Abstract
Radiation therapy (RT) plays a critical role in the local-regional control of head and neck squamous cell carcinoma (HNSCC). However, the efficacy of RT in treating HNSCC is limited by severe normal tissue toxicity, predominantly mucositis. One pharmacological approach for increasing the clinical response to RT is the use of radiation response modifiers that preferentially sensitize tumor cells. Previously we demonstrated that curcumin, a natural plant polyphenol, increased the radiation sensitivity of HNSCC cells and that the observed sensitization was dependent on curcumin-mediated inhibition of thioredoxin reductase 1 (TxnRd1) a key cytosolic regulator of redox-dependent signaling. Here, we examined curcumin-induced radiation sensitization in HNSCC cell lines with differing HPV status and expressing different levels of TxnRd1, in vitro. The intrinsic radiation resistance of the HPV- cell lines was significantly higher than the HPV+ cell lines used in our study. Notably, all of the HPV- cell lines expressed high levels of TxnRd1 and exhibited higher intrinsic resistance to RT. While curcumin was effective at increasing the radiation response of the resistant HPV- cell lines it had no effect on the HPV+ cells. Based on these findings we employed an orthotopic, HPV- HNSCC tumor model in athymic nude mice to examine the effect of combining curcumin with fractionated RT, in vivo. The combination of curcumin feeding and fractionated RT had a significant effect on tumor doubling time and overall animal survival. We therefore propose that curcumin and RT should be considered as a first line treatment of HPV- HNSCC.
Introduction
Head and neck cancer (HNC) is the sixth most common form of cancer worldwide and head and neck squamous cell carcinoma (HNSCC) accounts for > 90% of all HNC.Citation1,Citation2 Historically, tobacco and alcohol have been the primary risk factors associated with HNSCC, however, in the past two decades human papillomavirus (HPV) infection has surfaced as an additional important risk factor.Citation3-Citation6 While HPV-HNSCC represents a significant percentage of all cases of HNSCC, the percentage of HPV+ cases has increased during the past decade, likely due to a decrease in HPV- cancers associated with the declining use of tobacco products.Citation5-Citation7 HPV+ and HPV- HNSCC represent distinct subsets of HNSCC, based on epidemiology data (age at initial diagnosis, sex and geographical distribution), molecular profiles, and the clinical observation that HPV+ HNSCCs are more responsive to conventional radiation and chemotherapy and corresponding improved survival.Citation6–Citation10 HNSCCs are often advanced (stage III or IV) at initial diagnosisCitation1 and treatment typically involves surgery to remove the primary tumor followed by radiation or combined radio-chemotherapy.Citation11-Citation14 Alternatively, radio-chemotherapy is used alone for unresectable disease or in instances where surgery-related morbidities would be unacceptable.Citation14,Citation15 Five year survival rates for patients with late stage HNSCC have remained near 50% over the past three decades, substantiating the need for research into new or alternative treatment strategies.Citation11-Citation13,Citation16
Curcumin, a diphenolic compound that gives the spice turmeric its characteristic yellow color has an extensive history of use in Ayurvedic and ancient Chinese medicine.Citation17-Citation19 Indeed, modern scientific studies have confirmed that curcumin possesses diverse pharmacologic activities, including anti-cancer efficacy, as either a single agent or in combination with conventional radiation and chemotherapy.Citation20 As a natural product, curcumin has been granted “generally regarded as safe” status by the FDA. Moreover, evidence from preclinical studies and multiple phase I/II clinical trials have demonstrated that curcumin is safe when given orally at doses up to 12 g per day.Citation21,Citation22
We and others have demonstrated that curcumin can act as both a radiosensitizer and radioenhancer in squamous cell carcinoma cell lines, while not altering the sensitivity of normal or immortalized but untransformed cells to ionizing radiation.Citation23-Citation26 The anticancer efficacy of curcumin alone has been ascribed to its ability to interact with diverse cellular target molecules, such as NFκB, AP1, Nrf-2 and thioredoxin reductase 1 (TxnRd1).Citation27,Citation28 Thioredoxin reductases (TrxRs) are a family of NADPH-dependent flavoproteins with a penultimate selenocysteine residue at the carboxy-terminus. These enzymes exhibit broad substrate specificity, which is due to the accessibility of the C-terminal redox-active site, when reduced29. TxnRds are ubiquitous with defined roles in diverse redox-regulated cellular functions, including transcription, DNA damage recognition and repair, proliferation, apoptosis and neoangiogenesis.Citation29-Citation32 The cytosolic isoform, TxnRd1, has been shown to regulate ionizing radiation- and general redox stress-induced cytotoxicity.Citation24,Citation31,Citation32 Moreover, experimental evidence indicates that overactivation/dysfunction of TxnRd1 is found in multiple human cancers,Citation33,Citation34 including HNSCCs.Citation35,Citation36 Significantly elevated TxnRd1 levels of TxnRd1 have been associated with aggressive tumor phenotype and poor clinical outcomes in cancer patients.Citation29,Citation33,Citation35,Citation36 Using both genetic and pharmacological means, we unequivocally demonstrated that the radiation sensitizing efficacy of curcumin depended on its ability to inhibit TxnRd1.Citation24
Here we expand on our earlier work, demonstrating that TxnRd1 is highly expressed in radioresistant HPV- HNSCC cell lines but not in radiosensitive HPV+ HNSCC cells. Pretreatment with curcumin significantly increased the radiation sensitivity of the HPV- cells while having no significant effect on the radiation response in HPV+ cell lines. Based on these data, we determined the effect of dietary curcumin in an orthotopic intralingual xenograft model of HPV- human HNSCC, grown in athymic nude mice. The combination of curcumin feeding and ionizing radiation resulted in a significant inhibition of tumor growth with a concomitant increase in animal survival. These data suggest that curcumin should be critically examined as an adjuvant to ionizing radiation therapy for treating HPV- HNSCC in the clinical setting.
