Abstract
The cytokine-induced killer cells (CIK) have been reported to have potent cytotoxicity against a variety of tumor cells including multiple myleoma (MM) cells. The mechanisms that CIK cell recognizing MM cells remain unknown. Recent studies indicated that the interaction between NKG2D receptor and NKG2D ligands plays an important role in inducing cytotoxicity against various target cells by natural killer cells (NK). We suspect whether NKG2D receptor and NKG2D ligands interaction is also responsible for the killing of MM cells by CIK as the same way did NK cells. We expanded CIK cells from healthy controls with interferon (IFN)-γ, CD3 monoclonal antibodies (mAb) and interleukin-2 (IL-2), and checked expression of NK cell receptors on CIK cells by flow cytometry. About 86% bulk CIK cells expressed NKG2D receptor but not other NK receptors, such as CD158a, CD158b and NCRs. We analyzed NKG2D ligands expression in MM patients by flow cytometry, primary plasma cells from 8 out of 13 (62%) MM patients expressed different levels of ULBPs or MICA/B on the cell surface. Interestingly, when stimulated with MM cell line U266 that expressed some levels of MICA/B, only NKG2D expressing CIK cells released IFN-γ. CIK cells showed cytotoxicity against NKG2D ligands expressing U266 and primary MM cells, and the cytotoxicity was partially blocked by treating CIK with anti-NKG2D antibody. We conclude that NKG2D-NKG2D ligand interaction may be one of the mechanisms by which CIK cells kill MM cells.
Introduction
Activation of natural killer cells (NK) is regulated by interaction between inhibitory receptors for major histocomopatibility complex (MHC) class I molecules and activating receptors,Citation1,Citation2 NKG2D is an important activating receptor for tumor recognition and killing,Citation3-Citation5 and is one member of the c-type lectin-activating receptor family, its ligands include MHC-I-related molecules MICA/B and ULBPs (UL16-binding proteins). Interestingly the NKG2D ligands are relatively restricted to virus-infected tumor and stressed cells.Citation6,Citation7
Adoptive cellular immunotherapy aims at eliminating tumor cells by stimulating or restoring immune system, cytokine induced killer cells (CIK) derived from peripheral blood for treating various cancer have been received considerable attention. Previously studies showed the superiority of CIK cells over lymphokine-activated killer cells in terms of cytotoxicity against tumor cells.Citation8-Citation10 Eearly studies demonstrated that CIK cells showed cytotoxicity against various tumor cell lines in vitro in an MHC-unrestricted manner.Citation11-Citation14 Clinical trials have also reported the safety and efficacy of CIK cells in the treatment of patients with malignant neoplasms.Citation10,Citation15,Citation16 The expanded bulk CIK cell population consists of over 90% CD3+ cells, small numbers of them are CD56+ (T-NK phenotype) and the others are CD3+CD56- cells, the majority of which are CD8+ T cells.Citation12,Citation13 There is also a small subset of NK cells (CD3-CD56+). The NKG2D activating receptors are not only expressed on NK cells, but also expressed on some T-cell subsets including NKT cells.
NKG2D plays an important role in eliminating tumor cells by cytotoxic effector cells. Previous studies demonstrated that effector cells’ recognition and lysis of tumor cells was mainly mediated by NKG2D activating receptor.Citation3,Citation4,Citation17,Citation18 NKG2D-mediated cytotoxicity not only depends on the NKG2D receptors on surface of immune cells but also depends on NKG2D ligands expression on target cells.Citation19 Expression of NKG2D ligands is associated with malignant transformation, and target cells with higher levels of NKG2D ligands are more susceptible to NK cell-mediated lysis.Citation20-Citation22 Therefore, NKG2D is important both in tumor immunosurveillance to prevent tumor initiation and in immune-mediated rejection of tumor cells to prevent tumor progression.
