Abstract
Twist-1 protein (also called Twist) has been suggested to be involved in tumor epithelial-mesenchymal transition (EMT) related progression, however, the mechanism by which twist promotes lymph node metastasis is not fully understood. In the present study, we found that nuclear twist expression is clearly correlated with lymph node (LN) metastasis as determined by immunohistochemistry (IHC). A highly invasive EC109 cell subline, EC109-P, was established by repeated in vitro transwell isolations for the cell model. Immunofluorescence (IF) assay demonstrated that nuclear twist expression was markedly higher in the highly invasive EC109-P cell line when compared with EC109 and EC9706 cells. Based on our cell model, the function and mechanism by which twist regulates LN metastasis in ESCC was investigated. The results showed that the overexpression of Twist could significantly increase the invasion and VEGF-C expression of EC9706 cells, whereas the knockdown of twist expression results in the opposite effects. This finding was further strengthened by the results of the analysis of co-expression of twist and VEGF-C by IHC in ESCC clinical samples. In summary, our study indicates that nuclear twist plays an important role in ESCC lymphatic metastasis by increasing the expression of VEGF-C. The combination of twist and VEGF-C detection could be a reliable prediction of LN metastasis in ESCC.
Keywords: :
Introduction
Esophageal squamous cell cancer (ESCC) is one of the most common malignancy tumors, which morbidity ranks 6th among the most common types of cancers and is currently the 5th leading cause of death worldwide.Citation1 Although surgical advances, such as excision with clear margins, have been achieved in ESCC treatment, the overall 5-y ESCC survival rate is still unsatisfactory.Citation2 As it is well known that lymph node (LN) metastasis is a key prognostic factor for long-term ESCC outcomes,Citation3,Citation4 an understanding of the mechanisms is critical for the prediction, prevention and cure of ESCC LN metastasis.
Twist is a transcription factor that promotes the EMT-associated with tumor metastases, cancer stem cells and drug resistanceCitation5-Citation7 and acts cooperatively with Bmi1 to inhibit the expression of the epithelial marker E-cadherin, and leads to a poor prognosis in a variety of head and neck cancers.Citation8 Twist can also upregulate AKT2 to enhance tumor migration and resistance to paclitaxel,Citation9 and increasing twist expression was found to be correlated with lymph node metastases in colon cancer and ESCC.Citation10-Citation12 Nevertheless, the mechanism by which twist regulates lymph node metastasis in ESCC is unclear. VEGF-C, a member of the vascular endothelial growth factor (VEGF) family, can lead to lymphatic vascular endothelium growth and induce selective hyperplasia of the lymphatic vasculature in vivo.Citation13,Citation14 VEGF-C plays significant roles in cancer cell mobility and invasiveness by binding to its receptors.Citation15,Citation16 Therefore, VEGF-C promotes lymphangiogenesis and, in turn, increases lymphatic metastasis in different cancer types including ESCC.Citation17-Citation23 Although the level of twist was positively correlated with VEGF-C in supraglottic carcinoma,Citation24 the mechanisms by which twist and VEGF-C are involved in lymphatic metastasis in ESCC require to be further study.
To investigate the roles of the subcellular localization of twist in ESCC progression, especially in lymphatic metastasis, in the present study, IHC was used to detect the expression of twist in 168 ESCC tissues. We established a highly invasive cell subline, EC109-P using repetitive transwell assays. A twist overexpression and siRNA plasmids were transfected into ESCC cell lines, and transwell assays were used to detect the alteration of invasion ability induced by the up and downregulation of twist. Moreover, the co-expression of twist and VEGF-C in cell lines and clinical samples were evaluated by WB, IHC and real-time PCR.