Results
TxnRd1 expression and activity varies significantly in HPV+ and HPV- human HNSCC cell lines
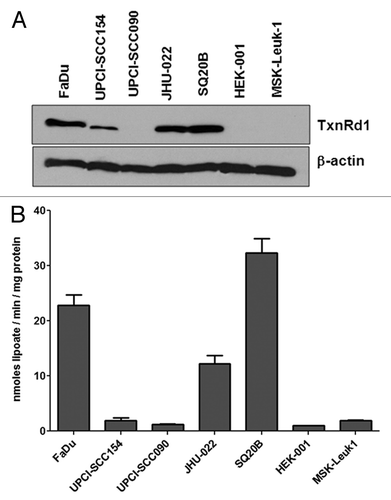
Previously, we demonstrated that curcumin inhibits whole TxnRd activity in HNSCC cells in culture. Moreover, using shRNA knockdown and overexpression methodologies we showed that curcumin-induced radiation sensitization depended on the levels of TxnRd1 expressed. These studies also demonstrated that TxnRd1 expression and activity were low in primary keratinocytes and minimally transformed cells, consistent with other reports.Citation24 Here we examined the expression and activity of TxnRd1 in a panel of HPV- and HPV+ human HNSCC cell lines as well as the immortalized and minimally transformed cell lines, HEK-001 and MSK-Leuk1 (). TxnRd1 expression was significantly higher in all three of the HPV- HNSCC cell lines that we examined (FaDu, 44.2 ± 4.2; JHU-022, 28.8 ± 3.6 and SQ20B 54.1 ± 6.1 fold when compared with HEK-001 cells, respectively). HPV+ HNSCC differs from the classic HPV- phenotype in several ways, most significantly HPV+ HNSCC are generally more responsive to both radiation and chemotherapy.Citation8-Citation10 Expression of TxnRd1 protein was lower in both HPV+ human HNSCC cell lines; the level in UPCI-SCC090 was similar to HEK-001, while expression was 3.2 ± 0.8 fold higher in UPCI-SCC154. MSK-Leuk1 cells are a minimally transformed line derived from an area of leukoplakia adjacent to a squamous cell carcinoma in the oral cavity of a female patient.Citation37 MSK-Leuk1 cells are immortalized but non-tumorigenic.Citation37 This cell line also did not show a significant increase in TxnRd1 levels when compared with HEK-001 cells. In addition to immunoblot analysis for TxnRd1 expression, we determined whole cell TxnRd activity. The assay used measures activity in intact cells,Citation38,Citation39 therefore we cannot directly rule out contributions of other TxnRd family members or closely related proteins in the total activity that is observed. However, as seen in , the whole cell assay exhibited a clear correlation with the level of TxnRd protein that is expressed in each line.
Figure 1. TxnRd1 protein and activity levels in cells with different transformation status correlate with response to curcumin. (A) TxnRd1 protein levels were determined by immunoblot analysis in a panel of human head and neck cancer cell lines. FaDu, UPCISCC090, UPCISCC154 (both HPV+) SQ20B and JHU022. TxnRd1 level;s were also measured in the immortalized keratinocyte cell line HEK-001 and the leukoplakia derived line MSK = Leuk 1. β-Actin was used as a loading control. (B) Basal levels of whole-cell TxnRd activity was measured as nanomoles dihydrolipoate formed per milligram cell protein.
Effect of curcumin on the radiation response of HNSCC cell lines, in vitro
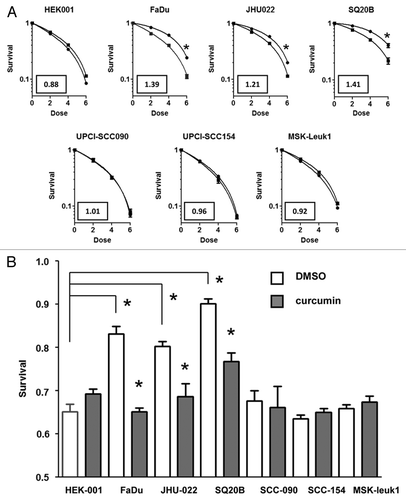
We next examined the ability of curcumin to affect radiation sensitivity in the same panel of human HNSCC lines by clonogenic survival assay. The intrinsic radiation sensitivity of the two HPV+ cell lines and the leukoplakia derived line did not differ significantly from that of HEK-001 cells, as shown in the survival curves presented in . In contrast, all three HPV- HNSCC cell lines were significantly more resistant to radiation compared with HEK-001 cells. We did not observe a direct correlation between EGFR expression and intrinsic radiation sensitivity in this panel of HNSCC lines (Fig. S1). Exposing the cells to 10 μM curcumin for 8 h produced minimal toxicity in all cell lines; surviving fractions were 96.2 ± 5.2 for SQ20B, 93.5 ± 4.8 for FaDu, 92.8 ± 6.4 for JHU022, 87.6 ± 6.6 for UPCI-SCC154, 83.8 ± 3.6 for UPCI-SCC090, 83.3 ± 5.1 for MSK-Leuk1 and 85.1 ± 4.7 for HEK-001 cells. We consistently observed a small increase in radiation resistance in HEK-001 cells after an 8 h pretreatment with 10 μM curcumin, however, this response did not reach a statistical level of significance (p = 0.057). There was also no significant difference in radiation response observed in UPCI-SCC154, UPCI-SCC090 or MSK-Leuk1 cells with vehicle control or curcumin. In contrast, we observed a significant increase in radiation sensitivity in all three HPV- HNSCC lines pretreated with 10 μM curcumin. Dose modifying ratios (DMRs) were calculated from the ratio of radiation dose that reduced survival to 0.5 in control and curcumin pretreated cells. The values obtained were 1.41 for SQ20B, 1.39 for FaDu and 1.21 for JHU-022, respectively. Surviving fraction at a dose of 2 Gy (SF2Gy) has been shown to be a useful predictor of HNSCC tumor response to radiation.Citation40 shows the effect of curcumin on the response of each HNSCC cell line to a single fraction of 2 Gy.
Figure 2. HPV- head and neck squamous carcinoma lines with TxnRd1 are sensitized to IR by curcumin. (A) Head and neck cell lines were treated with DMSO or 10 μM curcumin for 8 h followed by exposure to 0, 2, 4 or 6 Gy doses of IR. Survival was assessed by clonogenic assays. Points represent the average of a minimum of three independent experiments; bars ± SEM. Data were analyzed by pair wise comparison. * indicates a statistically significant difference in radiation sensitivity between curcumin treated and DMSO treated groups (p < 0.05). Values in boxes represent the dose modification factor observed at S.F. = 0.50. (B) Survival after a single dose of 2 Gy has been shown to predict intrinsic radiation sensitivity of tumor cells in vivo response. All HPV- HNSCC cell lines (FaDu, SQ20B and JHU-022, were significantly more resistant to 2 Gy of radiation then either HEK-001 or MSK-leuk-1 cells (*p < 0.05, t test). There was no difference in radiation sensitivity observed between the HPV+ cell lines and HEK-001 or MSK-Leuk 1. Pretreatment with curcumin, as indicated above, induced a significant increase in sensitivity in the three HPV- cell lines (* p < 0 0.05, t-test).
