Abstract
We investigated the role of vasculogenic mimicry (VM) in the progression of prostate cancer (PCa). Ninety-six patients who had undergone prostatectomy for treatment of PCa and for whom a complete record of clinical and follow-up data were available were reviewed. VM and matrix metalloproteinase-2 (MMP-2) were detected by immunohistochemical staining in frozen tissue sections. Relationship between VM and clinicopathological variables was analyzed statistically. Multivariate analyses were performed to assess the prognostic significance of VM. Results showed that out of the 96 PCa cases studied here, VM was detectable in 24 (25%) and was positively correlated with preoperative prostate-specific antigen (PSA) level, Gleason score, pathological stage, lymph node metastasis, seminal vesicle invasion, distant metastasis and PSA doubling time (PSADT). Univariate analysis showed that VM, PSA level, Gleason score, distant metastasis and PSADT were correlated with overall survival (OS), while VM, Gleason score, distant metastasis, local recurrence and PSADT were correlated with disease-free survival (DFS). Multivariable analysis indicated that the presence of VM, higher Gleason score and distant metastasis were the adverse predictors of OS and DFS. A higher widespread staining for MMP-2 was correlated with the VM-positive subgroup. In conclusion, VM mainly exists in the high risk PCa patients and is an independent marker of poor prognosis.
Introduction
It has long been thought that angiogenesis is the only way to get the blood supply to a tumor, thus making vascular endothelial cells as a target of anti-angiogenesis therapy.Citation1,Citation2 However, tumor angiogenesis is not the only mechanism of blood supply for tumor microcirculation. Some highly aggressive tumor cells can simulate the structure of endothelium-dependent vessels (EDV) to form a new tumor blood supply. These new vessels have no endothelial lining and are mainly composed of basement membrane-like material. These channels constitute the vasculogenic mimicry (VM) and the process is called “vasculogenesis.”Citation3,Citation4 Since its discovery, VM has been described in many kinds of tumors, such as melanoma,Citation5 synovial sarcoma,Citation6 rhabdomyosarcoma,Citation6 osteosarcoma,Citation7 inflammatory and ductal breast carcinomaCitation8 and ovarian carcinoma.Citation9,Citation10 Most of these studies have shown some association between angiogenesis or VM proliferation in relationship with the aggressiveness of the tumor. Therapeutic implications of VM have also been described in aggressive prostate cancer (PCa) in vitro.Citation11 However, VM as a prognostic marker remains debatable as there is at least one study showing that there is no significant correlation between VM channels and histological grading of PCa.Citation12 Studies on the relationship between VM and PCa are scarce.Citation13,Citation14 In the current study, we looked for VM in 96 cases of PCa retrospectively to determine the significance of VM in clinical pathology and prognosis. In addition, we also explored the possible molecular mechanism of VM formation by measuring levels of one of the known key factors, matrix metalloproteinase-2 (MMP-2), in the PCa patient samples. Establishing such correlations, particularly in advanced PCa, may assist in better patient stratification and also in the search for newer therapeutic strategies.
Results
Characteristics and follow up of PCa patients
A total of 96 cases, who underwent RRP operation for treatment of prostate cancer and for whom full clinical records were available were included in this retrospective study. The mean age of the study population at the time of RRP was 65 y (range 56–75 y). The median preoperative PSA level was 20.4 ng/ml (range 2.60–96.60 ng/ml). Twenty patients (20.83%) had a positive surgical margin, 14 patients (14.58%) had seminal vesicle invasion, and 12 patients (12.50%) had lymph node metastasis. Patients in pTNM stages T2a, T2b, T2c, T3a, T3b were 33 (34.37%), 26 (27.08%), 17 (17.71%), 10 (10.42%) and 10 (10.42%), respectively. The mean follow-up time was 88 months (range 12–165 months). Sixty-eight patients (70.83%) were alive, when the follow up ended and 28 patients (29.17%) died as a result of their malignancy. The mean disease-free survival (DFS) was 48 months. Local recurrence was observed in 32 patients (33.33%). The mean period from initial surgery to the first local recurrence or metastasis was 93.65 months (range 5–126 months). Twenty-two patients (22.91%) developed bone metastasis, 1 (1.04%) patient developed bone and liver metastasis. The median PSADT was 9.0 months (range 3–18 months).