Results
Nuclear expression of twist in ESCC tissues associated with LN metastasis
IHC was performed to detect the expression of twist in 168 ESCC tissues. The results showed that twist was expressed in the nucleus and cytoplasm in ESCC tissues (). Thus, we analyzed the twist subcellular localization, as correlated with the clinical significance. The nuclear localization of twist presented in 60.7% (102/168) of the ESCC tissues, and the positive rate in the cytoplasm was 35.1% (59/168). The twist expression in the nucleus and cytoplasm associated with clinicopathological characteristics of the patients, including gender, age, location, T stage and LN metastasis, are presented in . Positive expression of twist in the nucleus but not in the cytoplasm was significantly correlated with LN metastasis (p = 0.001). In contrast, the twist expression in both the nucleus and cytoplasm had no statistical correlation with age, sex, T stage and location (p > 0.05), indicating that twist expression in the nucleus was critical for lymph node metastasis of ESCC.
Figure 1. Expression of twist in ESCC tissues. (A) IHC analysis of twist in ESCC with or without LN metastasis. (a) Negative staining in normal esophageal tissue; (b-c) Strong nuclear staining in primary sites of metastatic ESCC cancer; (d-e) Moderate staining in the cytoplasm but negative nuclear staining in primary ESCC cancer sites without LN metastasis. (f) Negative control. (Original magnification, 200 × ). (B) WB analysis of twist expression in primary ESCC tissues (T) with or without LN metastasis and matched nontumorous tissues (N). Twist showed much higher expression in T compared with N and was stronger in primary ESCC with LN metastasis (T1, T2) than without LN metastasis (T3, T4). Four representatives are shown. β-actin was used as an internal control.
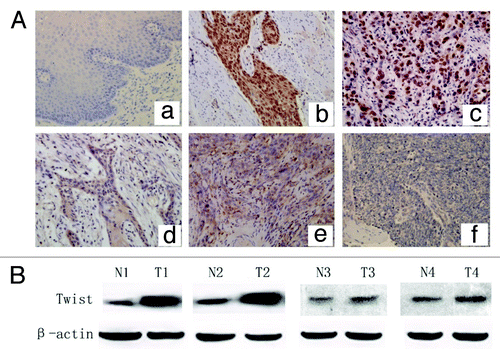
Table 1. The twist expression associated with patients’ clinical features
We also examined the twist protein level in fresh primary ESCC and matched adjacent tissues from 10 patients using WB. Twist showed much higher expression levels in the tumor tissues than the matched adjacent tissues, and the expression was stronger in primary ESCC with LN metastasis than that without LN metastasis ().
Nuclear expression of twist correlated with cell migration and invasion ability
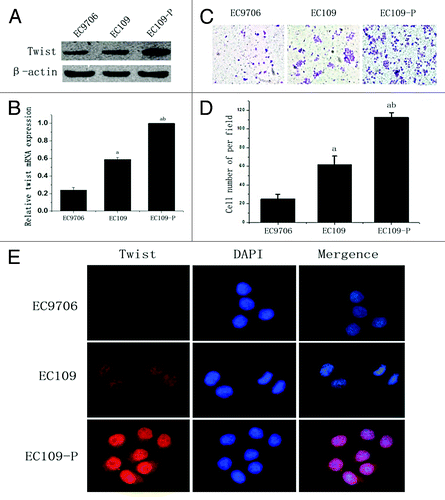
A highly invasive EC109 cell subline, EC109-P, was established for further study. WB and real time-PCR were used for the evaluation of twist in ESCC cell lines. As shown in , a notably higher expression level of twist was detected in EC109-P cells, a moderate level in EC109 cells, and the lowest level in EC9706 cells. The transwell assays demonstrated that EC109-P possessed an apparently higher invasion ability than the EC109 and EC9706 cells (), and IF assays showed that the nuclear expression of twist in EC109-P was markedly stronger than in the EC109 and EC9706 cells (). These data indicated that the nuclear expression of twist was correlated with the cell invasion.
Figure 2. Expression of twist in ESCC cell lines. (A)Western blot and (B) real time PCR analysis of twist expression in ESCC cell lines EC9706, EC109 and EC109-P. β-actin was used as a loading control. (C and D) Transwell assays analysis of the invasion ability of ESCC cell lines. (E) Immunofluorescence analysis of twist in ESCC cell lines. The values presented are the means of three determinations (Original magnification, 400x). abStatistical significance (ap < 0.05 vs. EC9706 and bp < 0.05 vs. EC109).