Uptake of curcumin in orthotopic HPV-FaDu cell tumors in nude mice
Based on the above results, we next sought to examine the effect of dietary curcumin on the radiation response of HPV- FaDu cells grown as an orthotopic xenograft tumor in athymic nude mice. To measure tumor growth in vivo, FaDu cells were transfected with firefly luciferase under control of the CMV promoter. The FaDu-CMV-Luci cells were then used to generate intralingual tumors as described in materials and methods. shows representative images made from 10 mm cross sections of tongues removed 10 d after implantation. shows an H&E stained section that shows sub-mucosal tumor growth and invasion into the intrinsic muscle of the tongue. is an image of a similar cross section stained for Ki67, showing that the tumor mass is largely composed of proliferating cells at this early time point. Once again, the tumor is seen to invade the tongue’s intrinsic musculature.
Figure 3. Growth of FaDu cells as an orthotopic intralingual model of HNSCC in nude mice and uptake of orally administered curcumin. (A) 10 μm cross-section of mouse tongue following H&E staining. (B) Immunohistochemical staining of a similar cross section for the proliferation marker Ki67. In both (A and B), the area labeled with T denotes tumor tissue; NS is normal stroma and NE is normal epithelium. (C) Uptake of curcumin into tumor tissues. Left panel shows a representative run for a tumor removed from an animal that was fed a 1% curcumin diet, (w/w) for 7 d, beginning 3 d after tumor implant. Right panel depicts a tumor-bearing mouse fed control lab chow. The peak that elutes at 40 min coincided with the peak obtained with the curcumin control. (D) Curcumin uptake was measured in three mice and the amount was determined by comparison to a curcumin standard. Error bars represent SEM values (p < 0.05; paired t-test). (E) TxnRd activity was measured in the tumor samples to insure that dietary curcumin was reaching the purported target in 3 mice fed 1% curcumin chow compared with 3 mice fed the control chow. Error bars represent SEM values (p < 0.05; paired t-test).
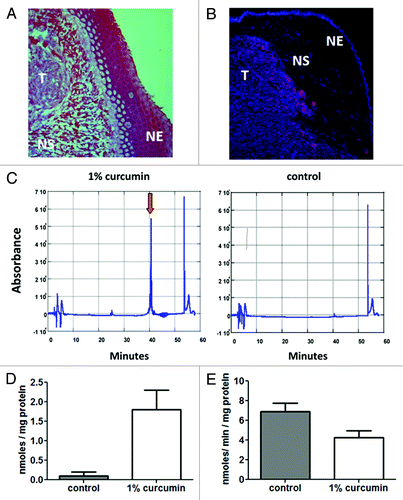
Uptake of curcumin into the tumor tissues was assessed via HPLC analysis as described in the materials and methods.Citation41 Tongues were removed after 7 d of feeding mice either control lab chow or a matched caloric diet containing 1% curcumin w/w. Anterior and posterior sections of the tongue, devoid of tumor were removed prior to the analysis. The remaining tumor-containing section, was then divided in half. As seen in left hand panel in , curcumin elutes from the column at 40 min. The amount of curcumin eluted from triplicate samples is shown in . To demonstrate that the 1% curcumin chow had biological activity in the tumor, the remaining tissue was minced and assayed for TxnRd1 activity using a modification of our lipoate reduction assay. As seen in , we observed a > 40% decrease in total TxnRd activity in samples from animals that were maintained on the 1% curcumin diet 10 d relative to animals fed the control diet. While this assay may not be a direct measure of the level of inhibition of TxnRd1 in vivo, the data do demonstrate that dietary curcumin is effective at inhibiting enzyme activity in the tumor containing tissues in vivo.
Effect of curcumin feeding and fractionated radiation therapy on growth of orthotopic FaDu tumors in nude mice
The intrinsic radiation sensitivity of the stable FaDu-CMV-Luci cells does not differ significantly from the parental line (). Moreover, the DMF obtained with curcumin pretreatment was similar between the parental FaDu and the FaDu-CMV-Luci cells. Initial bioluminescent images were taken on day 3 and 7, prior to the animal beginning experimental therapy. Animals without tumors visible in both images were excluded from the study. To measure tumor response to curcumin and radiation, two groups of 20 tumor bearing mice were randomly placed on either a control lab diet or a diet formulated with 1% w/w curcumin on day seven. After 3 d on their respective experimental diets, ten mice from each feeding arm were assigned to receive radiation therapy, 3 fractions of 2 Gy of 225 KeV X-rays. Post treatment bioluminescent imaging was performed twice weekly until animals lost > 20% of their pre-study body mass, or the completion of the study at day 90.
Figure 4. Effect of curcumin feeding with IR on orthotopic FaDu tumor growth was measured by bioluminescent image analysis. (A) FaDu cells transfected with CMV luciferase (FaDu-CMV-luci) were pretreated with 10 μM curcumin or DMSO for 8 h and then exposed to 0, 2, 4 or 6 Gy of radiation. Survival was measured by the clonogenic assay. The DMF = 1.42 for FaDu CMV-Luci cells, was similar to that of the parental FaDu cell line (). (B) Representative image from two groups of mice taken 10 d after completing 3 fractions of 2 Gy given at 48 h intervals. Left panel: from the group fed 1% curcumin chow; right panel: mice from the control chow fed group. (C) Tumor growth rates from each group of mice (10 mice/group at start of experiment) (control diet, 1% curcumin diet, 2Gy x 3 ionizing radiation alone or curcumin + ionizing radiation) based on twice-weekly bioluminescent imaging. Error bars represent SEM values from surviving mice at time of measurement. (D) Volume doubling time was calculated froom the time of the second pre-irradiation bioluminescent image intensity, obtained on day 7. Interaction was assessed by repeated measures ANOVA (p < 0.0001). The doubling time for the combined curcumin and radiation group was significantly larger than the unirradiated and irradiated mice on the control diet, and the curcumin-fed mice without radiation the radiation only treatment. Box represents minimum and maximum values for each group of animals with the median indicated by the interior bar. Error bars represent 95% confidence limits.