Morphologic findings of VM in PCa tissues
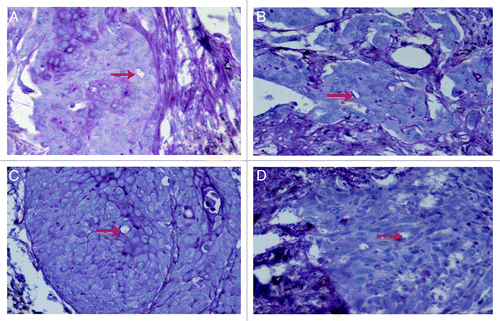
VM was detected preliminarily by simple hematoxylin-eosin staining of the frozen tissue sections from patients (data not shown). It could be seen as vessels formed by tumor cells but not the endothelial cells, without hemorrhage, necrosis, or inflammatory cells infiltrating around these structures. CD31/PAS dual-staining was then used to validate the presence of VM. CD31-negative, PAS-positive loops could be seen surrounding tumor cells (not endothelial cells), with or without red blood cells (). Twenty-four of 96 cases (25.0%) were VM-positive by PAS-CD31 dual staining. However, the membrane formed by the PAS-positive substance was not absolutely necessary for the existence of VM in the current study. Sometimes, VM are just small vessels surrounded by tumor cells and PAS-negative. The PAS-positive membrane could be seen as a complete loop (), arc (), line () or was absent (). Only ten cases (10.42%) showed complete loops in the current study.
Figure 1. Detection of vascular mimicry in prostate cancer tissues by using CD31-PAS dual staining. The VM channel (red arrow) is formed by tumor cells. There are red blood cells in the center of the channel. PAS-positive substance (rose red) lines the channel and forms a basement membrane-like structure. The absence of necrosis and hemorrhage in the tumor tissue near the VM channel is seen. Endothelium-dependent vessels (blue arrows) are lined by endothelial cells, which are stained by CD31 (brown). Original magnification: × 400.
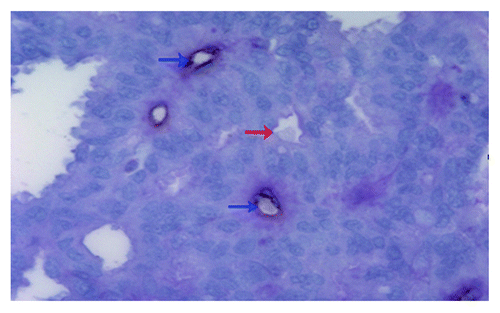
Figure 2. CD31-PAS dual staining to visualize different morphologies of the basement membrane-like structure formed by PAS-positive (rose red) substance in vascular mimicry in prostate cancer tissues. (A) Complete loop; (B) arc; (C) line; (D) absent, without PAS-positive substance. Original magnification: × 200.
