Abstract
We generated novel truncated insulin-like growth factor I receptors (IGF-IRs) designated as 126/STOP, 223/STOP and 325/STOP in order to establish shorter soluble IGF-IRs than previously reported 486/STOP without abrogating the same antitumor effects. Stable transfection of 223/STOP and 325/STOP, but not 126/STOP caused inhibition of anchorage-independent growth of CaOV-3 ovarian cancer cells in vitro. This antitumor effect was reproduced when we used recombinant proteins of these constructs, suggesting a bystander effect of these shorter truncated IGF-IRs. Tumorigenesis in vivo of CaOV-3 cells tranfected with 223/STOP or 325/STOP was strictly inhibited, and inoculation of these cells in nude mice caused massive apoptosis exclusively in vivo. Phosphorylations of IGF-IR and Akt, but not Erk were attenuated in 223/STOP- or 325/STOP-transfected CaOV-3 cells, and downregulations of IGF-IR and Akt phosphorylation seemed to play at least a partial role in the anti-tumor effect of these novel truncated IGF-IRs. Since 223/STOP and 325/STOP are smaller in size than previously reported 486/STOP, and they retain the same antitumor effects, they could be good candidates for clinical application in the future.
Introduction
The insulin-like growth factor (IGF) axis, including specific ligands IGF-I, IGF-II, and insulin at supra-physiological concentrations, and their relevant receptor (IGF-IR), play an essential role in establishment and maintenance of transformed phenotypes of various cancer-derived cell lines.Citation1,Citation2 In clinical fields, overexpression of IGF-IR and /or IGF-I have been reported in many cancer tissues,Citation3,Citation4 and more importantly, these overexpressions have been associated with poor prognosis.Citation5-Citation7 IGF-IR is a tyrosine kinase receptor that is located on the cellular membrane, and upon paracrine and autocrine stimulation with its ligands, it undergoes tyrosil-autophosphorylation, leading to the activation of two downstream pathways: the mitogen-activated protein kinase (MAP kinase) and phosphatidylinositol 3-kinase (P-I-3 kinase) pathways. Thus IGF-IR not only acts as a growth factor by activating mainly the MAP kinase pathway, but also transduces potent survival signals via the P-I-3 kinase pathway by activating the serine/threonine phosphorylation of Akt followed by serine phosphorylation of Bad, which protects cells from undergoing apoptosis.Citation8,Citation9
Inhibition of this IGF-I axis by an antisense or neutralizing antibody,Citation10-Citation13 myristylated c-terminus of IGF-IR,Citation14,Citation15 mutantCitation16 or truncated IGF-IRCitation5,Citation17-Citation20 as dominant negatives for IGF-IR have been reported to have anti-tumor effects. Recently, neutralizing antibodies to IGF-IR and small molecule specific tyrosine kinase inhibitors for IGF-IR have been introduced into clinical fields as translational research.Citation21,Citation22 We previously reported that an IGF-IR truncated at the 486th amino acid within its α-subunit exhibited marked inhibition of cellular transformation in vitro and induction of massive apoptosis exclusively in vivo in CaOV-3 ovarian cancer cells.Citation20 Although, the precise molecular mechanisms of this 486/STOP have not been fully elucidated, it has been shown to exhibit a bystander effect through its secretion outside the transfected cells,Citation17,Citation19,Citation20 and thus it is expected to be useful for clinical use. For further investigation of the soluble IGF-IR, we generated several shorter truncated IGF-IRs, which were 126, 223 and 325 amino acids in length (almost 150, 250 and 350 amino acids when including the first 30 amino acids signal peptides), respectively. Through analyses of the anti-tumor effects of these shorter and soluble IGF-IR mutants, we tried to identify smaller candidates for clinical use.
Results
Expression levels and sizes of novel truncated IGF-IRs in CaOV-3 stable transformants
CaOV-3 cells were transfected with pCvn126/STOP, pCvn223/STOP, pCvn325/STOP or pCvn empty vector, and stable transformants were established in the presence of 800 μg/ml G418. Expression levels and sizes of smaller truncated IGF-IRs in representative clones were shown in . Approximate sizes of the truncated IGF-IRs expressed in CaOV-3 clones were; 23 kDa for 126/STOP, 35 kDa for 223/STOP and 50 kDa for 325/STOP. These truncated IGF-IRs were overexpressed when compared with a faint expression of the endogenous α-subunit of IGF-IR confirmed only at longer exposure (not shown in ).
