-Ortega D, Sarmentero J, Bañez-Coronel M, Martín-Cantalejo Y, Lacal JC. Choline kinase is a novel oncogene that potentiates RhoA-induced carcinogenesis. Cancer Res 2005; 65:5647 - 53; http://dx.doi.org/10.1158/0008-5472.CAN-04-4416; PMID: 15994937
- Cammareri P, Scopelliti A, Todaro M, Eterno V, Francescangeli F, Moyer MP, et al. Aurora-a is essential for the tumorigenic capacity and chemoresistance of colorectal cancer stem cells. Cancer Res 2010; 70:4655 - 65; http://dx.doi.org/10.1158/0008-5472.CAN-09-3953; PMID: 20460511
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 1998; 281:1305 - 8; http://dx.doi.org/10.1126/science.281.5381.1305; PMID: 9721089
- Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, et al. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest 2008; 118:100 - 10; http://dx.doi.org/10.1172/JCI33061; PMID: 18079967
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5:157 - 63; http://dx.doi.org/10.1038/5517; PMID: 9930862
- Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene 2008; 27:6207 - 15; http://dx.doi.org/10.1038/onc.2008.298; PMID: 18931688
- Wu XX, Kakehi Y. Enhancement of lexatumumab-induced apoptosis in human solid cancer cells by Cisplatin in caspase-dependent manner. Clin Cancer Res 2009; 15:2039 - 47; http://dx.doi.org/10.1158/1078-0432.CCR-08-2667; PMID: 19276256
- Koschny R, Holland H, Sykora J, Haas TL, Sprick MR, Ganten TM, et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res 2007; 13:3403 - 12; http://dx.doi.org/10.1158/1078-0432.CCR-07-0251; PMID: 17545549
- Oikonomou E, Kothonidis K, Taoufik E, Probert E, Zografos G, Nasioulas G, et al. Newly established tumourigenic primary human colon cancer cell lines are sensitive to TRAIL-induced apoptosis in vitro and in vivo. Br J Cancer 2007; 97:73 - 84; http://dx.doi.org/10.1038/sj.bjc.6603835; PMID: 17551494
- Drosopoulos K, Pintzas A. Multifaceted targeting in cancer: the recent cell death players meet the usual oncogene suspects. Expert Opin Ther Targets 2007; 11:641 - 59; http://dx.doi.org/10.1517/14728222.11.5.641; PMID: 17465723
- Grandemange S, Herzig S, Martinou JC. Mitochondrial dynamics and cancer. Semin Cancer Biol 2009; 19:50 - 6; http://dx.doi.org/10.1016/j.semcancer.2008.12.001; PMID: 19138741
- Amm HM, Oliver PG, Lee CH, Li Y, Buchsbaum DJ. Combined modality therapy with TRAIL or agonistic death receptor antibodies. Cancer Biol Ther 2011; 11:431 - 49; http://dx.doi.org/10.4161/cbt.11.5.14671; PMID: 21263219
- Wang Y, Engels IH, Knee DA, Nasoff M, Deveraux QL, Quon KC. Synthetic lethal targeting of MYC by activation of the DR5 death receptor pathway. Cancer Cell 2004; 5:501 - 12; http://dx.doi.org/10.1016/S1535-6108(04)00113-8; PMID: 15144957
- Nesterov A, Nikrad M, Johnson T, Kraft AS. Oncogenic Ras sensitizes normal human cells to tumor necrosis factor-alpha-related apoptosis-inducing ligand-induced apoptosis. Cancer Res 2004; 64:3922 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-03-2219; PMID: 15173003
- Drosopoulos KG, Roberts ML, Cermak L, Sasazuki T, Shirasawa S, Andera L, et al. Transformation by oncogenic RAS sensitizes human colon cells to TRAIL-induced apoptosis by up-regulating death receptor 4 and death receptor 5 through a MEK-dependent pathway. J Biol Chem 2005; 280:22856 - 67; http://dx.doi.org/10.1074/jbc.M412483200; PMID: 15757891
- Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 2006; 131:1096 - 109; http://dx.doi.org/10.1053/j.gastro.2006.08.011; PMID: 17030180
- Céspedes MV, Espina C, García-Cabezas MA, Trias M, Boluda A, Gómez del Pulgar MT, et al. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol 2007; 170:1077 - 85; http://dx.doi.org/10.2353/ajpath.2007.060773; PMID: 17322390
- Al-Saffar NM, Jackson LE, Raynaud FI, Clarke PA, Ramírez de Molina A, Lacal JC, et al. The phosphoinositide 3-kinase inhibitor PI-103 downregulates choline kinase alpha leading to phosphocholine and total choline decrease detected by magnetic resonance spectroscopy. Cancer Res 2010; 70:5507 - 17; http://dx.doi.org/10.1158/0008-5472.CAN-09-4476; PMID: 20551061
- Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res 2008; 68:2850 - 60; http://dx.doi.org/10.1158/0008-5472.CAN-07-5256; PMID: 18413753
- Gaspar N, Sharp SY, Pacey S, Jones C, Walton M, Vassal G, et al. Acquired resistance to 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) in glioblastoma cells. Cancer Res 2009; 69:1966 - 75; http://dx.doi.org/10.1158/0008-5472.CAN-08-3131; PMID: 19244114
- Gaspar N, Sharp SY, Eccles SA, Gowan S, Popov S, Jones C, et al. Mechanistic evaluation of the novel HSP90 inhibitor NVP-AUY922 in adult and pediatric glioblastoma. Mol Cancer Ther 2010; 9:1219 - 33; http://dx.doi.org/10.1158/1535-7163.MCT-09-0683; PMID: 20457619
- Chan F, Sun C, Perumal M, Nguyen QD, Bavetsias V, McDonald E, et al. Mechanism of action of the Aurora kinase inhibitor CCT129202 and in vivo quantification of biological activity. Mol Cancer Ther 2007; 6:3147 - 57; http://dx.doi.org/10.1158/1535-7163.MCT-07-2156; PMID: 18089709
- Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A. Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha-RAS-transformed human colon cells. Carcinogenesis 2007; 28:1021 - 31; http://dx.doi.org/10.1093/carcin/bgl232; PMID: 17148506
- Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther 2007; 6:2591 - 9; http://dx.doi.org/10.1158/1535-7163.MCT-07-0001; PMID: 17876056
- Oikonomou E, Koc M, Sourkova V, Andera L, Pintzas A. Selective BRAFV600E inhibitor PLX4720, requires TRAIL assistance to overcome oncogenic PIK3CA resistance. PLoS One 2011; 6:e21632; http://dx.doi.org/10.1371/journal.pone.0021632; PMID: 21738740
- Rodríguez-González A, Ramírez de Molina A, Fernández F, Ramos MA, del Carmen Núñez M, Campos J, et al. Inhibition of choline kinase as a specific cytotoxic strategy in oncogene-transformed cells. Oncogene 2003; 22:8803 - 12; http://dx.doi.org/10.1038/sj.onc.1207062; PMID: 14654777
- Rodríguez-González A, Ramírez de Molina A, Fernández F, Lacal JC. Choline kinase inhibition induces the increase in ceramides resulting in a highly specific and selective cytotoxic antitumoral strategy as a potential mechanism of action. Oncogene 2004; 23:8247 - 59; http://dx.doi.org/10.1038/sj.onc.1208045; PMID: 15378008
- Bañez-Coronel M, Ramírez de Molina A, Rodríguez-González A, Sarmentero J, Ramos MA, García-Cabezas MA, et al. Choline kinase alpha depletion selectively kills tumoral cells. Curr Cancer Drug Targets 2008; 8:709 - 19; http://dx.doi.org/10.2174/156800908786733432; PMID: 19075594
- Quintás-Cardama A, Cortes J. Homoharringtonine for the treatment of chronic myelogenous leukemia. Expert Opin Pharmacother 2008; 9:1029 - 37; http://dx.doi.org/10.1517/14656566.9.6.1029; PMID: 18377344
- Vaculova A, Kaminskyy V, Jalalvand E, Surova O, Zhivotovsky B. Doxorubicin and etoposide sensitize small cell lung carcinoma cells expressing caspase-8 to TRAIL. Mol Cancer 2010; 9:87; http://dx.doi.org/10.1186/1476-4598-9-87; PMID: 20416058
- Kaminskyy VO, Surova OV, Vaculova A, Zhivotovsky B. Combined inhibition of DNA methyltransferase and histone deacetylase restores caspase-8 expression and sensitizes SCLC cells to TRAIL. Carcinogenesis 2011; 32:1450 - 8; http://dx.doi.org/10.1093/carcin/bgr135; PMID: 21771726
- Símová S, Klíma M, Cermak L, Sourková V, Andera L. Arf and Rho GAP adapter protein ARAP1 participates in the mobilization of TRAIL-R1/DR4 to the plasma membrane. Apoptosis 2008; 13:423 - 36; http://dx.doi.org/10.1007/s10495-007-0171-8; PMID: 18165900
- Oikonomou E, Kosmidou V, Katseli A, Kothonidis K, Mourtzoukou D, Kontogeorgos G, et al. TRAIL Receptor Upregulation Correlates to KRAS/ BRAF Mutations in Human Colon Cancer Tumors and Respective Normal Tissue. Int J Cancer 2009; 125:2127 - 35; http://dx.doi.org/10.1002/ijc.24613; PMID: 19637313