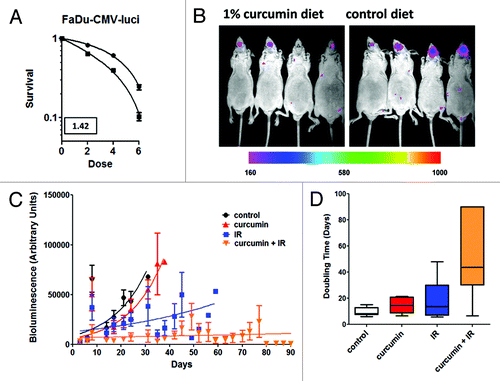
shows a representative image for two groups of four mice 10 d after the final X-ray treatment. The four animals in the right hand panel are from the control diet arm of the study. While there is some variation in tumor size, all of the animals in this treatment group exhibit bioluminescent tumors growing within the tongue. The mean tumor volume was noticeably smaller in the curcumin fed group in the left hand panel. In fact, tumor volumes were either similar to the volumes observed at the start of radiation treatment or in some cases tumor volume had regressed significantly by this time, note apparent absence of signal in animal three in the curcumin fed group.
shows the tumor growth data obtained for each experimental group of 10 animals, and doubling times were computed for each group of 10 animals (). These data were analyzed using one-way ANOVA and Tukey’s multiple comparison test.Citation42 A significant difference was found in the mean doubling time between the combined curcumin + radiation (mean of 51.98 d) and the radiation alone (mean of 18.54 d) or curcumin alone (mean of 14.39 d) or control (mean of 9.84 d) groups. Differences in tumor doubling time between the curcumin alone, radiation alone, and control groups did not did not differ significantly.
Effect of curcumin and fractionated radiation therapy on animal survival in orthotopic FaDu tumor bearing nude mice
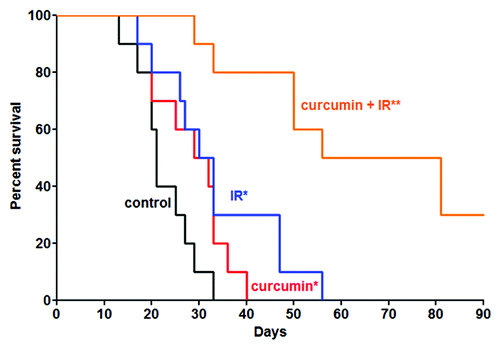
After approximately 6 doublings, the tumor volume began to interfere with ability of mice to obtain adequate nutrition and at that point we observed a rapid decrease in body mass. Animals were removed from the protocols and euthanized when their weight dropped below 80% of the pre-study weight. Kaplan-Meier plot of the data are shown in . Mantel Cox log rank test was used to compare animal survival for the four experimental groups.Citation43 There was a significant difference in mean survival between the radiation alone and control diet alone groups, likewise there was a significant difference between the radiation alone and curcumin alone groups. The difference in mean survival was highly significant for the curcumin with radiation group compared with any of the other three experimental conditions. Moreover, 30% of the animals in the curcumin with radiation group were still alive at day 90, the final day of the experiment, with no evidence of bioluminescent detectable tumor regrowth at that time.
Figure 5. Effect of combined curcumin and IR on animal survival. Animals were removed from the study and euthanized when their body mass decreased by > 20% based on their weight at beginning of experiment. There was a statistical difference in mean survival between the curcumin alone or IR alone-treated groups when compared with the untreated animals (*p = 0.05). The difference in mean survival between the combined treatments group and all other groups was highly significant (**p = 0.0001). Notably, 3 animals from the combined treatment group survived beyond the last data point collected at day 90.
Discussion
TxnRd1 is an essential regulator of cellular resistance to radiation and electrophillic cytotoxins. Upregulation of cytosolic TxnRd1 has been observed in diverse human cancers,Citation29,Citation32-Citation34 including HNSCC.Citation35,Citation36 and elevated expression imparts a selective advantage by increasing proliferative capacity,Citation29,Citation30,Citation33-Citation35 as well as resistance to conventional radiation and chemotherapy.Citation29,Citation35,Citation36 TxnRd1 protein levels were significantly higher in the HPV- cell lines (SQ20B, FaDu and JHU022) compared with the HPV+ HNSCC lines (UPCI-SCC090 and UPCI-SCC154). Expression of TxnRd1 was also low in HEK-001, a keratinocyte cell line immortalized with HPV 16 E6 and E7 proteins.Citation44 Previously, we demonstrated that stable expression of shRNA against TxnRd1 in FaDu cells produced clones that were far more sensitive to ionizing radiation then clones transfected with a non targeting shRNA.Citation24 Conversely, overexpressing TxnRd was sufficient to increase radiation resistance in HEK-293 cells, a transformed cell line with low basal expression of TxnRd1.Citation24 All of the HPV- HNSCC cell lines exhibited higher intrinsic resistance to ionizing radiation compared with the HPV+ cell lines, including HEK-001 and in MSK-Leuk1. While EGFR has been shown to be a marker for poor response to radiotherapy in patients with HNSCC, a positive HPV status is associated with a more favorable radiation response. As expected, high levels of expression of EGFR is not typically seen in HPV+ tumors.Citation45,Citation46 We saw no direct correlation between EGFR expression and the intrinsic radiation response in the panel HNSCC cell lines that were used in this study. These data strongly suggest that high levels of TxnRd1 correlate with increased radioresistance in HNSCC cell lines. However, due to the small number of HPV+ samples we have thus far examined, we cannot make an unequivocal connection between HPV status and TxnRd1 levels.
Curcumin has the unique property of protecting non-transformed cells and tissues from the damaging effects of ionizing radiation, while acting as a radiation sensitizer of malignant cancer cells.Citation24,Citation47 Previously, we demonstrated that the reactive Michael function of curcumin’s α,β unsaturated carbon bond and adjacent ketone was absolutely necessary for its radiation sensitizing efficacy in human squamous cell carcinoma cells, in vitro. Tetrahydrocurcumin (THC), a natural metabolite, is identical in structure to the parent compound, except that the unsaturated bond is reduced. THC had no effect on the radiation response of FaDu or HeLa cells, even when much higher doses were used.Citation24 Michael acceptors are potent electrophiles that readily react with cysteine and histidine residues in a large number of proteins.Citation48,Citation49 Nevertheless, there are a number of purported target proteins that appear to be relevant to the pharmacologic properties of curcumin.Citation27,Citation28 Curcumin-dependent inhibition of NFκB signaling, via inhibition of IκB-kinase, has been shown to play a direct role in the antiproliferative effects observed in a wide range of human cancers,Citation28 including HNSCC.Citation50 Transactivation of ARE response genes, mediated through dissociation of Nrf2 from Keap-1, a cytosolic negative regulator, plays a role in the chemo and radioprotective effect of curcumin observed in normal tissues.Citation51 Curcumin inhibits TxnRd1 activity in FaDu, with an IC50 of 10 μM.Citation24,Citation49,Citation52 Previously, we observed significant radiation sensitivity in FaDu cells pretreated with curcumin.Citation24 Ultimately, we demonstrated that the ability of curcumin to enhance radiation sensitivity was eliminated if TxnRd1 was knocked down by stable transfection with shRNA.Citation24 Here we demonstrate that curcumin’s effectiveness as a radiation sensitizer depends on the elevated expression of TxnRd1, as seen in the HPV- HNSCC cell lines. Therefore, while curcumin appears to interact with pleiotropic target molecules and induce in transformed cells and affect multiple pathways, in HNSCC, its radiosensitizing properties appear to depend on TxnRD1 inhibition.