The influence of the alteration twist expression on the invasion of ESCC
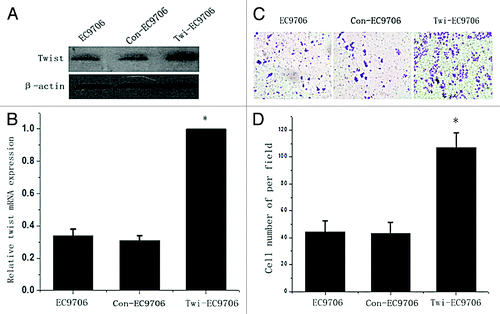
Twist siRNA plasmid was transfected into cell line EC109-P, which has a high level of endogenous twist expression. Both WB and real time PCR confirmed that twist was significantly downregulated in the siRNA-transfected cells compared with the controls (). The transwell assays showed that the knocking down of twist notably reduced the invasion of the EC109-P cells (). To study whether twist is involved in ESCC cell invasion further, a twist overexpression plasmid was transfected into a cell line EC9706, which has a low endogenous twist expression, and the expression of twist was markedly upregulated (). The transwell assays showed that the enhanced twist expression notably increased the invasion ability of EC9706 (). These data are consistent with the observation that twist promotes ESCC lymph node metastasis in tissues.
Figure 3. Decreased invasion ability of EC109-P cells by inhibiting twist expression. (A) western blot and (B) real time PCR analysis of twist expression in siRNA transfected EC109-P cells and controls. (C and D) Transwell assays for siRNA transfected cells and controls. A representative of each one is presented (Original magnification, 100x). The mean values of three repetitions are presented. *Statistical significance (p < 0.05, Si-EC109-P vs. EC109-P or Con-EC109-P cells).
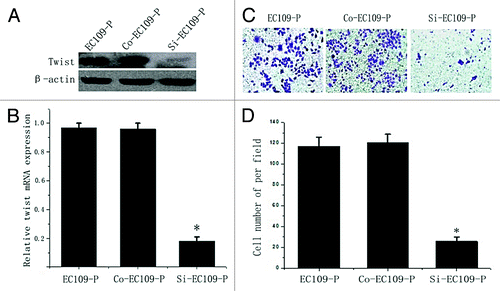
Figure 4. Enhancing the invasion ability of EC9706 cells by the upregulation of twist expression. (A) western blot and (B) real time PCR analysis of twist expression in twist overexpression plasmid transfected EC9706 cells and controls. (C) Transwell assays for twist overexpressing EC9706 cells and controls. A representative of each one is shown. (D) The mean values of three times was presented. *Statistical significance (p < 0.05, Twi-EC9706 vs. EC9706 or Con- EC9706 cells).
Twist promotes ESCC lymph node metastasis through increasing VEGF-C
The present study confirmed that twist was critical in LN metastasis of ESCC. Therefore, we further investigated the mechanism of twist in the invasion of ESCC by determining whether twist promotes ESCC invasion through regulating VEGF-C.WB and real time PCR showed that the overexpression of twist markedly upregulated the mRNA and protein expression of VEGFC in EC9706 cells (). Conversely, the knockdown endogenous twist expression in the EC109-P cells markedly inhibited the expression of VEGF-C protein and mRNA (). These data indicated that twist promoted ESCC invasion partly through increasing VEGF-C expression.
Figure 5. Twist regulates VEGF-C expression in ESCC cells and tissues. (A and B) WB analysis of VEGF-C protein expression in twist down and upregulated cells. β-actin was used as a loading control. (C and D) Relative VEGF-C mRNA expression in twist down and upregulated cells by real time PCR analysis. (E) Co-expression of twist and VEGF-C in ESCC tissues. (a) Negative twist expression in the nucleus correlated with (b) lower VEGF-C expressionin the same patients. (c) Strong twist expression in the nucleus correlated with (d) high VEGF-C expression in the same samples (Original magnification, 200x).