Previous studies showed that CIK cells exert their functions in the similar way as NK cells, their cytotoxicity against target cells does not require prior immunogenic priming, and they are CD3+ like T cells.Citation10 But how the CIK cells recognize and kill MM cells is still unclear. Understanding the mechanism of MM cell recognition is important for the optimal application of CIK cell therapy in clinical practice. In this study, we analyzed NKG2D expression on bulk CIK cells and NKG2D ligands on multiple myeloma patients, our results demonstrated that CIK cells recognize and kill multiple myeloma cells partially depending on NKG2D-NKG2D ligand interaction, which highlighted the utility of CIK cells in clinical practice.
Results
Characterization and phenotypes of CIK cells
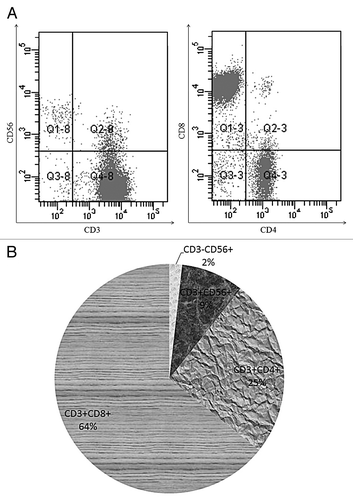
PBMCs were cultured with IFN-γ, OKT-3 and interleukin (IL)-2 for 3 weeks, the expanded bulk CIK cells was analyzed by flow cytometry. Bulk CIK cells consisted of a small percentage of CD3-CD56+ cells (1.8 ± 1.1%) and about 90% CD3+ cells (93 ± 5.6%) (), of CD3+ cells, 8.4 ± 3.1% cells co-expressed CD56 while the remaining cells were CD3+CD56- (84.6 ± 4.9%), of which 60.9 ± 8.2% are CD3+CD8+ T cells. The percentage of CD8+ T cells was higher than CD4+ T cells (), the CD4/CD8 ratio (0.39 ± 0.11) decreased compared with that before culturing (1.44 ± 0.24).
Figure 1. Phenotypic features of bulk CIK cells. PBMCs from heathy individuals were exposed to IFN-γ, OKT3 and IL-2 for 3 weeks. (A) A representative experiment shows the phenotype of bulk CIK cells. (B) Average percentage of different cell subset in bulk CIK cells was measured by flow cytometry (n = 35).
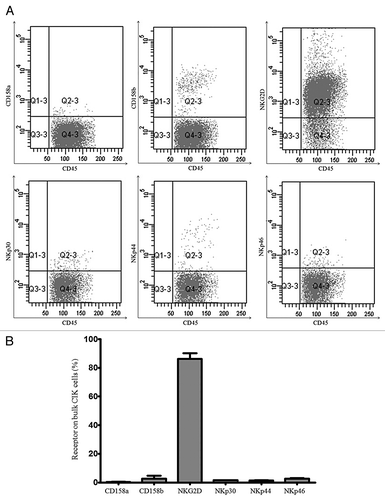
Next, we analyzed the expression of NK-cell inhibitory and activating receptors on bulk CIK cells (). We found only a small percentage of CIK cells expressed inhibitroy receptors such as CD158a (0.44 ± 0.18%) and CD158b (2.60 ± 2.10%), while 86.3 ± 3.9% of the bulk CIK cells expressed NKG2D activating receptors, the levels were higher than that before culturing (50.26 ± 6.54%). Almost no natural killer cytotoxicity receptors (NCRs) including NKp30, NKp44 and NKp46 were detected on bulk CIK cells ().
Figure 2. Expression of NK-cell activating and inhibitory receptors on bulk CIK cells. (A) Cultured bulk CIK cells labeled with mAb recognizing NK activating receptors (NKG2D, NCRs) and inhibitory receptors (CD158a, CD158b). A representative experiment shows the different receptors on surface of bulk CIK cells. (B) Percentages of different receptors on bulk CIK cells were measured by flow cytometry (n = 35).