Figure 1. Protein expression levels of CaOV-3 and CHO-Ras stable transformants. CaOV-3 cells were stably transfected with an empty vector, 126/STOP, 223/STOP or 325/STOP, and expression levels of representative clones were shown by western blot. (A) Lane 1, empty vector, Lane 2, 126/STOP #12; Lane 3, 126/STOP #16; Lane 4, 223/STOP #8; Lane 5, 223/STOP #13; Lane 6, 325/STOP #21; Lane 7, 325/STOP #22. CHO-Ras cells were stably transfected with the same plasmids to get high protein expression levels. Conditioned media from representative CHO-Ras stable transformants were separated and stained with anti-IGF-IR α-subunit. (B) Lane 1, empty vector, Lane 2, 126/STOP #14; Lane 3, 126/STOP #16; Lane 4, 223/STOP #23; Lane 5, 223/STOP #26; Lane 6, 325/STOP #12; Lane 7, 325/STOP #15; Lane 8, 486/STOP #13.
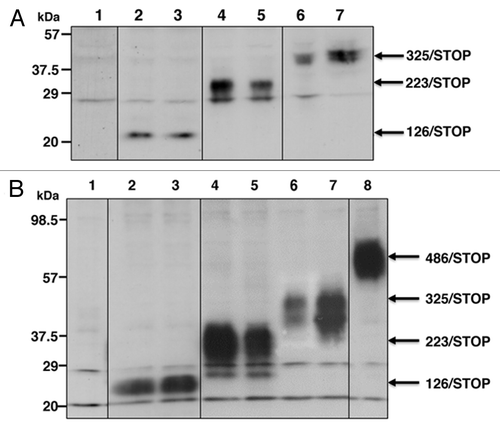
High dose expression of recombinant proteins in conditioned media from CHO-Ras clones
In order to produce enough amount of recombinant proteins for evaluating bystander effects of these truncated IGF-IRs, the same constructs were co-transfected with pPDV6+ hygromycin resistant gene into CHO-Ras cellsCitation23 (because G418 selection was already used for transfection of c-Ha-ras), highly expressing clones were selected in the presence of 30 μg/ml of hygromycin. High dose expressions of the recombinant proteins in conditioned media of these clones () as well as in whole cell lysates of these clones (data not shown) were confirmed. Because of the high protein expressions, we did not have to concentrate conditioned media as previously reported,Citation20 and we used crude conditioned media as recombinant protein solutions. was cropped from two consecutive blots performed simultaneously, and fused as one blot. The volume of total cell lysates loaded in and those of conditioned media loaded in were almost the same; therefore expression levels of these proteins were obviously higher in CHO-Ras clones.
Cell growth in monolayer culture
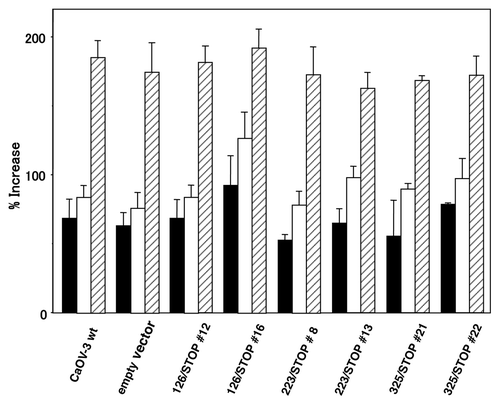
Cell growth in a monolayer culture of the representative CaOV-3 clones in SFM, SFM supplemented with 20 ng/ml IGF-I, SFM supplemented with 10% FBS are shown in . As we showed previously,Citation10,Citation20 there was little difference in monolayer cell growth by addition of IGF-I at this concentration, and expression of these truncated IGF-IRs did not cause significant growth retardation in a monolayer culture.
Figure 2. Cell growth in a monolayer culture. Representative CaOV-3 stable clones were plated at a density of 5 × 104 cells in 6-well plates and incubated for 24 h in DMEM with 10% FBS. The medium was replaced either with SFM (closed columns), SFM+IGF-I (open columns) and SFM+10% FBS (striped columns). The number of cells was determined after an additional 48 h of incubation in each medium and is expressed as percentage increase. Columns, mean; bars, SD.
Cell growth in soft agar
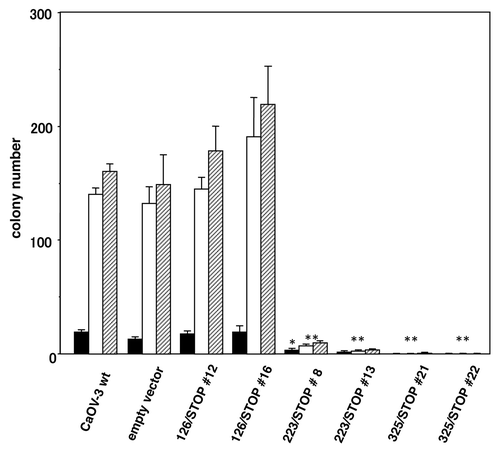
Each stably transfected CaOV-3 clones and wild-type CaOV-3 cells were seeded in soft agar as described in Materials and Methods to evaluate inhibition of anchorage-independent growth (). Clones transfected with 126/STOP retained the identical anchorage-independent growth as wild-type CaOV-3 cells and an empty vector transfected clone that we used as negative controls. Whereas, anchorage-independent cell growth of 223/STOP and 325/STOP clones were markedly inhibited for up to 5 weeks () and longer thereafter.