Based on this evidence, we determined the efficacy of dietary curcumin combined with fractionated radiation therapy on the growth of HPV- FaDu tumors in an orthotopic xenograft in athymic nude mice. Previously, Khafif et al.Citation26 examined the combination of curcumin feeding and ionizing radiation in an orthotopic nude mouse model of HNSCC. In that report they indicate that there was a trend toward radiation sensitization; however, the data did not reach statistical significance. The use of bioluminescent imaging in our study allowed for multiple tumor volume measurements in each animal, rather than a single caliper-based volume measurement or measurement of tumor mass as was reported in the earlier study.Citation26 Clinical radiation therapy for HNSCC is given in small fractions delivered daily over several weeks.Citation11-Citation15 To more closely model a clinical protocol, the 6 Gy dose of radiation was therefore delivered in three 2 Gy fractions. Pretreatment and post treatment bioluminescent imaging were conducted at twice weekly intervals and there was no need to euthanize animals until overt signs of tumor related toxicity was observed. Using this methodology, we were able to follow 3 out of 10 animals in the combined curcumin and radiation treatment arm until the conclusion of the study at day 90. We found a highly significant effect of the combination of curcumin and radiation on mean time to tumor doubling when compared with the untreated controls, curcumin feeding alone and radiation therapy alone arms of the study. When animal survival was analyzed, radiation alone produced a significant increase in time to 50% survival compared with the curcumin fed alone or the untreated cohort of animals. The increase in time to 50% survival in the combined treatment arm was highly significant compared with all of the other treatment cohorts. Notably, three out of the total of 10 animals in the combined treatment cohort were effectively cured, i.e., they were still alive at day 90 and had no detectable bioluminescent tumor present, and no overt signs of treatment or disease related toxicity.
One of the limitations of using curcumin in the clinic for solid malignancies is its poor bioavailability and short half-life in plasma. Levels of curcumin are reportedly in the low μM range in both patient and experimental animal serum.Citation53 However, curcumin has been shown to be effectively absorbed across the colon mucosa.Citation53,Citation54 Therefore, we hypothesized that direct absorption across the mucosal lining of the oral cavity when administered as 1% w/w in the lab chow, would lead to sufficient uptake to have biological and therapeutic efficacy in our orthotopic-intralingual tumor model. Our HPLC data demonstrate that curcumin is directly absorbed through the oral mucosa and the TxnRd1 activity assay confirms that curcumin was taken up by the tumor at levels sufficient to have therapeutic potential. Therefore, in the clinical setting for HNSCC, we propose that curcumin be administered locally (e.g., as an oral rinse) to bypass issues of low systemic bioavailability.
The current standard of care for advanced stage HNSCC utilizes combinations of radiation and cisplatin.Citation14-Citation16,Citation55 Recent studies suggest adding fluorouracil or taxanes to this regimen could potentially enhance therapeutic efficacy.Citation15,Citation55 While aggressive chemo-radio therapy has improved loco-regional control, it has come at the expense of increased morbidity. Most prevalent is an increase in severe (grades 3–4) mucositis that can severely impact a patient’s quality of life.Citation56,Citation57 Our data demonstrate that curcumin, a natural product with proven low systemic toxicity,Citation21,Citation22 sensitizes HPV- HNSCC cells, expressing high levels of TxnRd1 to ionizing radiation, in vitro and in vivo. Moreover, limited experimental evidence suggests curcumin might also protect against radiation induced mucositis.Citation58,Citation59 At this point, we cannot exclude the possibility that the increased survival we observed in the cohort of mice treated with curcumin and radiation might have also been affected by curcumin-induced protection of normal mucosa, in addition to tumor radiosensitization. Based on these results, we propose that initiation of phase I/II clinical trials to test curcumin given with radiation therapy as a first line treatment for HNSCC is warranted. The design should include prescreening patients for both HPV status and expression of TxnRd1 prior to enrolling patients in the trial.
Materials and Methods
Cell lines and culture conditions
FaDu, SQ20B and the immortalized keratinocyte cell line HEK-001 cells were purchased from the ATCC. JHU-022 was isolated by one of us as previously described.Citation60 The HPV+ cell lines, UPCI-SCC090 and UPCI-SCC154 were provided by Dr. Susan Gollen, the Department of Genetics at the University of Pittsburgh Cancer Institute.Citation61,Citation62 The MSK-Leuk1 is a minimally transformed cell line derived from an oral leukoplakia and was provided by Dr. Peter Saks and the Memorial Sloan Kettering Hospital.Citation36 FaDu, SQ20B and JHU-022 were all carried in dMEM supplemented with 10% FBS, 20 mM Hepes (pH = 7.5) and 1% penicillin/streptomycin. HEK-001 and MSK-Leuk1 were carried in defined keratinocyte basal medium (Lonza cat# 00192151). The HPV+ HNSCC cells were carried in eagles MEM, supplemented with 10% FBS, 1% NEAA, 1 mM sodium pyruvate, 25 mM Hepes (pH = 7.5) 1% penicillin/streptomycin and 5 μgm/ml gentamycin. All cell lines were maintained in logarithmic growth at 37°C in a humidified atmosphere in 5% CO2.
Clonogenic survival analysis
Two x 105 cells were plated and allowed to attach overnight. Media was replaced with 1 ml of complete growth media containing 10 μM curcumin from a 10 mM stock made in DMSO. Controls contained an equivalent volume of DMSO alone. Cells were returned to the incubator for 8 h. They were then irradiated using a Phillips MARK V cesium source irradiator. Cells were again returned to the incubator for 1 h before subculturing. After rinsing each dish with 1 ml of dPBS, cells were dissociated by trypsinization, and cell density was determined using a coulter counter. After serial dilution, a known number of cells were plated onto 60 mm culture dishes in 3 ml of complete growth media. Dishes were incubated for 10–21 d, depending on the cell line. Colonies were stained with 1% methylene blue and 1% crystal violet in 70% EtOH. All colonies containing a minimum of 50 cells were scored as survivors.