Correlation of VM with clinicopathological variables
VM expression and its correlation with clinicopathological variables are listed in . The positive correlation of VM was significantly higher in cases with higher pre-operative PSA level, higher Gleason score and higher pathological stage than those with lower pre-operative PSA level, lower Gleason score or lower pathological stage (all p < 0.001). According to the subgroup analysis, incidence of VM was 9.1% (2/22), 18.0% (9/50) and 54.2% (13/24) in PSA subgroups of ≤ 10, 10–20 and ≥ 20 ng/L, respectively; 6.3% (1/16), 9.6% (5/52) and 64.3% (18/28) in Gleason score subgroups of ≤ 6, = 7 and ≥ 8 respectively; and 11.8% (9/76) and 75% (15/20) in pT2 and pT3 subgroups respectively. Clinically, occurrence of VM was significantly greater in patients with local lymph node metastases than those without local lymph node metastasis (75% vs. 17.86%) (p = 0.000). The incidence of VM was higher in patients who developed distant metastasis during the follow-up period than those without distant metastasis (41.67% vs. 16.67%) (p = 0.012). In addition, the positive correlation of VM was higher in the cases with seminal vesicle invasion than those without seminal vesicle invasion (41.67% vs. 17.07%) (p = 0.000). Notably, the positive correlation of VM was higher in the cases with shorter PSADT (≤ 6 months) than those with longer PSADT (> 6 months) (48.15% vs. 18.97%) (p = 0.001). On the other hand, the occurrence of VM did not vary with age (mean, 65 years; range 56–75 years), surgical margin status or local recurrence.
Table 1. VM expression and correlation with clinicopathological variables in tissue sections from PCa patients (n = 96)
Survival analysis
Univariate analysis showed that the OS of VM-positive patients was significantly poorer than that of VM-negative patients (p = 0.026) (). Furthermore, pre-operative PSA level (p = 0.017), Gleason score (p = 0.021), distant metastasis (p = 0.019) and PSADT (p = 0.021) were negatively correlated with OS. However, there was no significant association between OS and age, pathological stage, surgical margin status, seminal vesicle invasion, lymph node metastasis and local recurrence (). Cox proportional hazard regression analysis indicated that the presence of VM [risk ratio (RR) = 3.049, p = 0.041], higher Gleason score (RR = 2.316, p = 0.038) and distant metastasis (RR = 1.912, p = 0.040) were negative predictors of OS.
Figure 3. Overall survival (OS) and disease-free survival (DFS) based on occurrence of vascular mimicry in prostate cancer tissues from patients (n = 96). (A) Overall survival in VM-positive and VM-negative subgroups (p = 0.026). (B) Disease-free survival in VM-positive and VM-negative subgroups (p = 0.013).
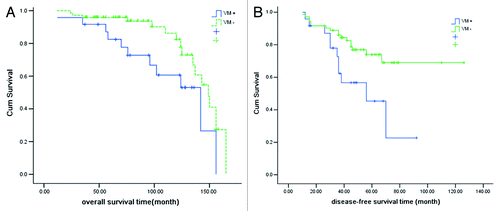
Table 2. Univariate analysis of the relationship between clinicopathological variables and survival of PCa patients (n = 96)
Univariate analysis showed that DFS was significantly related with VM (p = 0.013) (), Gleason score, distant metastasis, local recurrence and PSADT (). Multivariate analysis indicated that presence of VM (RR = 1.920, p = 0.045), higher Gleason score (RR = 2.056, p = 0.039) and distant metastasis (RR = 2.253, p = 0.028) were adverse predictors for DFS.
MMP-2 expression and its correlation with VM
We determined the expression of MMP-2 in the same PCa tissues. MMP-2 immunoreactivity was presented as diffuse cytoplasmic staining in tumor cells. Results showed that the VM-positive subgroup had a widespread MMP-2 positive signal (). To elucidate the relationship between VM and MMP-2, the MMP-2 expression levels between the VM-positive and VM-negative subgroups were compared. This analysis indicated that tissues from VM-positive subgroup were more likely to have a widespread MMP-2 expression (> 10% cells positive for MMP-2) than the VM-negative subgroup (83.33% vs. 48.61%, χ2 = 8.869, p = 0.003) ().
Figure 4. Immunohistochemistry staining of MMP-2 in prostate cancer tissues. (A) A widespread signal of MMP-2 (brown staining) in the cytoplasm of VM-positive prostate cancer tissues. (B) A weak signal of MMP-2 in the cytoplasm of VM-negative PCa tissues. Original magnification: × 200.