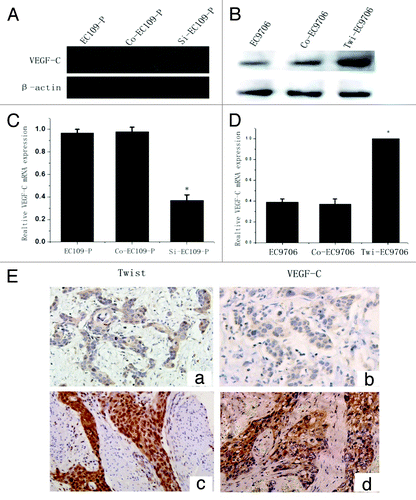
Twist expression in the nucleus is associated with VEGF-C in ESCC tissues
To determine whether the correlation of twist and VEGF-C levels was associated with the development of lymph node metastasis in ESCC, we further examined VEGF-C in serial sections of ESSC tissues from 168 patients by IHC. The results showed no statistical correlation between twist expression in the cytoplasm and VEGF-C expression (r = 0.121, p = 0.118, Spearman’s rank test), but a significant correlation was found between nuclear twist and VEGF-C expression ( and ) (r = 0.356, p < 0.001, Spearman’s rank test). Moreover, the regression analysis results suggested that patients with higher nuclear twist levels and VEGF-C expression had a greater risk of developing lymph node metastasis (OR = 2.53, p < 0.01 vs. OR = 4.37, p < 0.001, respectively; Binary Logistic analysis).
Table 2. The association of twist and VEGF-C expression in ESCC tissues
Discussion
ESCC is one of the most malignant cancers and had a large prevalence worldwide.Citation1,Citation2 As lymph node metastasis is a critical factor in determining ESCC prognosis, to understand the mechanisms of lymphatic metastasis, it is necessary for providing better diagnostic and therapeutic strategies for ESCC. Although several lines of evidence have confirmed the correlation of twist with cancer progression, especially in LN metastasis, the mechanisms required further study.
In this study, we demonstrated for the first time that a nuclear location of twist played an important role in ESCC lymphatic metastasis. The result of IHC of ESCC tissues from 168 patients showed that 60.7% of the samples possessed a nuclear localization of twist, whereas 35.1% was in the cytoplasm. The cytoplasmic expression of twist did not correlate with any of the clinicopathological data. In contrast, twist expression in the nucleus was statistically correlated with LN metastasis, indicating that twist expression in the nucleus was critical for the lymph node metastasis of ESCC.WB also showed that twist presented notably elevated expression, especially in primary tumor tissues, with LN metastasis compared with matched adjacent tissues. We established a highly invasive cell subline, EC109-P, using repetitive transwell assays. Consistent with the twist expression in tissues, the most interesting results showed that the nuclear expression of twist was markedly stronger in the highly invasive EC109-P cells compared with its parental EC109 cells, indicating that the nuclear expression of twist was correlated with the migration and invasion ability of the cells. This phenomenon was further supported by the results that inhibiting twist expression significantly suppressed the invasion of EC109-P cells and enhancing twist expression showed the opposite effect in EC9706 cells.
VEGF-C plays significant roles in lymphatic vascular endothelium growth and the induction of selective hyperplasia of the lymphatic vasculature.Citation13,Citation14 To clarify whether VEGF-C is involved in twist-mediated LN metastasis in ESCC, WB and real time PCR were used to evaluate the co-expression of twist and VEGF-C in cells transfected with siRNA and overexpression plasmids and corresponding controls. The results showed that knocking down of twist can repress both the protein and mRNA expression of VEGF-C in EC109-P cells, whereas increased twist expression had an opposite effect in EC9706 cells. Additionally, we also detected the VEGF-C expression in serial sections from 168 ESCC patients, and the results showed that the presence of high twist expression in the nucleus correlated significantly with strong VEGF-C expression. Moreover, the regression analysis showed that patients with higher twist in the nucleus and VEGF-C co-expression had a greater risk of developing lymph node metastasis. Because twist has been demonstrated to be a key transcription factor which induces the epithelial-mesenchymal transition,Citation5-Citation7 we speculated that twist accumulation in the nucleus and the alteration of VEGF-C might be related to its transcriptional activity.