Immunoblotting
Whole-cell lysates were obtained as described previously.Citation63 For detection of TxnRd1 and β-actin, 20 to 50 μg of total protein was resolved on 10% SDS-PAGE gels, transferred to polyvinylidene difluoride membrane, blocked for 30 min in Tris-buffered saliine with 0.1% tween (TBST) and 5% milk, and incubated for 1 to 2 h with the following antibodies in TBST containing 1% milk: anti-TxnRd1 (AbCam) anti-EGFR (Millipore) anti-Ku80 (Calbiochem) and anti-β-actin (Sigma). All membranes were incubated with horseradish peroxidase–conjugated anti-mouse or anti-rabbit secondary antibodies (Santa Cruz Biotechnology), and immunoreactive bands were detected using ECL Plus chemiluminescence (GE Healthcare).
TxnRd1 activity assay (in vitro)
We used a whole cell TxnRd activity assay based on the reduction of lipoateCitation60 that has been described in detail elsewhere.Citation24,Citation38,Citation39 Briefly, cells were plated to 90% confluence, allowed to attach overnight, and treated with 10 μM curcumin for 7 h. Following treatment, media was aspirated and replaced with complete media with Na-R-Lipoate (Na-RALA, GeroNova Research, Inc.) at a final concentration of 1 mM. Plates were returned to the incubator for one hour; an aliquot was removed from the media overlying the cell monolayer and spun at 1,000 × g for 5 min to get rid of cellular debris. 50 μL of cleared media were then added to 100 μL of 5,5-dithiobis(2-nitrobenzoic acid) in 1M PBS (pH = 7.5) in triplicate wells of a 96-well plate. The plates were incubated for 10 min in the dark. Absorbance was measured at 450 nm using a multiwell plate reader. TxnRd activity was defined by the concentration of dehydrolipoate formed per milligram cellular protein.
HPLC assay for curcumin uptake
Six tumor bearing mice, fed either control or 1% (w/w) curcumin containing chow (TestDiets/Purina) for 7 d, were sacrificed and the fore tongue and base of tongue were removed to obtain a section that contained a minimal margin of normal tissue. The tissue was rinsed three times in dPBS with 10% ethanol, to remove residual chow from the surface. Samples were homogenized in 10 volumes (v/w) of 0.2% NP-40 in Hepes buffered saline (pH = 7.4). 0.5 ml of ethyl acetate:2-propanol (9:1) was then mixed into each sample by gentle inversion. Following centrifugation at 10,000 x g, 0.4 ml of the organic phase was removed and dried under a stream of nitrogen + 5% CO2. The dried samples were reconstituted in acetonitrile and subjected to HPLC analysis as described by Garcea et al.Citation41 The amount of curcumin eluted was calculated by comparing AUC for each sample to a curcumin standard eluted under identical conditions.
TxnRd1 Activity Assay (in vivo)
To confirm that the 1% curcumin chow had biological activity a piece of fresh tumor tissue was minced to a very fine paste in ice cold tissue culture media, the sample was then mixed into an equal volume of media containing 1 mM Na-R-lipoate. Samples were then transferred to a water bath at 37°C for two hours and total lipoate reduced was determined as indicated above.
Transfections
FaDu cells were plated into 6 well plates at 1 x 105 per well. Cells were transfected with CMV-luciferase (Genlantis) using lipofectamine 2000. Stable transfectants were selected in media containing 500 μg/ml puromycin beginning 24 h after transfection. Clones were propagated in duplicate 24 well plates, luciferase expression was determined by bioluminescent image analysis in the presence of luciferin potassium salt (Regis Inc.) on one of the duplicate samples. Three distinct luciferase expressing clones (FaDu-CMV-luci) were pooled and the pooled population frozen for subsequent use in tumor growth experiments in vivo.
In vivo tumor growth and animal survival
The orthotopic nude mouse model of oral cancer described by Myers et al.Citation64 was modified by the use of a firefly luciferase expressing human HNSCC cell line. Briefly, 5 x 105 FaDu-CMV-luci cells were injected into the intrinsic muscle beneath the dorsal mucosal layer of the tongue in athymic nude mice under general anesthesia. At day 3 and 6 after tumor implant mice received 0.2 ml of luciferin potassium salt (Regis, Inc.) by IP injection and bioluminescent images were obtained after 10 min. A cohort of 3 animals were sacrificed at day 10, tongues were removed and sectioned for H&E staining and immunohistochemical staining for Ki67, using a rabbit polyclonal antibody to Ki67 (AbCam) and an Alexa 460 goat anti-rabbit secondary. Nuclei are stained with Hoescht 33342 dye. Mice exhibiting discernable bioluminescent tumors in both images were randomly placed on either a control diet or 1% (w/w) curcumin-containing diet (20 mice per feeding cohort) beginning on day 7. Each group of animals was further divided into either the control or irradiated arms of the study. Mice were anesthetized and placed into a jig to immobilize them during irradiation. Irradiated animals received 3 doses of 2 Gy of 225 KeV X-rays with 48 h in between each successive dose. Irradiation was delivered through a 0.5 x 0.5 cm port in 5 mm lead plate designed to shield vital organs, including the brain from radiation exposure. Following treatment, bioluminescent images were obtained twice weekly, until animals were removed from the study due to excessive weight loss (> 20% of pre-study body mass) or the completion of the study at 90 d.