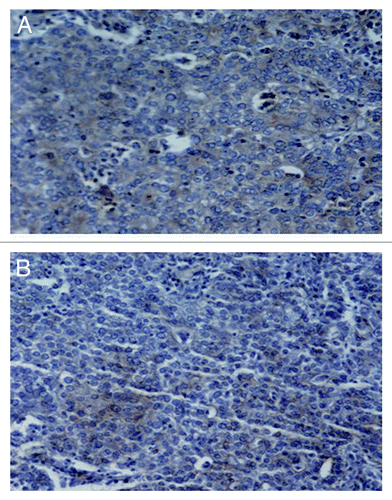
Table 3. Comparison of MMP-2 expression levels in the tissue sections from PCa patients (n = 96) that were either VM-positive or VM-negative
Discussion
PCa continues to be one of the biggest health problems in the aging male, with an estimated 217,730 new cases and 32,050 PCa-related deaths was predicted in 2010 in the United States alone.Citation18 PCa has become one of the most common malignancies seen in men worldwide, with strongly varying rates of tumor progression and responses to treatment. If the tumor is restricted to the prostate, patients can be treated by surgical removal of the tumor or by radiation, with high efficacy. By contrast, therapy of non-organ confined tumors still represents a major problem. Standard treatment for these patients is anti-androgens to achieve total androgen blockade.Citation19 Unfortunately, tumor progression and thus treatment failure occur very frequently and to date there are no alternative modalities to effectively treat PCa at this hormone-refractory stage, although a few chemotherapeutics may be available with the urologists. Therefore, reliable early diagnostic markers and novel interventional therapeutic targets for PCa are urgently required.
Tumors require a blood supply to sustain growth and to metastasize.Citation2 They initially co-opt with existing vessels to survive and grow, but the concept that they eventually send signals for new blood vessels to sprout (angiogenesis) is widely accepted as the mechanism by which most tumors metastasize.Citation1 The literature on this subject is immense and is still widely researched. Maniotis and coworkersCitation4 suggested a new mechanism by which some aggressive tumors may acquire a blood supply: the tumor cells themselves literally metamorphose into vessels that either carry blood or connect to the host’s blood supply. The formation of such a network of vessels is called VM. It refers to the de novo generation of tumor microcirculation without participation of endothelial cells, and is thus independent of angiogenesis. Furthermore, it is not a vasculogenic event as true vasculogenesis involves de novo formation of endothelial cell-lined vessels. Since its discovery, VM has been described in many kinds of tumors. To date, the literature on VM in prostate cancer is scanty. In the current study, we not only confirmed occurrence of VM in PCa, but also found a close relationship between VM and some of the established clinicopathological variables. These results might explain why anti-angiogenesis treatments remain clinically less effective.
VM was detected in tissue samples of PCa patients by PAS-CD31 dual staining (). The PAS-positive basement membrane was not always present, but when present different morphologies could be seen, such as a complete loop, arc or line (). These observations indicate that a PAS-positive membrane is not the absolute evidence for identifying VM.
In the current study the overall incidence of VM in PCa tissues was 25.0% (24/96 cases), which is different from another study on PCaCitation12 that reported a positive rate of VM as 60.0% (12/20) in low grade PCa tissues and 70.0% (14/20) in high grade PCa tissues. Furthermore, there was no significant difference between the two groups. In our study, the PCa tissues were classified into different subgroups according to different clinicopathological variables () and were correlated with VM. Incidence of VM was significantly higher in cases with higher pre-operative PSA level, Gleason score or pathological stage. Thus, VM occurred mainly in high risk PCa patients (). This conclusion was similar to that from an in vitro study.Citation11 In a comprehensive multidisciplinary study, Sharma et al. observed the ability of carcinoma cells from dunning rat and human prostate to form VM channels in 3-dimensional cell cultures.Citation11 They concluded that the potential for forming VM networks was seen in aggressive prostate cancer in vitro. The presence of VM is thus a signature of high malignancy.