Expression of NKG2D ligands and HLA class I molecules on patient’s myleoma cells
Previous studies indicated that immune cells could eradicate tumor cells through interaction between activating receptors and corresponding ligands, ligands of NKG2D expressed on tumor cells could stimulate anti-tumor activity by lymphocytes, and the cytotoxicity was correlated with the ratio of NKG2D ligands to HLA class I molecules.Citation19 We examined NKG2D ligands and HLA class I molecules expression on myeloma cells of MM patients and human myeloma cell line U266. Blood samples of 13 patients with MM were obtained prior to chemotherapy. Isolated MM patient’s BM cells were stained with mAbs against CD138, MICA/B, ULBP-1, -2, -3 and HLA-ABC. In these 13 investigated patients, 62% (8/13) of patients expressed NKG2D ligands on the CD138+ plasma cells, while all MM patients showed some levels of HLA class I molecules on the CD138+ plasma cell surface (). The results suggested that myeloma cells in most MM patients expressed certain levels of NKG2D ligands on cell surface, the levels of HLA class I and NKG2D ligands were not equivalent.
Table 1. Expression of NKG2D ligands and HLA class I on human myeloma cells and U266
NKG2D is required for IFN-γ secretion of bulk CIK cell
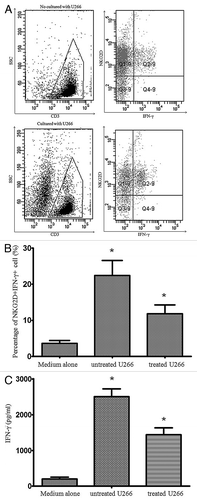
Interferon-γ is an important cytokine in cellular immunity and cytotoxic T lymphocytes function. Immune cells secrete IFN-γ in response to 2 types of stimuli: target cell ligands or antibodies engaging activating receptors. To investigate the involvement of NKG2D-NKG2D ligands interaction in IFN-γ secretion by CIK cells, we stimulated bulk CIK cells with U266 that expresses NKG2D ligands for 10 h and measured IFN-γ secretion by intracellular staining. Because about 93% bulk CIK cells expressed CD3 molecules, we first gated CD3+ cells, then analyzed IFN-γ secretion on CD3+ cells. We found IFN-γ secretion by CIK cells stimulated with U266 cells were increased compared with unstimulated CIK cells (), and interestingly, IFN-γ secretion was only detected in NKG2D+ CIK cells (). When we treated U266 cells with anti-MICA/B antibodies, bulk CIK cells showed marked reduction of IFN-γ secretion compared with CIK cells incubated with untreated U266 cells (). We also measured IFN-γ concentrations in the culture supernatants by ELISA. IFN-γγ concentrations of unstimulated, stimulated with U266 and subsequently treated with anti-MICA/B Abs were 205 ± 35, 2650 ± 145, and 1560 ± 152 pg/mL, respectively (). This result demonstrated that NKG2D+ CIK cells are potent effector cells and IFN-γ secretion partially depends on NKG2D-NKG2D ligands interaction.
Figure 3. NKG2D receptor was required for IFN-γ secretion. IFN-γ secretion was measured in bulk CIK cells by intracellular staining. (A) The represent experiment show IFN-γ secretion in NKG2D+ CIK cells after coculturing without or with U266 for 10 h. (B) U266 cells were treated with anti-MICA/B for 30 min then used for stimulating bulk CIK cells, compared with medium alone. *p < 0.05.C. IFN-γ concentrations in the culture supernatants were measured by ELISA. *p < 0.05.