Statistical analysis
Statistical analysis was performed using the embedded statistical package in Prism Graph Pad 5.0. For tumor growth studies, statistical significance between treatment groups was determined by one-way ANOVA analysis using the Tukey post-test for pairwise comparison.Citation42 For animal survival data, a log rank (Mantel-Cox) test was used to determine interaction and an unpaired t-test was used to determine whether there was a significant difference in time to reach 50% survival between treatment groups.Citation43 All other data were analyzed using a paired t-test. Significance is reported for p values < 0.05.
| Abbreviations: | ||
| head and neck squamous cell carcinoma | = | HNSCC |
| human papillomavirus | = | HPV |
| ionizing radiation | = | IR |
| thioredoxin reductase | = | TxnRd1 |
Additional material
Download Zip (83.7 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer 2005; 5:127 - 35; http://dx.doi.org/10.1038/nrc1549; PMID: 15685196
- Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11:9 - 22; http://dx.doi.org/10.1038/nrc2982; PMID: 21160525
- Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010; 11:781 - 9; http://dx.doi.org/10.1016/S1470-2045(10)70017-6; PMID: 20451455
- Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol 2004; 31:744 - 54; http://dx.doi.org/10.1053/j.seminoncol.2004.09.011; PMID: 15599852
- Hobbs CGL, Sterne JAC, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 2006; 31:259 - 66; http://dx.doi.org/10.1111/j.1749-4486.2006.01246.x; PMID: 16911640
- Ragin CCR, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 2007; 121:1813 - 20; http://dx.doi.org/10.1002/ijc.22851; PMID: 17546592
- Vu HL, Sikora AG, Fu S, Kao J. HPV-induced oropharyngeal cancer, immune response and response to therapy. Cancer Lett 2010; 288:149 - 55; http://dx.doi.org/10.1016/j.canlet.2009.06.026; PMID: 19628331
- Gupta AK, Lee JH, Wilke WW, Quon H, Smith G, Maity A, et al. Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys 2009; 74:928 - 33; http://dx.doi.org/10.1016/j.ijrobp.2009.03.004; PMID: 19480971
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363:24 - 35; http://dx.doi.org/10.1056/NEJMoa0912217; PMID: 20530316
- Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 2001; 92:805 - 13; http://dx.doi.org/10.1002/1097-0142(20010815)92:4<805::AID-CNCR1386>3.0.CO;2-9; PMID: 11550151
- Tobias JS, Monson K, Gupta N, Macdougall H, Glaholm J, Hutchison I, et al, UK Head and Neck Cancer Trialists’ Group. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol 2010; 11:66 - 74; http://dx.doi.org/10.1016/S1470-2045(09)70306-7; PMID: 19875337
- Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al, Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004; 350:1937 - 44; http://dx.doi.org/10.1056/NEJMoa032646; PMID: 15128893
- Adelstein DJ, Li Y, Adams GL, Wagner H Jr., Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21:92 - 8; http://dx.doi.org/10.1200/JCO.2003.01.008; PMID: 12506176
- Specenier PM, Vermorken JB. Current concepts for the management of head and neck cancer: chemotherapy. Oral Oncol 2009; 45:409 - 15; http://dx.doi.org/10.1016/j.oraloncology.2008.05.014; PMID: 18715812
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349:2091 - 8; http://dx.doi.org/10.1056/NEJMoa031317; PMID: 14645636
- Marur S, Forastiere AA. Update on role of chemotherapy in head and neck squamous cell cancer. Indian J Surg Oncol 2009; 1:85 - 95; http://dx.doi.org/10.1007/s13193-010-0021-y
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 2008; 65:1631 - 52; http://dx.doi.org/10.1007/s00018-008-7452-4; PMID: 18324353
- Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 2005; 41:1955 - 68; http://dx.doi.org/10.1016/j.ejca.2005.05.009; PMID: 16081279
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 2008; 267:133 - 64; http://dx.doi.org/10.1016/j.canlet.2008.03.025; PMID: 18462866
- Steward WP, Gescher AJ. Curcumin in cancer management: recent results of analogue design and clinical studies and desirable future research. Mol Nutr Food Res 2008; 52:1005 - 9; http://dx.doi.org/10.1002/mnfr.200700148; PMID: 18186103
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 2004; 10:6847 - 54; http://dx.doi.org/10.1158/1078-0432.CCR-04-0744; PMID: 15501961
- Lao CD, Ruffin MT 4th, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 2006; 6:10; http://dx.doi.org/10.1186/1472-6882-6-10; PMID: 16545122
- Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol 2008; 73:1491 - 501; http://dx.doi.org/10.1124/mol.107.043554; PMID: 18252805
- Javvadi P, Hertan L, Kosoff R, Datta T, Kolev J, Mick R, et al. Thioredoxin reductase-1 mediates curcumin-induced radiosensitization of squamous carcinoma cells. Cancer Res 2010; 70:1941 - 50; http://dx.doi.org/10.1158/0008-5472.CAN-09-3025; PMID: 20160040
- Khafif A, Hurst R, Kyker K, Fliss DM, Gil Z, Medina JE. Curcumin: a new radio-sensitizer of squamous cell carcinoma cells. Otolaryngol Head Neck Surg 2005; 132:317 - 21; http://dx.doi.org/10.1016/j.otohns.2004.09.006; PMID: 15692547
- Khafif A, Lev-Ari S, Vexler A, Barnea I, Starr A, Karaush V, et al. Curcumin: a potential radio-enhancer in head and neck cancer. Laryngoscope 2009; 119:2019 - 26; http://dx.doi.org/10.1002/lary.20582; PMID: 19655336
- Shehzad A, Lee YS. Curcumin: Multiple molecular targets mediate multiple pharmacological actions: A review. Drugs Future 2010; 35:113
- Ravindram J, Prasad S, Aggarwal BB. Curcmin and cancer cells: How many ways can curry kill tumor cells selectively?. AAPS J 2009; 3:495 - 510; http://dx.doi.org/10.1208/s12248-009-9128-x
- Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun 2010; 396:120 - 4; http://dx.doi.org/10.1016/j.bbrc.2010.03.083; PMID: 20494123
- Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol 2007; 7:392 - 7; http://dx.doi.org/10.1016/j.coph.2007.04.003; PMID: 17611157
- Nguyen P, Awwad RT, Smart DDK, Spitz DR, Gius D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett 2006; 236:164 - 74; http://dx.doi.org/10.1016/j.canlet.2005.04.028; PMID: 15955621
- Biaglow JE, Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther 2005; 4:6 - 13; http://dx.doi.org/10.4161/cbt.4.1.1434; PMID: 15684606