VM plays an important role in tumor aggression.Citation4 Here, we have shown for the first time in PCa patients that VM was more often seen in those with seminal vesicle invasion, lymph node metastasis, distant metastasis tissues or shorter PSADT (). All these variables are important prognostic factors of PCa. This observation is different from that of a previous studyCitation20 that reported that breast carcinomas engaged in VM had a higher rate of distant metastasis, but there was no significant correlation between VM and lymph node metastasis. Another study on laryngeal squamous cell carcinoma reported a significant correlation between VM and lymph node metastasis status, but failed to find a significant correlation with distant metastasis.Citation16 Though more studies with larger sample size are needed to elucidate the correlation of VM and local lymph node and distant metastasis in different types of tumors, our findings suggest a close relationship between VM and PCa invasion and metastasis.
We analyzed the relationship of OS and DFS with the clinicopathological variables (). Univariate analysis results showed that VM, as well as pre-operative PSA level, Gleason score, distant metastasis and PSADT, were correlated with OS (range of p values, 0.017–0.031); while VM, Gleason score, distant metastasis, local recurrence and PSADT were correlated with DFS (range of p values, 0.013–0.045). Higher Gleason score, distant metastasis and shorter PSADT are all known adverse prognostic factors of PCa.Citation21 Here, we have shown that VM is also an adverse prognostic factor of PCa. Furthermore, multivariate analysis indicated that in addition to VM, higher Gleason score and distant metastasis were also adverse predictors for OS and DFS. Our study shows that VM is a new independent marker of poor prognosis in PCa.
The expression of MMP-2 was determined in the same PCa cases to explore the possible mechanism of VM formation at a preliminary stage. Results showed that the VM-positive group had a significantly higher MMP-2 expression levels (). These results were consistent with previous studies,Citation22,Citation23 showing that MMP-2 plays an important role in the formation of VM by degrading collagen IV. Moreover, Sharma et al.Citation11 have demonstrated that Metastat, an inhibitor of matrix metalloproteinase (MMPs) decreased the formation of VM networks in aggressive prostate cancer, indicating its possible therapeutic implications. However, further studies are needed to elucidate the mechanism of formation of VM in detail.
In conclusion, we have statistically correlated histological data with clinicopathological data from 96 PCa cases and thereby confirmed that VM occurs in PCa and that MMP-2 likely plays a role in the formation of VM. The presence of VM was mainly in high risk PCa patients and was closely correlated with PCa invasion and metastasis. Retrospective survival analysis indicated that VM is an independent marker of poor prognosis in PCa. These findings not only present VM as a prognostic tool in PCa, but also open avenues to develop new therapeutic strategies targeting formation of VM to effectively inhibit the progression of PCa, especially in its advanced stages.
Materials and Methods
Patients
This retrospective study included a total of 96 patients with histopathologically diagnosed PCa, who were treated with radical retropubic prostatectomy (RRP) at the Department of Urology of the Second Hospital of Tianjin Medical University between January 1995 and December 2007. None of the patients had received neoadjuvant hormone therapy before the operation. All included cases had a complete record of clinical and follow-up data, including age at diagnosis, prostate-specific antigen (PSA) level at diagnosis, pathological tumor-node-metastasis (pTNM) stage, Gleason score, seminal vesicle status, surgical margin status, lymph node status, distant metastasis, recurrence and PSA doubling time (PSADT). Follow-up began post-operation and ended in November 2010. Thus, all surviving cases were followed-up for at least 36 mo. The tumors were classified according to the 2002 American Joint Committee on Cancer (AJCC) staging system. The PSADT was calculated by assuming first-order kinetics and by using a minimum of three detectable postoperative PSA measurements after a previous undetectable level, each separated by a minimum of 3 months and each with a PSA increase of at least 0.1 ng/ml.Citation15 The ethics committee of Second Hospital of Tianjin Medical University approved the study protocol.