Involvement of NKG2D in the CIK-mediated myleoma cell lysis
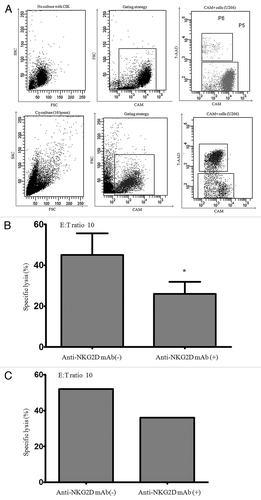
NKG2D is the identified cell surface molecule that plays a significant role in NK or CD8+ T cell-mediated target recognition and cytolysis. To investigate whether bulk CIK cells’ recognition and cytolysis of MM cells is in an NKG2D-NKG2D ligands dependent fashion, we checked the cytotoxicity of CIK cells against MM cell line U266 that expressed NKG2D ligands, U266 cells were labled with the fluorescent probe CAM and then co-cultured with CIK cells. Cells emerging from co-culture were gated based on CAM fluorescense and CAM+/7-AAD+ cells were considered as apototic cells (). In blocking experiment, anti-NKG2D antibodies were added at 10 μg/mL to CIK cells and incubated 30 min before the addiation of U266. We cocultured CIK cells with MM cell line U266 at an effector-target ratio (E/T ration) of 10:1. The expanded CIK cells mediated cytolysis against the U266 with 45% (45 ± 10.6%) specific killing, and this specific killing decreased (19 ± 5.2%) when CIK cells were pretreated with anti-NKG2D mAbs (, p < 0.05). Primary MM cells obtained from MM patient (patient 6) that expressed high levels of ULBPs molecules were also susceptible to cytotoxicity by patient’s autogenous CIK cells and this cytotoxicity was partially blocked by pretreating CIK cells with anti-NKG2D antibodies (), suggesting that CIK’s killing of MM cells is partially in an NKG2D-NKG2D ligand dependent fashion.
Figure 4. NKG2D mediated CIK cells cytotoxicity against myeloma cells. (A) The gating strategy for the analysis of CIK-mediate cytotoxicity is shown in a representive experiment. The 7-AAD expression on CAM labeled U266 after incubation without or with bulk CIK cells for 10 h (E/T ratio = 10). (B) Cultuered bulk CIK cells were incubated in the presence of anti-NKG2D antibodies or medium alone for 30 min and then used for cytotoxicity assay against CAM labeled U266 compare with medium alone. *p < 0.05. (C) The cytotoxicity of cultured bulk CIK cells against primary MM cells derived from MM patient (patient 6) was assessed by flow cytometry.
Discussion
NKG2D is expressed on the surface of all NK cells and some T cells.Citation2,Citation6 Previous studies showed NKG2D function as triggering receptors involved in natural cytotoxicity mediated by immune cells against a variey of tumor cells,Citation5,Citation17,Citation23 but NKG2D-mediated cytotoxicity not only depends on the NKG2D receptors on surface of immune cells but also NKG2D ligands expressed on target cells.Citation19 CIK cells expanded with IFN-γ, OKT3 and IL-2 expressed higher level of NKG2D receptors and low levels of KIR such as CD158a and CD158b, but almost no expression of NCRs. NKG2D receptor expressed on CIK cells may be important in eradiating malignant cells. Previous studies support the concept that NKG2D ligands result in different functional outcomes in immune cells.Citation7,Citation17,Citation19 More specifically, in immune cells, NKG2D triggering induces activating signals. NKG2D ligands were detected on the surface of MM cell line U266. And we also demonstrated that freshly isolated primary myeloma cells from patients expressed NKG2D ligands on the cell surface. Among 13 pateints with MM, we detected significant surface levels of MICA/B in 4 patients, and significant expression of ULBPs molecules in 6 patients with MM. Overall, in 62% (8/13) of the invistigated patients malignant cells expressed one or more ligands for NKG2D on the cell surface, nonmalignant PBMCs (CD138- or CD34- cells) from the same patients did not show expression of NKG2D ligands, which is consistent with previous studies that no expression of NKG2D ligands was detected on lymphocyte of healthy individuals.Citation6,Citation7 Previous studies showed that NK cell-mediated cytotoxicity was correlated with the ratio of NKG2D ligands to HLA class I molecules, but not with the amounts of MICA on the cell surface of tumor cells,Citation19 we also detected the expression of HLA class I molecules, we found that primary myeloma cells derived from MM patients expressed high levels of HLA class I molecules and some levels of NKG2D ligands which might be very important for the recognition of MM cells by immune cells.