Figure 3. Colony formation in soft agar. Transfected CaOV-3 clones and CaOV-3 wild-type cells and an empty vector transfected clone were seeded at 3 × 104 cells/35mm plate in 0.2% soft agar plates. Colonies grew > 200 μm in diameter were counted after 3 (closed columns), 4 (open columns) and 5 (striped columns) weeks incubation. *p < 0.01; **p < 0.001 vs. empty vector.
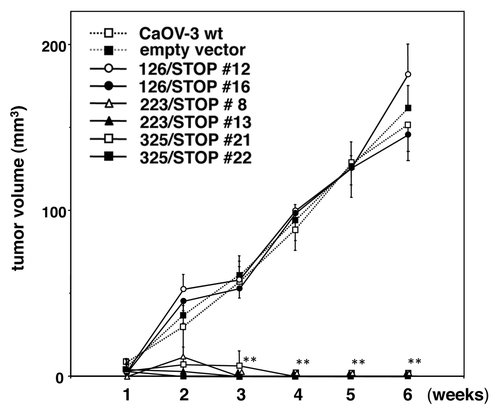
Tumorigenicity in nude mice
Tumorigenicity of wild-type CaOV-3 cells, an empty vector clone, 126/STOP, 223/STOP and 325/STOP clones are shown in . Both of the 126/STOP transfected clones and an empty vector clone formed palpable tumors in nude mice like wild-type CaOV-3 cells, but the tumorigenicity of 223/STOP and 325/STOP transfected clones were completely abrogated during 6 weeks observation.
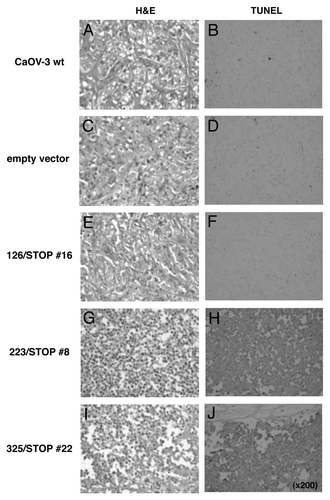
Detection of apoptosis in vivo.
Behavior of transfected clones with various truncated IGF-IRs as well as wild-type CaOV-3 cells in nude mice was checked by H&E or TUNEL staining. Formed palpable tumors were recovered 72 h after inoculation, and used for the study. Wild-type CaOV-3 cells, empty vector cells and 126/STOP cells formed tumors with viable cells (), and only small percentage of cells were stained positively with TUNEL (). In contrast, 223/STOP and 325/STOP cells were morphologically shrunk in shape with condensed nuclei () and TUNEL staining suggested massive apoptosis in vivo ().
Bystander effects of the recombinant protein
Bystander effects of the novel soluble IGF-I receptors to wild-type CaOV-3 cells obtained by addition of conditioned media in soft agar are shown in . Again, there was no inhibitory effect on anchorage-independent growth after addition of conditioned medium from the empty vector clone or 126/STOP clones in soft agar, whereas addition of conditioned media containing 223/STOP and 325/STOP recombinant proteins strongly inhibited anchorage-independent growth of wild-type CaOV-3 cells. Because we could add conditioned media only once in soft agar when we seeded cells, we evaluated anchorage-independent growth of wild-type CaOV-3 cells after 1 and 2 weeks. Wild-type CaOV-3 cells even treated with 223/STOP and 325/STOP gradually formed colonies thereafter.
Figure 6. Bystander effects of the novel soluble IGF-I receptors to wild-type CaOV-3 cells. Conditioned media from CHO-Ras clones expressing various soluble IGF-IRs or the conditioned medium from an empty vector clone were prepared, then these conditioned media were mixed with the same amount of DMEM with 10% FBS for soft agar assay. Wild-type CaOV-3 cells at 3 × 104 cells/35 mm plate were seeded in 0.2% agar (0.5% underlay) made of these mixed conditioned media. Definite colonies larger than 200 μm in diameter were counted after 1 week [open column] and 2 weeks [striped column]. *p < 0.01; **p < 0.001 vs. empty vector.