Immunohistochemistry
Tumor samples
Tissue samples were fixed in formaldehyde and embedded in paraffin for microscopic evaluation. Heat-induced epitope retrieval pretreatment in citrate buffer (0.01 mol/L; pH 6.0) was applied to all the tissue sections mounted on slides before immunohistochemical staining.
Major reagents
Anti-CD31 monoclonal mouse anti-human primary antibody and anti-MMP-2 polyclonal rabbit anti-human primary antibody were purchased from Zhongshan Golden Bridge Biotechnology Co. Ltd. Periodic acid and Schiff (PAS) solutions were purchased from Yili Fine Chemicals Biotechnology Co. Ltd.
PAS-CD31 dual staining for VM
VM was first identified with hematoxylin-eosin staining of the tissue sections (data not shown). CD31-PAS dual-staining was then used to validate presence of VM,Citation16 as CD31 marks endothelial cells and is absent from VM. Immunohistochemical staining was performed using the labeled streptavidin biotin method. First, tissue sections were allowed to react with anti-CD31 primary antibody, then with the secondary biotin-conjugated rabbit anti-goat antibody and followed by the horseradish peroxidase-labeled streptavidin at 37°C for 20 minutes each. (SP-9000 kit, Zhongshan Golden Bridge Biotechnology Co. Ltd.). Then the sections were treated with 0.5% periodic acid solution for ten minutes and rinsed with distilled water for two to three minutes. In a dark chamber, the sections were treated with Schiff’s solution for 15–30 minutes. After distilled water rinsing, sections were counterstained with hematoxylin. For each patient, three tissue sections were analyzed.
MMP-2 staining and semi-quantitative evaluation
Staining with primary antibodies against MMP-2 was performed on tissue sections with the SP-9000 kit, similar to CD31 staining procedure. The same sections were processed similarly but without primary antibodies as a negative control. MMP-2 staining in the cytoplasm of cells was estimated semi-quantitatively on the basis of number of positively stained tumor cells. To determine the positive rate, at least four high power microscope fields were randomly selected. Cells with pale-yellow to yellow-brown staining in cytoplasm were considered positive. The positive rate was calculated by dividing the number of positive cells by the total cell number. The stainability was scored as positive if ≥ 10% of the cells were stained positive for MMP-2.Citation17 For each patient, three tissue sections were analyzed.
Statistical analysis
The Chi-square (χ2) test was used to evaluate differences between the categorical variables. Differences in variables with parametric and nonparametric continuous distributions were evaluated with the independent-samples t-test and Mann-Whitney U-test. Overall survival (OS) curve was plotted using the Kaplan-Meier method and survival differences were analyzed with log-rank test. Cox proportional hazard regression model was used to simultaneously examine all factors found to be prognostic of survival in univariate analysis. SPSS software version 13.0 (SPSS, Inc.) was used for these analyses. Significance level was set at 0.05. p values presented are two-tailed.
Acknowledgments
This study was supported by grant from Tianjin Municipal Science and Technology Commission (NO: 033111311).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engström A, et al. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood 2000; 95:3403 - 11; PMID: 10828022
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285:1182 - 6; http://dx.doi.org/10.1056/NEJM197111182852108; PMID: 4938153
- Bissell MJ. Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch. A rose by any other name?. Am J Pathol 1999; 155:675 - 9; http://dx.doi.org/10.1016/S0002-9440(10)65164-4; PMID: 10487823
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999; 155:739 - 52; http://dx.doi.org/10.1016/S0002-9440(10)65173-5; PMID: 10487832
- Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS 2004; 112:508 - 25; http://dx.doi.org/10.1111/j.1600-0463.2004.apm11207-0810.x; PMID: 15563313
- Hao X, Sun B, Zhang S, Zhao X. [Microarray study of vasculogenic mimicry in bi-directional differentiation malignant tumor]. Zhonghua Yi Xue Za Zhi 2002; 82:1298 - 302; PMID: 12509930
- Cai XS, Jia YW, Mei J, Tang RY. Tumor blood vessels formation in osteosarcoma: vasculogenesis mimicry. Chin Med J (Engl) 2004.