- Ramírez de Molina A, Sarmentero-Estrada J, Belda-Iniesta C, Tarón M, Ramírez de Molina V, Cejas P, et al. Expression of choline kinase alpha to predict outcome in patients with early-stage non-small-cell lung cancer: a retrospective study. Lancet Oncol 2007; 8:889 - 97; http://dx.doi.org/10.1016/S1470-2045(07)70279-6; PMID: 17851129
- Sayan AE, Sayan BS, Gogvadze V, Dinsdale D, Nyman U, Hansen TM, et al. P73 and caspase-cleaved p73 fragments localize to mitochondria and augment TRAIL-induced apoptosis. Oncogene 2008; 27:4363 - 72; http://dx.doi.org/10.1038/onc.2008.64; PMID: 18362891
- Briassouli P, Chan F, Savage K, Reis-Filho JS, Linardopoulos S. Aurora-A regulation of nuclear factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer Res 2007; 67:1689 - 95; http://dx.doi.org/10.1158/0008-5472.CAN-06-2272; PMID: 17308110
- Lucken-Ardjomande S, Montessuit S, Martinou JC. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ 2008; 15:929 - 37; http://dx.doi.org/10.1038/cdd.2008.9; PMID: 18259190
- Lucken-Ardjomande S, Montessuit S, Martinou JC. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ 2008; 15:484 - 93; http://dx.doi.org/10.1038/sj.cdd.4402280; PMID: 18084240
- Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 2010; 142:889 - 901; http://dx.doi.org/10.1016/j.cell.2010.08.017; PMID: 20850011
- Olsson M, Vakifahmetoglu H, Abruzzo PM, Högstrand K, Grandien A, Zhivotovsky B. DISC-mediated activation of caspase-2 in DNA damage-induced apoptosis. Oncogene 2009; 28:1949 - 59; http://dx.doi.org/10.1038/onc.2009.36; PMID: 19347032
- Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell 2008; 14:250 - 62; http://dx.doi.org/10.1016/j.ccr.2008.08.002; PMID: 18772114
- Vaughan CK, Mollapour M, Smith JR, Truman A, Hu B, Good VM, et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell 2008; 31:886 - 95; http://dx.doi.org/10.1016/j.molcel.2008.07.021; PMID: 18922470
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5:157 - 63; http://dx.doi.org/10.1038/5517; PMID: 9930862
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3:673 - 82; http://dx.doi.org/10.1016/1074-7613(95)90057-8; PMID: 8777713
- de Jong S, Timmer T, Heijenbrok FJ, de Vries EG. Death receptor ligands, in particular TRAIL, to overcome drug resistance. Cancer Metastasis Rev 2001; 20:51 - 6; http://dx.doi.org/10.1023/A:1013112624971; PMID: 11831647
- Gura T. How TRAIL kills cancer cells, but not normal cells. Science 1997; 277:768; http://dx.doi.org/10.1126/science.277.5327.768; PMID: 9273698
- Voelkel-Johnson C, Hannun YA, El-Zawahry A. Resistance to TRAIL is associated with defects in ceramide signaling that can be overcome by exogenous C6-ceramide without requiring down-regulation of cellular FLICE inhibitory protein. Mol Cancer Ther 2005; 4:1320 - 7; http://dx.doi.org/10.1158/1535-7163.MCT-05-0086; PMID: 16170023
- White-Gilbertson S, Mullen T, Senkal C, Lu P, Ogretmen B, Obeid L, et al. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene 2009; 28:1132 - 41; http://dx.doi.org/10.1038/onc.2008.468; PMID: 19137010
- Rodríguez-González A, Ramirez de Molina A, Fernández F, Lacal JC. Choline kinase inhibition induces the increase in ceramides resulting in a highly specific and selective cytotoxic antitumoral strategy as a potential mechanism of action. Oncogene 2004; 23:8247 - 59; http://dx.doi.org/10.1038/sj.onc.1208045; PMID: 15378008
- Zhivotovsky B, Orrenius S. Clinical perspectives of cell death: where we are and where to go.. Apoptosis 2009; 14:333 - 5; http://dx.doi.org/10.1007/s10495-009-0326-x; PMID: 19238546
- Workman P, Collins I. Probing the probes: fitness factors for small molecule tools. Chem Biol 2010; 17:561 - 77; http://dx.doi.org/10.1016/j.chembiol.2010.05.013; PMID: 20609406
- Workman P, de Bono J. Targeted therapeutics for cancer treatment: major progress towards personalised molecular medicine. Curr Opin Pharmacol 2008; 8:359 - 62; http://dx.doi.org/10.1016/j.coph.2008.07.007; PMID: 18678282
- Yap TA, Sandhu SK, Workman P, de Bono JS. Envisioning the future of early anticancer drug development. Nat Rev Cancer 2010; 10:514 - 23; http://dx.doi.org/10.1038/nrc2870; PMID: 20535131