![Figure 6. Bystander effects of the novel soluble IGF-I receptors to wild-type CaOV-3 cells. Conditioned media from CHO-Ras clones expressing various soluble IGF-IRs or the conditioned medium from an empty vector clone were prepared, then these conditioned media were mixed with the same amount of DMEM with 10% FBS for soft agar assay. Wild-type CaOV-3 cells at 3 × 104 cells/35 mm plate were seeded in 0.2% agar (0.5% underlay) made of these mixed conditioned media. Definite colonies larger than 200 μm in diameter were counted after 1 week [open column] and 2 weeks [striped column]. *p < 0.01; **p < 0.001 vs. empty vector.](/cms/asset/aa981e79-cb7f-484a-94f7-f2ff702c2a86/kcbt_a_10919609_f0006.gif)
Analysis of possible molecular mechanisms for antitumor effects
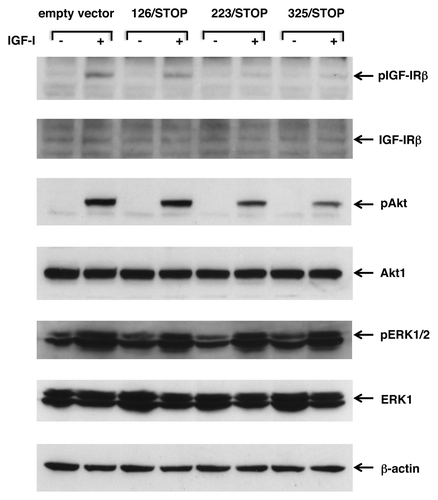
First, we checked whether the anti-tumor effect of these truncated IGF-IRs was via direct inhibition of IGF-IR property or not. Tyrosil autophosphorylation of IGF-IR after stimulation with IGF-I were evaluated by western blot by using phosphorylation-specific antibodies. Since establishment of stable transformants with cytotoxic constructs frequently causes unexpected clonal variations for compensation, we transiently transfected truncated IGF-IRs to CaOV-3 cells. We could not use conditioned media for this purpose because CHO-Ras cells could not survive without FBS supplementation. Autophosphorylation of the β-subunit of IGF-IR was attenuated by 223/STOP and 325/STOP transient transfections, but 126/STOP transfected CaOV-3 cells retained the same phosphorylation status as empty vector transfected cells (). Then two of the main downstream pathways were evaluated. Serine phosphorylation of Akt after stimulation with IGF-I was attenuated in 223/STOP and 325/STOP transfected cells when compared with empty vector transfected cells and 126/STOP transfected cells, but there was no difference in threonine/tyrosine phosphorylation of ERK1/2 ().
Figure 7. Transient expression of 233/STOP and 325/STOP in CaOV-3 cells downregulates IGF-I induced phosphorylation of IGF-IR and Akt. After transient transfection of empty vector or various soluble IGF-IRs, cells were kept in SFM for overnight, and then stimulated with 20 ng/ml IGF-I for 10 min at 37°C. The blots were probed with phosphorylation-specific antibodies, then stripped and re-probed with responsible antibodies. Expression levels of β-actin are shown as an internal control.
Discussion
In growth-regulated cells such as 3T3 cells, cell growth in a monolayer culture in SFM usually requires supplementation of more than one growth factor, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF) and IGF-I. Thus, cell growth promotion in a monolayer culture by addition of solely IGF-I in SFM is modest when compared with that by addition of 10% FBS.Citation10,Citation20 In the present study, truncated IGF-IRs 223/STOP and 325/STOP strongly inhibited anchorage-independent cell growth of CaOV-3 cells ( and ), but there was little difference in monolayer cell growth in 10% FBS between these inhibited clones and ineffective 126/STOP clones, an empty vector clone and CaOV-3 wild-type cells (). So the difference in the result of the soft agar assay was mainly due to the inhibition of the transforming ability of CaOV-3 cells by capable truncated IGF-IRs, but it was not merely due to the inhibition of mitogenicity. The IGF axis also transmits strong survival signals via the P-I-3 kinase pathways that are essential for cancer cells and that are driven by several activated oncogenes and/or abrogation of tumor-suppressor genes. These survival signals through the IGF axis have been reported to protect cancer cells from apoptosis induced by many causes, such as ionizing radiation,Citation24 chemotherapeutic reagents,Citation25,Citation26 UV-B exposures,Citation8 interleukin 3 withdrawal,Citation27 and okadaic acid.Citation28 When these survival signal pathways from IGF-IR are blocked or attenuated, this can result in massive apoptosis exclusively in vivo. In malignant tumor cells, the expression level of IGF-IR is often upregulated by de-repression of IGF-IR gene from p53-MDM2 related transcriptional downregulation.Citation29,Citation30 For these reasons, IGF-IR targeting seems ideal for clinical application, because the IGF axis is upregulated and also is required for cell survival.
Several mutant IGF-IRs were tested to clarify their anti-tumor effects.Citation16 Some of them exhibited inhibitory effects on anchorage-independent growth, and it was speculated that those mutant IGF-IRs might act as dominant negatives. But none of them caused apoptosis in C6 rat glioblastoma cells.Citation16 However, two truncated IGF-IRs designated as 952/STOPCitation18 and 486/STOPCitation17,Citation19,Citation20 were reported to have pro-apoptotic ability, and 486/STOP was also reported to be secreted from transfected cells, and to have a bystander effect.Citation17,Citation19,Citation20,Citation31 In the present study, we made several novel truncated IGF-IRs which were shorter than 486/STOP in length. This is because shorter truncation is favorable for polypeptide administration in clinical use, and the series of truncated IGF-IRs would be useful for investigating the molecular mechanisms of the antitumor effects of the truncated IGF-IRs, which have not yet been elucidated.