- Hendrix MJ, Seftor EA, Kirschmann DA, Seftor RE. Molecular biology of breast cancer metastasis. Molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res 2000; 2:417 - 22; http://dx.doi.org/10.1186/bcr88; PMID: 11250735
- Sood AK, Fletcher MS, Coffin JE, Yang M, Seftor EA, Gruman LM, et al. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol 2004; 190:899 - 909; http://dx.doi.org/10.1016/j.ajog.2004.02.011; PMID: 15118611
- Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol 2001; 158:1279 - 88; http://dx.doi.org/10.1016/S0002-9440(10)64079-5; PMID: 11290546
- Sharma N, Seftor RE, Seftor EA, Gruman LM, Heidger PM Jr., Cohen MB, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate 2002; 50:189 - 201; http://dx.doi.org/10.1002/pros.10048; PMID: 11813211
- Ahmadi SA, Moinfar M, Gohari Moghaddam K, Bahadori M. Practical application of angiogenesis and vasculogenic mimicry in prostatic adenocarcinoma. Arch Iran Med 2010; 13:498 - 503; PMID: 21039005
- Chung LW, Huang WC, Sung SY, Wu D, Odero-Marah V, Nomura T, et al. Stromal-epithelial interaction in prostate cancer progression. Clin Genitourin Cancer 2006; 5:162 - 70; http://dx.doi.org/10.3816/CGC.2006.n.034; PMID: 17026806
- Liu C, Huang H, Doñate F, Dickinson C, Santucci R, El-Sheikh A, et al. Prostate-specific membrane antigen directed selective thrombotic infarction of tumors. Cancer Res 2002; 62:5470 - 5; PMID: 12359755
- Lin DD, Schultz D, Renshaw AA, Rubin MA, Richie JP, D’Amico AV. Predictors of short postoperative prostate-specific antigen doubling time for patients diagnosed during PSA era. Urology 2005; 65:528 - 32; http://dx.doi.org/10.1016/j.urology.2004.10.041; PMID: 15780370
- Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res 2010; 29:60; http://dx.doi.org/10.1186/1756-9966-29-60; PMID: 20525189
- Périgny M, Bairati I, Harvey I, Beauchemin M, Harel F, Plante M, et al. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am J Clin Pathol 2008; 129:226 - 31; http://dx.doi.org/10.1309/49LA9XCBGWJ8F2KM; PMID: 18208802
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277 - 300; http://dx.doi.org/10.3322/caac.20073; PMID: 20610543
- Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989; 321:419 - 24; http://dx.doi.org/10.1056/NEJM198908173210702; PMID: 2503724
- Shirakawa K, Wakasugi H, Heike Y, Watanabe I, Yamada S, Saito K, et al. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int J Cancer 2002; 99:821 - 8; http://dx.doi.org/10.1002/ijc.10423; PMID: 12115483
- Swanson GP, Basler JW. Prognostic factors for failure after prostatectomy. J Cancer 2010; 2:1 - 19; PMID: 21197260
- Sun B, Qie S, Zhang S, Sun T, Zhao X, Gao S, et al. Role and mechanism of vasculogenic mimicry in gastrointestinal stromal tumors. Hum Pathol 2008; 39:444 - 51; http://dx.doi.org/10.1016/j.humpath.2007.07.018; PMID: 18261629
- Sun B, Zhang S, Zhang D, Yin X, Wang S, Gu Y, et al. Doxycycline influences microcirculation patterns in B16 melanoma. Exp Biol Med (Maywood) 2007; 232:1300 - 7; http://dx.doi.org/10.3181/0705-RM-145; PMID: 17959842