- Lincoln DT, Ali Emadi EM, Tonissen KF, Clarke FM. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Res 2003; 23:3B 2425 - 33; PMID: 12894524
- Yoo MH, Carlson BA. Tsuji, Irons R, Gladyshev VN, Hatfield DL. Alterartion of Thioredoxin Reductase 1 levels in elucidation cancer etiology. Methods Enzymol 2010; 474:255 - 75; http://dx.doi.org/10.1016/S0076-6879(10)74015-5; PMID: 20609915
- Zhu X, Huang C, Peng B. Overexpression of thioredoxin system proteins predicts poor prognosis in patients with squamous cell carcinoma of the tongue. Oral Oncol 2011; 47:609 - 14; http://dx.doi.org/10.1016/j.oraloncology.2011.05.006; PMID: 21652258
- Lincoln DT, Al-Yatama F, Mohammed FMA, Al-Banaw A, Al-Bader M, Burge M, et al. Thioredoxin and thioredoxin reductase expression in thyroid cancer depends on tumor aggressiveness. Anticancer Res 2010; 30:67 - 76
- Sacks PG. Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer Metastasis Rev 1996; 15:27 - 51; http://dx.doi.org/10.1007/BF00049486; PMID: 8842478
- Biaglow JE, Donahue J, Tuttle S, Held K, Chrestensen C, Mieyal J. A method for measuring disulfide reduction by cultured mammalian cells: relative contributions of glutathione-dependent and glutathione-independent mechanisms. Anal Biochem 2000; 281:77 - 86; http://dx.doi.org/10.1006/abio.2000.4533; PMID: 10847613
- Biaglow JE, Ayene IS, Koch CJ, Donahue J, Stamato TD, Mieyal JJ, et al. Radiation response of cells during altered protein thiol redox. Radiat Res 2003; 159:484 - 94; http://dx.doi.org/10.1667/0033-7587(2003)159[0484:RROCDA]2.0.CO;2; PMID: 12643793
- Eschiweg F, Bourhis J, Girinski T, Laritigau E, Deble D, Kepa L, et al. Predictive assays of radiation response in patients with head and neck squamous cell carcinoma: A review of the institute gustave roussy experience. IJROBP 1997; 39:845 - 53
- Garcea G, Jones DJL, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 2004; 90:1011 - 5; http://dx.doi.org/10.1038/sj.bjc.6601623; PMID: 14997198
- Gustafson DL, Frederick B, Merz AL, Raben D. Dose scheduling of the dual VEGFR and EGFR tyrosine kinase inhibitor vandetanib (ZD6474, Zactima) in combination with radiotherapy in EGFR-positive and EGFR-null human head and neck tumor xenografts. Cancer Chemother Pharmacol 2008; 61:179 - 88; http://dx.doi.org/10.1007/s00280-007-0460-5; PMID: 17393165
- Bianco C, Tortora G, Bianco R, Caputo R, Veneziani BM, Caputo R, et al. Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor ZD1839 (Iressa). Clin Cancer Res 2002; 8:3250 - 8; PMID: 12374696
- Sugerman PB, Bigby M. Preliminary functional analysis of human epidermal T cells. Arch Dermatol Res 2000; 292:9 - 15; http://dx.doi.org/10.1007/PL00007461; PMID: 10664009
- Reimers N, Kasper HU, Weissenborn SJ, Stützer H, Preuss SF, Hoffmann TK, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer 2007; 120:1731 - 8; http://dx.doi.org/10.1002/ijc.22355; PMID: 17236202
- Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2009; 74:553 - 61; http://dx.doi.org/10.1016/j.ijrobp.2009.02.015; PMID: 19427557
- Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal 2005; 7:1630 - 47; http://dx.doi.org/10.1089/ars.2005.7.1630; PMID: 16356126
- Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A 2001; 98:3404 - 9; http://dx.doi.org/10.1073/pnas.051632198; PMID: 11248091
- Cai W, Zhang L, Song Y, Wang B, Zhang B, Cui X, et al. Small molecule inhibitors of mammalian thioredoxin reductase. Free Radic Biol Med 2012; 52:257 - 65; http://dx.doi.org/10.1016/j.freeradbiomed.2011.10.447; PMID: 22064364
- Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer 2004; 111:679 - 92; http://dx.doi.org/10.1002/ijc.20333; PMID: 15252836
- Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res 2008; 52:Suppl 1 S128 - 38; PMID: 18327872
- Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem 2005; 280:25284 - 90; http://dx.doi.org/10.1074/jbc.M414645200; PMID: 15879598
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007; 4:807 - 18; http://dx.doi.org/10.1021/mp700113r; PMID: 17999464
- Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett 2007; 255:170 - 81; http://dx.doi.org/10.1016/j.canlet.2007.03.005; PMID: 17448598
- Forastiere AA, Trotti A, Pfister DG, Grandis JR. Head and neck cancer: recent advances and new standards of care. J Clin Oncol 2006; 24:2603 - 5; http://dx.doi.org/10.1200/JCO.2006.07.1464; PMID: 16763271
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003; 66:253 - 62; http://dx.doi.org/10.1016/S0167-8140(02)00404-8; PMID: 12742264
- Sonis ST. The pathobiology of mucositis. Nat Rev Cancer 2004; 4:277 - 84; http://dx.doi.org/10.1038/nrc1318; PMID: 15057287
- Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, et al. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys 2006; 65:890 - 8; http://dx.doi.org/10.1016/j.ijrobp.2006.03.025; PMID: 16751071
- Akpolat M, Kanter M, Uzal MC. Protective effects of curcumin against gamma radiation-induced ileal mucosal damage. Arch Toxicol 2009; 83:609 - 17; http://dx.doi.org/10.1007/s00204-008-0352-4; PMID: 18754102
- Rhee JG, Li D, O’Malley BW Jr., Suntharalingam M. Combination radiation and adenovirus-mediated P16(INK4A) gene therapy in a murine model for head and neck cancer. ORL J Otorhinolaryngol Relat Spec 2003; 65:144 - 54; http://dx.doi.org/10.1159/000072252; PMID: 12925815
- White JS, Weissfeld JL, Ragin CCR, Rossie KM, Martin CL, Shuster M, et al. The influence of clinical and demographic risk factors on the establishment of head and neck squamous cell carcinoma cell lines. Oral Oncol 2007; 43:701 - 12; http://dx.doi.org/10.1016/j.oraloncology.2006.09.001; PMID: 17112776
- Ferris RL, Martinez I, Sirianni N, Wang J, López-Albaitero A, Gollin SM, et al. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer 2005; 41:807 - 15; http://dx.doi.org/10.1016/j.ejca.2004.11.023; PMID: 15763658
- Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 2002; 22:7405 - 16; http://dx.doi.org/10.1128/MCB.22.21.7405-7416.2002; PMID: 12370288
- Arnér ES, Nordberg J, Holmgren A. Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase. Biochem Biophys Res Commun 1996; 225:268 - 74; http://dx.doi.org/10.1006/bbrc.1996.1165; PMID: 8769129
- Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res 2002; 8:293 - 8; PMID: 11801572