NKG2D is involved in CIK cells cytotoxicity against MM cells. Immune cells kill malignant cells by directly killing and IFN-γ secretion. We stimulated CIK cells with NKG2D ligands expressing the U266 cell line and we found that only NKG2D expressing CIK cells released IFN-γ. However, when we treated U266 cell with anti-MICA/B antibodies, IFN-γ secreting NKG2D+ CIK cells were significantly decreased. Next, we detected the cytotoxicity of bulk CIK cells in vitro, bulk CIK cells showed cytotoxicity against U266 cell line and myeloma cells derived from MM patients, and the cytotoxicity of CIK cells decreased when treated with anti-NKG2D antibodies, these findings suggest that bulk CIK cells may kill myeloma cells in the way of interaction between NKG2D and NKG2D ligands. The blocking antibodies against the NKG2D receptor could not block the cytotoxicity of bulk CIK cells completely even when very high doses of NKG2D blocking antibody were used. Some studies indicated that NK cytotoxicity against target cells depends on the synergistic effect of both NCR and NKG2D,Citation17 but in our study almost no NCRs expression was detected on bulk CIK cells, suggesting mechanisms other than NKG2D-NKG2D ligand interaction may also be involved in the cytotoxicity of CIK cells against MM cells.
Malignant cells can usually survive traditional treatment strategies such as surgery, radiation and chemotherapy, and the latter two therapies carry many side effects. Most importantly, small lesions and metastatic cells often remain and cause recurrence of disease. Immunotherapy is a new and promising treatment for a number of cancers. It has the advantages to stimulate and restore the body’s natural abilities of the immune system which can recognize and kill tumor cells.Citation10,Citation14 Thus, CIK cell-based immunotherapy is a promising new therapy with potential to kill a wide spectrum of tumor cells. A combination of IFN-γ, OKT-3 and IL-2 induced the expansion of immune effector cells with high expression of NKG2D on the cell surface, which might bind to NKG2D ligands on MM cells, so that mediated cytolysis of MM cells. CIK cellsinfusion is an attractive antitumor immunotherapy for the treatment of MM patients. The mechanism of CIK cells cytolysis seems to be mediated at least in part by the interaction of NKG2D-NKG2D ligands. Our studies have practical significance in supporting clinical trial of CIK transfusion in the treatment of patients with MM.
Materials and Methods
Myeloma cells
The MM cell line U266 was obtained from Suzhou University, and was maintained in RPMI 1640 supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum (FBS). Human myeloma cells were isolated from bone marrow of patients with MM (containing > 30% myeloma cells) using the density gradient method. The myeloma cells were cryopreserved at -80°C until use. All patients provided their informed consent for the use of their samples and this study protocol was approved by the institutional ethical committee of Nanjing Medical University.
Generation of CIK cells
Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation. The CIK cells were cultured as previously described.Citation8 In brief, PBMCs were cultured in 10% FBS/RPMI-1640 with 1000 U/mL interferon γ (IFN-γ; Saida) added at the initiation of culture. Fifty ng/mL recombinant humanized anti-CD3 monoclonal antibody (OKT3; Wuhan Institute of Biological Products) and 1000 U/mL recombinant human inerleukin-2 (rhIL-2; Saida Shanghai, China) were added 24 h later and the culture was maintained by adding fresh medium every 3 d and IL-2 every 6–7 d. The CIK cells were harvested and used for assays at week 3 of culture.
Phenotype analysis of CIK and human myeloma cells
The phenotypes of cultured cells were determined by flow cytometry (BD FACS Canto II). The cells were stained with various monoclonal antibodies (mAbs) including CD3, CD4/8 (BD Multitest), CD45, CD56, CD138, CD158a, CD158b (BD PharMingen), NKG2D, NKp30, NKp44 and NKp46 (Beckman Coulter). The expression of NKG2D ligands on human myeloma cells from MM patients was examined using mAbs specific to MICA⁄B (clone 6D4, eBioscience), ULBP1, ULBP2 and ULBP3 (R&D Systems). The expression of HLA class I on human myeloma cells from MM patients was determined using anti-HLA-ABC antibody (BD PharMingen), anti-human NKG2D and MICA/B (R&D Systems).