Anchorage-independent growth in soft agar () and tumorigenicity in nude mice () of the CaOV-3-derived stable transformants with 223/STOP and 325/STOP were almost completely inhibited. But transfection of 126/STOP did not exhibit any antitumor effects at all. Exactly the same inhibition of anchorage-independent growth was obtained by administration of the conditioned medium to CaOV-3 wild-type cells (). In this experiment, we could also confirm bystander effects of these two effective constructs. Taken together, these results indicate that novel truncated IGF-IR 223/STOP and 325/STOP could be good candidates for clinical use in the future because they are shorter than 486/STOP in size, and they retain the same anti-tumor effects.
By using this series of novel truncated IGF-IRs, we attempted to identify alterations of the signal transduction pathways. The transient transfection assays indicated that tyrosil auto-phosphorylation of the kinase domain within the β-subunit of the endogenous IGF-IR of 223/STOP- and 325/STOP-transfected CaOV-3 cells was clearly downregulated, and serine phosphorylation of Akt was also downregulated (). However, there was no downregulation observed in threonine/tyrosine phosphorylation of Erk1/2 in our transient transfection assays (). We repeated the experiment from five independent transfections, and the results were quite reproducible. These decreased activations of IGF-IR and Akt were identical to the biological antitumor effects of these truncated IGF-IRs, so it seemed possible to assume that the anti-tumor effects of these truncated IGF-IRs were due to the downregulation of IGF-IR autophosphorylation and subsequent downregulation of the serine phosphorylation of Akt.
Although we can confirm downregulation of IGF-IR and Akt phosphorylation, it is true that the levels of these downregulations are not overwhelming enough to support the notion that these truncated IGF-IRs act as dominant negatives. It may be due to the transfection efficiency of the assay, but these downregulations should play at least a partial role in the antitumor effect of these truncated IGF-IRs.
Speculations in regard to the pro-apoptotic ability of the truncated IGF-IRs have been made in previous studies on 486/STOPCitation17,Citation19,Citation20 and in studies on the c-terminus of IGF-IR.Citation14,Citation15 The facts that a deletion mutant of IGF-IR lacking the c-terminal region has greater anti-apoptotic ability,Citation32 and that transfection of the c-terminus constructCitation14,Citation15 or administration of the synthetic peptide of the c-terminal region of IGF-IRCitation33 induces massive apoptosis in vivo, lead us to speculate that the c-terminus of IGF-IR possesses a pro-apoptotic signal, but this signal is usually neutralized by a strong anti-apoptotic signal by the whole IGF-IR.Citation34 Since 486/STOP has been shown to form hetero-dimers with endogenous IGF-IR,Citation34 hetero-dimeric IGF-IRs with truncated IGF-IRs may transmit pro-apoptotic signals, possibly via conformational changes of the complex. The numbers of cysteine residues included in the sequences of 126/STOP, 223/STOP, 325/STOP and 486/STOP are 3, 17, 29 and 31, respectively. These cysteines are essential to form disulfide bonds between two α-subunits of the IGF-IR. Judging from the possible disulfide bond numbers for hetero-dimer formation, 3 cysteines in 126/STOP might not be sufficient to form hetero-dimers. This hypothesis may be a clue to explain for the strong pro-apoptotic ability of these truncated IGF-IRs shown in , but the precise mechanism for these pathways are still to be elucidated.
In conclusion, we confirmed that novel shorter soluble IGF-IR 223/STOP and 325/STOP possessed almost the same antitumor effects as reported for 486/STOP. In addition, we confirmed that these two novel truncated IGF-IRs retained bystander effects. These antitumor effects are, at least in part, due to the attenuated phosphorylation of IGF-IR and Akt. Because these truncated IGF-IRs are shorter than 486/STOP, they are suitable for future clinical applications. Although the significance and the precise mechanism for the pro-apoptotic ability of the IGF-IR are not clarified yet, these series of truncated IGF-IRs may be a good tool to search for its pro-apoptotic signal transduction pathways.
Materials and Methods
Site directed mutagenesis
Novel truncated IGF-IR mutants 126/STOP, 223/STOP and 325/STOP were generated by site-directed mutagenesis as previously described.Citation35 The sequences of mutagenic primers were; 5′-CTCCACTGTCGACTGATCCDTGATC-3′ (126/STOP) or 5′-GTAGCATGCCGCCACTGATACTATGCC-3′ (223/STOP) or 5′-CCAAGGGTGCACCATCTGAAAGGCCAAT-3′ (325/STOP), respectively. These mutagenic primers were designed to cause early stop codon formation (bold letters) and unique extra cutting sites (underlined) with SalI, SphI and ApaLI, respectively. Substituted nucleotides are described in Italics. Mutated fragments were subcloned into TA cloning vectors (pCR II; Invitrogen), and correct mutations were checked by digestion with specific restriction enzymes and also by dideoxy sequencing. These three mutated cDNAs were then subcloned into the pCvn IGF-IR plasmid (Ullrich et al. 1986) and were used for expression vectors.