In conclusion, twist nuclear accumulation can promote LN metastasis and regulate this metastasis partly through altering VEGF-C expression in both ESCC tissues and cell lines. The combination of twist and VEGF-C detection could be help in predicting the risk of LN metastasis for ESCC patients. Further experiments, such as luciferase reporter assays and chromatin immunoprecipitation, should be utilized to obtain more information about the precise mechanism of twist and VEGF-C which is involved in the progression of ESCC LN metastasis.
Materials and Methods
Tissues
A total of 168 primary Parafðn-embedded tissues were obtained from ESCC patients, ranging from 37 to 77 y old (58.9 ± 9.8), who underwent surgery in Xijing Hospital of Digestive Diseases, Xian, China, from 2008–11 to 2011–05. Fresh surgical tissues from 10 ESCC patients (7 males and 3 females) who had undergone esophagectomy in our hospital between February and September of 2010 were stored at -80°C. All of the patients underwent transthoracic esophagectomy with three-field or two-field lymphadenectomy, and were confirmed by clinical and pathological diagnosis. Full clinical history records of these patients, including age, gender, location, T stage and LN metastasis, were also collected. None of the patients received preoperative chemotherapy or radiotherapy. Agreements for the tissue use were signed with informed consent by all of the patients and this study was approved by the Ethical Committee of Xijing Hospital.
IHC staining
The IHC staining was performed to test the twist and VEGF-C expression in serial sections of tissues from 168 ESCC patients, as described in our previous study.Citation25 Briefly, the endogenous peroxidase activity was blocked using 3% H2O2 for 10 min at room temperature. The sections were incubated with twist (1:500, Abcam) and VEGF-C (1:250, Abcam) antibodies in PBS overnight at 4°C. Sections incubated in PBS with anti-mouse/goat IgG (1:2000, Zhongshan) but without primary antibody were used as negative controls. After three-washes with PBS-T, the sections were incubated with anti-mouse/goat IgG antibodies (1:2000, Zhongshan). Following another three-washes with PBS-T, the antibodies binding were visualized using 3, 3′-diaminobenzidine (DAB) (Zhong-Shan Goldbridge Co.). The intensity was scored 0 (no immunoreactivity), 1 (weak), 2 (moderate) or 3 (intense immunoreactivity), and the proportion was scored 0 (0%), 1 (0–30%), 2 (30–60%) or 3 (> 60%), respectively. The two scores were then added to obtain the final results: negative 0–2 (-) and positive 3–6 (+).
ESCC cell lines and construction of a highly invasive EC109 subline
The human ESCC cell lines EC109 and EC9706 were obtained from the Chinese Academy of Medical Science.Citation26 The cells were cultured in RPMI-1640 medium (GIBCO) containing 10% heat-inactivated fetal calf serum (GIBCO) in a humidified incubator at 37°C in the presence of 5% CO2. The highly invasive EC109 cell subline was constructed as our previous reportCitation27 and described mainly below: EC109 cells in 1ml serum-free 1640 were placed in the top chamber of an 8-μm-pore transwell (Corning) coated with 200 mg/ml Matrigel (BD Biosciences), the underlayer well was filled with 2.0 mL 1640 medium with 20% serum. After cultured for 24 h, the cells on the lower membrane were collected and expanded for four-round isolations.
Protein collection and western blot analysis
The protein in fresh tissues and cell lines were extracted as described in our previous work.Citation25 After loading the proteins (30 μg) onto an 8% SDS-PAGE, electrophoresis was performed at 20 mA for 60 min under denaturing conditions, and the proteins were then transferred to a nitrocellulose membrane. The membrane was incubated in 5% fat-free milk for 1h. The primary twist antibody (1:500, Abcam), VEGF-C (1:500, Abcam) and β-actin (1:2000, Sigma) were added. After three washes with PBS-T, the membranes were incubated with horseradish peroxidase-conjugated anti-goat or mouse secondary Ab (1:3000; Sigma-Aldrich). Protein bands were detected using an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech). Each blot was repeated three times.