IFN-γ detection
Intracellular staining of IFN-γ was performed as described previously.Citation24 106 expended CIK cells were stimulated with or without 5 × 105 U266 cells in 12-well plates. After 2 h, monensin (2 μM) was added to block cytokine transportation. After additional 8 h of culture, cells were collected and were labeled with anti-CD3-FITC and anti-NKG2D-PE. Cells were then permeabilized, and stained with anti-IFN-γ–allophycocyanin (APC) (BD Bioscience), followed by flow cytometric analysis (BD FACS Canto II). A similar experiment was set up except without adding monensin, IFN-γ concentrations in the culture supernatants were measured using ELISA kit (Saida).
Cytotoxicity assay
CIK cells against MM cells were assessed by flow cytometry, as described previously.Citation25 After 21 d culture, CIK cells were used for cytotoxicity assays. Calcein acetoxymethyl ester (CAM) (Sigma-Aldrich) was dissolved in DMSO to a final concentration of 1 mM and stored in aliquots at -80°C. Target cells including U266 or human primary myeloma cells were incubated with CAM at final concentration of 0.1 μM for 15 min at 37°C in the dark. The labeled cells were washed twice in ice-cold medium supplemented with 10% FBS, re-suspended in 10% FBS/RPMI-1640 and then plated in round bottom 96-well plates at 5 × 105 cells/well in duplicate. CIK cells were added at an effector-to-target (E:T) ratio of 10, in a final volume of 200 μl, and were incubated for 10 h at 37°C in the dark. Cells were then washed with PBS and resuspended in 100 μl of 1 x binding buffer (10 mM Hepes/NaOH, 140 mM NaCl, 2.5 mM CaCl2, pH7.4), and stained with 10 μl of 7-amino-actinomycin D (7-AAD) (Sigma-Aldrich). After 15 min incubation at room temperature in the dark, the cells were analyzed using flow cytometry. The cytotoxic activity against the target cells was determined on the basis of the enumeration of viable cells by flow cytometry. The percentage of specific lysis (PSL) was calculate as follows: % specific lysis = (CT - TE/CT) × 100 [where CT is the percentage of viable fluorescent target cells in control tubes and TE is the percentage of viable fluorescent target cells in test (target + effector) tubes]. In blocking experiments, anti-NKG2D Abs were added to the CIK cell suspension at 10 μg ⁄mL and incubated at 37°C for 30 min before the addition of target cells.
Acknowledgments
This work was supported by China Postdoctoral Science Foundation (20110491337), Postdoctoral Science Foundation of Jiangsu (1101012C), Major projects of Changzhou City Health Bureau (ZD201010) and the Key Medical Subject Fundation of Jiangsu.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633 - 40; http://dx.doi.org/10.1016/S1471-4906(01)02060-9; PMID: 11698225
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44 - 9; http://dx.doi.org/10.1126/science.1198687; PMID: 21212348
- Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005; 105:251 - 8; http://dx.doi.org/10.1182/blood-2004-04-1422; PMID: 15328155
- Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol 2006; 16:333 - 43; http://dx.doi.org/10.1016/j.semcancer.2006.07.008; PMID: 16914326
- Zafirova B, Wensveen FM, Gulin M, Polić B. Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci 2011; 68:3519 - 29; http://dx.doi.org/10.1007/s00018-011-0797-0; PMID: 21898152
- Obeidy P, Sharland AF. NKG2D and its ligands. Int J Biochem Cell Biol 2009; 41:2364 - 7; http://dx.doi.org/10.1016/j.biocel.2009.07.005; PMID: 19631280
- Mistry AR, O’Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology 2007; 121:439 - 47; http://dx.doi.org/10.1111/j.1365-2567.2007.02652.x; PMID: 17614877