Cell lines and transfections
CaOV-3 is a human ovarian carcinoma-derived cell line obtained from American Type Culture Collection, and maintained in DMEM (Invitrogen) with 10% FBS. CaOV-3 cells were transfected with pCvn126/STOP, pCvn223/STOP or pCvn325/STOP (encoding 126, 223 or 325/STOP under the control of the CMV promoter) or empty pCvn vector by using Transfectam (Promega) according to the manufacture’s protocol, and stable transformants were selected in the presence of 800 μg/ml G418. CHO-Ras is a Chinese Hamster Ovary (CHO) clone transfected with constitutively active Ras (c-Ha-ras) under the control of the CMV promoter, and was selected in the presence of 1 mg/ml G418.Citation23 CHO-Ras cells were established to produce high amount of recombinant proteins, and maintained in αMEM (Promega) with 10% fetal calf serum. CHO-Ras cells were co-transfected with various truncated IGF-IRs including 486/STOPCitation20 (or an empty vector) and a hygromycin resistant gene (pPDV6+) by using Transfectam, and stable transformants were selected in the presence of 30 μg/ml hygromycin. For transient transfection assay, CaOV-3 cells were transfected with various truncated IGF-IR expression plasmids by using Lopofectamine 2000 (Invitrogen) according to the manufacture’s protocol. After 48 h incubation in DMEM + 10% FBS at 37°C, transfected cells were washed three times with PBS and incubated further 8 h in SFM. Then cells were stimulated with or without 20 ng/ml of IGF-I (Invitrogen) for 5 min at 37°C and total cell lysates were collected and used for western blot analyses.
Western blot
For detecting various truncated IGF-IR proteins, subconfluent CaOV-3 derived clones were lysed in a lysis buffer as previously described.Citation20 Fifty micrograms of total cell lysates from CaOV-3 clones or 20 μl of conditioned media from CHO-Ras clones were separated on a 5–15% gradient SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and stained with anti-IGF-IR α-subunit (N20) (sc-712, 1:200, Santa Cruz Biotechnology). For analyzing signal transduction pathways, CaOV-3 cells were transiently transfected with various truncated IGF-IR genes and were incubated for 48 h, stimulated with or without 20 ng/ml of IGF-I for 10 min at 37°C and whole cell lysates were collected. Fifty micrograms of each lysates were uses for western blot analyses and stained with anti-phosphorylated IGF-IR β-subunit (pY1135/1136) (#3024, 1:1,000, Cell Signaling Technology), anti-IGF-IR β-subunit (C20) (sc-713, 1:200, Santa Cruz Biotechnology), anti-phosphorylated Akt (Ser473) (#9270, 1:1,000, Cell Signal Technology), anti-Akt1 (C-20) (sc-1618, 1:200, Santa Cruz Biotechnology), anti-phosphorylated ERK1/2 (pT202/pY204) (E23920, 1:1,000, BD Transduction Laboratories), anti-ERK1 (M12320, 1:5,000, BD Transduction Laboratories) and anti-β-actin (C4) (sc-47778, 1:200, Santa Cruz Biotechnology).
Preparation of conditioned media
Various truncated IGF-IR expressing CHO-Ras clones and an empty vector transfected clone were seeded at 60% confluency and kept in αMEM supplemented with 10% FBS for 3 d at 37°C. Media were recovered and the supernatants after a brief spin were used as conditioned media.
Cell growth in monolayer culture
CaOV-3 wild-type cells and stable clones were plated at 5 × 104 cells/6-well plates in DMEM with 10% FBS and incubated for 24 h. Then cells were washed three times with PBS and shifted to SFM, SFM with 20 ng/ml IGF-I, or SFM with 10% FBS. Cell growth is indicated as percent increase after 48 h incubation. All the points were results of triplicate experiments.
Soft agar assay
CaOV-3 derived clones were plated at 3 × 104 cells/35mm plate in 0.2% agar (0.5% agarose underlay) containing DMEM and 10% FBS. Cells were incubated at 37°C and colonies grew larger than 200 μm in diameter were counted after 3, 4 and 5 weeks. To evaluate a bystander effect by recombinant protein administrations, conditioned media from CHO-Ras clones expressing various truncated IGF-IR or an empty vector clone (kept in αMEM + 10% FBS without hygromycin) were prepared, then these conditioned media were mixed with the same amount of DMEM with 10% FBS for soft agar assay. Wild-type CaOV-3 cells at 3 × 104 cells/35 mm plate were seeded in 0.2% agar (0.5% underlay) made of these mixed conditioned media. Definite colonies larger than 200 μm in diameter were counted after 1 week and 2 weeks incubation at 37°C.