- Linn YC, Lau SK, Liu BH, Ng LH, Yong HX, Hui KM. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology 2009; 126:423 - 35; http://dx.doi.org/10.1111/j.1365-2567.2008.02910.x; PMID: 18778291
- Bonanno G, Iudicone P, Mariotti A, Procoli A, Pandolfi A, Fioravanti D, et al. Thymoglobulin, interferon-γ and interleukin-2 efficiently expand cytokine-induced killer (CIK) cells in clinical-grade cultures. J Transl Med 2010; 8:129; http://dx.doi.org/10.1186/1479-5876-8-129; PMID: 21138560
- Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer 2011; 2:363 - 8; http://dx.doi.org/10.7150/jca.2.363; PMID: 21716717
- Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood 1998; 92:3318 - 27; PMID: 9787169
- Linn YC, Lau LC, Hui KM. Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. Br J Haematol 2002; 116:78 - 86; http://dx.doi.org/10.1046/j.1365-2141.2002.03247.x; PMID: 11841399
- Chan JK, Hamilton CA, Cheung MK, Karimi M, Baker J, Gall JM, et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clin Cancer Res 2006; 12:1859 - 67; http://dx.doi.org/10.1158/1078-0432.CCR-05-2019; PMID: 16551871
- Thanendrarajan S, Nowak M, Abken H, Schmidt-Wolf IG. Combining cytokine-induced killer cells with vaccination in cancer immunotherapy: more than one plus one?. Leuk Res 2011; 35:1136 - 42; http://dx.doi.org/10.1016/j.leukres.2011.05.005; PMID: 21652069
- Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol 2011; 137:305 - 10; http://dx.doi.org/10.1007/s00432-010-0887-7; PMID: 20407789
- Niam M, Linn YC, Fook Chong S, Lim TJ, Chu S, Choong A, et al. Clinical scale expansion of cytokine-induced killer cells is feasible from healthy donors and patients with acute and chronic myeloid leukemia at various stages of therapy. Exp Hematol 2011; 39:897 - 903, e1; http://dx.doi.org/10.1016/j.exphem.2011.06.005; PMID: 21703986
- Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol 2001; 31:1076 - 86; http://dx.doi.org/10.1002/1521-4141(200104)31:4<1076::AID-IMMU1076>3.0.CO;2-Y; PMID: 11298332
- Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 2004; 103:3065 - 72; http://dx.doi.org/10.1182/blood-2003-06-2125; PMID: 15070686
- Fuertes MB, Girart MV, Molinero LL, Domaica CI, Rossi LE, Barrio MM, et al. Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell-mediated cytotoxicity. J Immunol 2008; 180:4606 - 14; PMID: 18354183
- Lu X, Ohata K, Kondo Y, Espinoza JL, Qi Z, Nakao S. Hydroxyurea upregulates NKG2D ligand expression in myeloid leukemia cells synergistically with valproic acid and potentially enhances susceptibility of leukemic cells to natural killer cell-mediated cytolysis. Cancer Sci 2010; 101:609 - 15; http://dx.doi.org/10.1111/j.1349-7006.2009.01439.x; PMID: 20028385
- Kato N, Tanaka J, Sugita J, Toubai T, Miura Y, Ibata M, et al. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia 2007; 21:2103 - 8; http://dx.doi.org/10.1038/sj.leu.2404862; PMID: 17625602
- Kloss M, Decker P, Baltz KM, Baessler T, Jung G, Rammensee HG, et al. Interaction of monocytes with NK cells upon Toll-like receptor-induced expression of the NKG2D ligand MICA. J Immunol 2008; 181:6711 - 9; PMID: 18981088
- Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev 2010; 235:267 - 85; PMID: 20536569
- Tassi I, Cella M, Presti R, Colucci A, Gilfillan S, Littman DR, et al. NK cell-activating receptors require PKC-theta for sustained signaling, transcriptional activation, and IFN-gamma secretion. Blood 2008; 112:4109 - 16; http://dx.doi.org/10.1182/blood-2008-02-139527; PMID: 18784374
- Cholujová D, Jakubíková J, Kubes M, Arendacká B, Sapák M, Ihnatko R, et al. Comparative study of four fluorescent probes for evaluation of natural killer cell cytotoxicity assays. Immunobiology 2008; 213:629 - 40; http://dx.doi.org/10.1016/j.imbio.2008.02.006; PMID: 18765168