Tumor formation in nude mice
CaOV-3 stable clones as well as CaOV-3 wild-type cells were kept in SFM for 24 h and washed twice with PBS. As a xenograft, 5 × 106 cells suspended in 100 μl of PBS were injected s.c. in 5- to 6-week-old male BALB/c nude mice (Charles River Japan).
Detection of apoptosis in vivo.
Apoptosis of wild-type CaOV-3 cells, an empty vector clone, 126/STOP, 223/STOP and 325/STOP clones in vivo was detected by ApopTag Peroxidase In Situ Apoptosis Detection kit (Intergen) according to the manufacturer’s protocol as previously described.Citation20 Morphological change of these inoculated cells was also checked by H&E staining. The animal experiment protocol in the present study was approved by the Ethics Review Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (Okayama, Japan).
Statistical Analysis
Groups were compared using a 2-tailed unpaired Students t-test. Statistical analysis was performed using IBM SPSS Statistics 20 software.
| Abbreviation: | ||
| FBS | = | fetal bovine serum |
| SFM | = | serum-free medium |
| PBS | = | phosphate buffered saline |
| H&E | = | hematoxylin and eosin |
| TUNEL | = | terminal deoxynucleotidyl transferase-mediated nick end labeling |
Acknowledgments
We thank Dr. Sanetaka Shirahata (Graduate School of Genetic Resources Technology, Kyusyu University) for providing CHO-Ras cells, and Dr. Yasushi Adachi (First Department of Internal Medicine, Sapporo Medical University) for a fruitful discussion. This study was supported in part by Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan 13671719 and 16591661 (A.H.). Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan 13671719 and 16591661.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta 1997; 1332:F105 - 26; PMID: 9196021
- Rubini M, Hongo A, D’Ambrosio C, Baserga R. The IGF-I receptor in mitogenesis and transformation of mouse embryo cells: role of receptor number. Exp Cell Res 1997; 230:284 - 92; http://dx.doi.org/10.1006/excr.1996.3430; PMID: 9024787
- Werner H, Bruchim I. The insulin-like growth factor-I receptor as an oncogene. Arch Physiol Biochem 2009; 115:58 - 71; http://dx.doi.org/10.1080/13813450902783106; PMID: 19485702
- Kuramoto H, Hongo A, Liu YX, Ojima Y, Nakamura K, Seki N, et al. Immunohistochemical evaluation of insulin-like growth factor I receptor status in cervical cancer specimens. Acta Med Okayama 2008; 62:251 - 9; PMID: 18766208
- Imsumran A, Adachi Y, Yamamoto H, Li R, Wang Y, Min Y, et al. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis 2007; 28:947 - 56; http://dx.doi.org/10.1093/carcin/bgl247; PMID: 17183068
- Kalla Singh S, Tan QW, Brito C, De León M, De León D. Insulin-like growth factors I and II receptors in the breast cancer survival disparity among African-American women. Growth Horm IGF Res 2010; 20:245 - 54; http://dx.doi.org/10.1016/j.ghir.2010.03.001; PMID: 20347606
- Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, et al. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res 1997; 57:3079 - 83; PMID: 9242428
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol 1997; 17:1595 - 606; PMID: 9032287
- Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, et al. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol 1999; 19:7203 - 15; PMID: 10490655
- Nakamura K, Hongo A, Kodama J, Miyagi Y, Yoshinouchi M, Kudo T. Down-regulation of the insulin-like growth factor I receptor by antisense RNA can reverse the transformed phenotype of human cervical cancer cell lines. Cancer Res 2000; 60:760 - 5; PMID: 10676665
- Liu X, Turbyville T, Fritz A, Whitesell L. Inhibition of insulin-like growth factor I receptor expression in neuroblastoma cells induces the regression of established tumors in mice. Cancer Res 1998; 58:5432 - 8; PMID: 9850076
- Burfeind P, Chernicky CL, Rininsland F, Ilan J, Ilan J. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo.. Proc Natl Acad Sci U S A 1996; 93:7263 - 8; http://dx.doi.org/10.1073/pnas.93.14.7263; PMID: 8692980
- Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML, Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science 1993; 259:94 - 7; http://dx.doi.org/10.1126/science.8418502; PMID: 8418502
- Hongo A, Yumet G, Resnicoff M, Romano G, O’Connor R, Baserga R. Inhibition of tumorigenesis and induction of apoptosis in human tumor cells by the stable expression of a myristylated COOH terminus of the insulin-like growth factor I receptor. Cancer Res 1998; 58:2477 - 84; PMID: 9622092
- Liu Y, Lehar S, Corvi C, Payne G, O’Connor R. Expression of the IGF-I receptor C terminus as a myristylated protein leads to induction of apoptosis in tumor cells. Cancer Res 1988; 58:570 - 6
- Burgaud JL, Resnicoff M, Baserga R. Mutant IGF-I receptors as dominant negatives for growth and transformation. Biochem Biophys Res Commun 1995; 214:475 - 81; http://dx.doi.org/10.1006/bbrc.1995.2311; PMID: 7677754
- D’Ambrosio C, Ferber A, Resnicoff M, Baserga R. A soluble insulin-like growth factor I receptor that induces apoptosis of tumor cells in vivo and inhibits tumorigenesis. Cancer Res 1996; 56:4013 - 20; PMID: 8752172
- Prager D, Li HL, Asa S, Melmed S. Dominant negative inhibition of tumorigenesis in vivo by human insulin-like growth factor I receptor mutant. Proc Natl Acad Sci U S A 1994; 91:2181 - 5; http://dx.doi.org/10.1073/pnas.91.6.2181; PMID: 8134369
- Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R, et al. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res 1998; 58:3353 - 61; PMID: 9699666
- Hongo A, Kuramoto H, Nakamura Y, Hasegawa K, Nakamura K, Kodama J, et al. Antitumor effects of a soluble insulin-like growth factor I receptor in human ovarian cancer cells: advantage of recombinant protein administration in vivo. Cancer Res 2003; 63:7834 - 9; PMID: 14633710
- Chitnis MM, Yuen JSP, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res 2008; 14:6364 - 70; http://dx.doi.org/10.1158/1078-0432.CCR-07-4879; PMID: 18927274
- Atzori F, Traina TA, Ionta MT, Massidda B. Targeting insulin-like growth factor type 1 receptor in cancer therapy. Target Oncol 2009; 4:255 - 66; http://dx.doi.org/10.1007/s11523-009-0123-z; PMID: 19876700
- Katakura Y, Seto P, Miura T, Ohashi H, Teruya K, Shirahata S. Productivity enhancement of recombinant protein in CHO cells via specific promoter activation by oncogenes. Cytotechnology 1999; 31:103 - 9; http://dx.doi.org/10.1023/A:1008048928053; PMID: 19003130
- Nakamura S, Watanabe H, Miura M, Sasaki T. Effect of the insulin-like growth factor I receptor on ionizing radiation-induced cell death in mouse embryo fibroblasts. Exp Cell Res 1997; 235:287 - 94; http://dx.doi.org/10.1006/excr.1997.3683; PMID: 9281378
- Sell C, Baserga R, Rubin R. Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent etoposide-induced apoptosis. Cancer Res 1995; 55:303 - 6; PMID: 7812962
- Dunn SE, Hardman RA, Kari FW, Barrett JC. Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of HBL100 human breast cancer cells by inhibition of apoptosis induced by diverse anticancer drugs. Cancer Res 1997; 57:2687 - 93; PMID: 9205078
- Prisco M, Hongo A, Rizzo MG, Sacchi A, Baserga R. The insulin-like growth factor I receptor as a physiologically relevant target of p53 in apoptosis caused by interleukin-3 withdrawal. Mol Cell Biol 1997; 17:1084 - 92; PMID: 9032235
- D’Ambrosio C, Valentinis B, Prisco M, Reiss K, Rubini M, Baserga R. Protective effect of the insulin-like growth factor I receptor on apoptosis induced by okadaic acid. Cancer Res 1997; 57:3264 - 71; PMID: 9242459
- Werner H, Le Roith D. New concepts in regulation and function of the insulin-like growth factors: implications for understanding normal growth and neoplasia. Cell Mol Life Sci 2000; 57:932 - 42; http://dx.doi.org/10.1007/PL00000735; PMID: 10950308
- Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer 2005; 92:2097 - 101; http://dx.doi.org/10.1038/sj.bjc.6602627; PMID: 15956962
- Reiss K, D’Ambrosio C, Tu X, Tu C, Baserga R. Inhibition of tumor growth by a dominant negative mutant of the insulin-like growth factor I receptor with a bystander effect. Clin Cancer Res 1998; 4:2647 - 55; PMID: 9829727
- O’Connor R, Kauffmann-Zeh A, Liu Y, Lehar S, Evan GI, Baserga R, et al. Identification of domains of the insulin-like growth factor I receptor that are required for protection from apoptosis. Mol Cell Biol 1997; 17:427 - 35; PMID: 8972223
- Reiss K, Yumet G, Shan S, Huang Z, Alnemri E, Srinivasula SM, et al. Synthetic peptide sequence from the C-terminus of the insulin-like growth factor-I receptor that induces apoptosis and inhibition of tumor growth. J Cell Physiol 1999; 181:124 - 35; http://dx.doi.org/10.1002/(SICI)1097-4652(199910)181:1<124::AID-JCP13>3.0.CO;2-0; PMID: 10457360
- Reiss K, Tu X, Romano G, Peruzzi F, Wang JY, Baserga R. Intracellular association of a mutant insulin-like growth factor receptor with endogenous receptors. Clin Cancer Res 2001; 7:2134 - 44; PMID: 11448933
- Hongo A, D’Ambrosio C, Miura M, Morrione A, Baserga R. Mutational analysis of the mitogenic and transforming activities of the insulin-like growth factor I receptor. Oncogene 1996; 12:1231 - 8; PMID: 8649825