Total RNA extraction and real-time PCR
Total tissue RNA was isolated from the cell lines using the TRIzol reagent (Invitrogen) as recommended by the manufacturer, and DNase was used to block the contamination of genomic DNA. Reverse transcription reactions were performed with 2 mg RNA using the Prime Script RT reagent kit and SYBR premix Ex Taq (Takara) with the following program: initial denaturation at 95°C for 2 min, followed by 45 cycles of 95°C for 15 sec, 56°C for 20 sec and 72°C for 15 sec. GAPDH was used as the housekeeping gene. The forward (F) and reverse (R) primer sequences were as follows: twist: F, 5′-GGCCAGGTACATCGACTTCC-3′ and R, 5′-CCGCTCGTGAGCCACATA-3′; VEGF-C: F, 5′-TCAGGCAGCGAACAAGAC- 3′ and R, 5′-GCATCCGAGGAAA ACA-3′; GAPDH: F, 5′-GCACCGTCAA GGCTGAGAAC-3′ and R, 5′-TGGTGAA G ACGCCAGTGGA-3′. Each reaction was performed in triplicate.
Immunofluorescence
EC9706, EC109 and EC109-P cells were plated on sterilized coverslips in a 24-well plate. Following fixation with 4% paraformaldehyde, the cells were treated with 0.25% Triton for 15 min and blocked in 10% goat serum for 30 min. The cells were incubated with the primary twist antibody (1:500, Abcam) overnight at RT. Donkey anti-mouse IgG-Cy3 (Molecular Probes, Invitrogen) was used as the secondary antibody. Lastly, the cells were counterstained with DAPI (Sigma) and examined using a fluorescence microscope (Olympus BX51, Olympus).
Plasmids and transfection
Twist overexpression and siRNA plasmids used in our lab, have been demonstrated high efficiencies of up and downregulating twist expression.Citation28 The twist-pcDNA 3.1 plasmid was transfected into EC9706 cells and the Psilence-Twist plasmid was transfected into EC109-P cells. The control plasmids, pcDNA 3.1 and Psilence, were transfected into the corresponding cells. The cell lines were named Si-EC109-P, Con-EC109-P, Twi-EC9706 and Con-EC9706 respectively. WB and real time PCR were used to detect the twist protein and mRNA expression, and transwell assays were performed to evaluate the invasion ability of these cell lines. Each was repeated in triplicate.
Transwell assays
Twenty-four-well polycarbonate transwell plates with 8 μm pores (Corning) were used to test the ESCC cell line migration and invasion. Briefly, for the transwell migration assays, tumor cells were placed in the top and bottom chambers at a concentration of 5 × 104 in DMEM medium with 5% FBS. For the invasion assays, the top chambers were coated with 200 mg/mL Matrigel and dried at 4°C overnight. Afterward, 5 × 104 cells were placed in the top chamber, and the cells migrating across the transwell membrane were stained with 1% crystal violet 24 h after placement. The migrated cell number was manually counted using a light microscope (Olympus BX51, Olympus) at × 200 magnification for ten random fields in each well.
Statistical analysis
All of the significant differences were analyzed using the SPSS 16.0 software package. A Kruskal-Wallis H test was performed to analyze the IHC of twist expression with clinical parameters. The one-way ANOVA was used for the analysis of three comparisons, including the results of the real time PCR and transwell assays. Correlations were assessed using bivariate correlation, and regression analysis was performed using Binary Logistic analysis. A p value < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by the grant from China Postdoctoral Science Foundation (grand no. 20090461447) and the National Natural Science Foundation of China (no. 81172288).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69 - 90; http://dx.doi.org/10.3322/caac.20107; PMID: 21296855
- Ren Y, Cao B, Law S, Xie Y, Lee PY, Cheung L, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res 2005; 11:6190 - 7; http://dx.doi.org/10.1158/1078-0432.CCR-04-2553; PMID: 16144920
- Lerut T, Nafteux P, Moons J, Coosemans W, Decker G, De Leyn P, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004; 240:962 - 72, discussion 972-4; http://dx.doi.org/10.1097/01.sla.0000145925.70409.d7; PMID: 15570202
- Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002; 236:177 - 83; http://dx.doi.org/10.1097/00000658-200208000-00005; PMID: 12170022
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 2008; 14:79 - 89; http://dx.doi.org/10.1016/j.ccr.2008.06.005; PMID: 18598946
- Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res 2006; 12:5369 - 76; http://dx.doi.org/10.1158/1078-0432.CCR-05-2722; PMID: 17000670
- Valdés-Mora F, Gómez del Pulgar T, Bandrés E, Cejas P, Ramírez de Molina A, Pérez-Palacios R, et al. TWIST1 overexpression is associated with nodal invasion and male sex in primary colorectal cancer. Ann Surg Oncol 2009; 16:78 - 87; http://dx.doi.org/10.1245/s10434-008-0166-x; PMID: 19002529
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res 2007; 67:1979 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-06-1479; PMID: 17332325
- Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 2010; 12:982 - 92; http://dx.doi.org/10.1038/ncb2099; PMID: 20818389
- Xie F, Li K, Ouyang X. Twist, an independent prognostic marker for predicting distant metastasis and survival rates of esophageal squamous cell carcinoma patients. Clin Exp Metastasis 2009; 26:1025 - 32; http://dx.doi.org/10.1007/s10585-009-9292-5; PMID: 19816777
- Sasaki K, Natsugoe S, Ishigami S, Matsumoto M, Okumura H, Setoyama T, et al. Significance of Twist expression and its association with E-cadherin in esophageal squamous cell carcinoma. J Exp Clin Cancer Res 2009; 28:158; http://dx.doi.org/10.1186/1756-9966-28-158; PMID: 20025748
- Lee KW, Kim JH, Han S, Sung CO, Do IG, Ko YH, et al. Twist1 is an independent prognostic factor of esophageal squamous cell carcinoma and associated with its epithelial-mesenchymal transition. Ann Surg Oncol 2012; 19:326 - 35; http://dx.doi.org/10.1245/s10434-011-1867-0; PMID: 21732143
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001; 7:192 - 8; http://dx.doi.org/10.1038/84643; PMID: 11175850
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997; 276:1423 - 5; http://dx.doi.org/10.1126/science.276.5317.1423; PMID: 9162011
- Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell 2006; 9:209 - 23; http://dx.doi.org/10.1016/j.ccr.2006.02.018; PMID: 16530705
- Das S, Ladell DS, Podgrabinska S, Ponomarev V, Nagi C, Fallon JT, et al. Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases. Cancer Res 2010; 70:1814 - 24; http://dx.doi.org/10.1158/0008-5472.CAN-09-3675; PMID: 20179201
- Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, et al. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat 2005; 91:125 - 32; http://dx.doi.org/10.1007/s10549-004-5783-x; PMID: 15868440
- Yu XM, Lo CY, Chan WF, Lam KY, Leung P, Luk JM. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin Cancer Res 2005; 11:8063 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-05-0646; PMID: 16299237
- Kitadai Y, Amioka T, Haruma K, Tanaka S, Yoshihara M, Sumii K, et al. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer 2001; 93:662 - 6; http://dx.doi.org/10.1002/ijc.1379; PMID: 11477575
- Kinoshita J, Kitamura K, Kabashima A, Saeki H, Tanaka S, Sugimachi K. Clinical significance of vascular endothelial growth factor-C (VEGF-C) in breast cancer. Breast Cancer Res Treat 2001; 66:159 - 64; http://dx.doi.org/10.1023/A:1010692132669; PMID: 11437102
- Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, et al. Serum midkine depends on lymph node involvement and correlates with circulating VEGF-C in oesophageal squamous cell carcinoma. Biomarkers 2007; 12:403 - 13; http://dx.doi.org/10.1080/13547500701192470; PMID: 17564845
- Kimura H, Kato H, Tanaka N, Inose T, Faried A, Sohda M, et al. Preoperative serum vascular endothelial growth factor-C (VEGF-C) levels predict recurrence in patients with esophageal cancer. Anticancer Res 2008; 28:1A 165 - 9; PMID: 18383841
- Okazawa T, Yoshida T, Shirai Y, Shiraishi R, Harada T, Sakaida I, et al. Expression of vascular endothelial growth factor C is a prognostic indicator in esophageal cancer. Hepatogastroenterology 2008; 55:1503 - 8; PMID: 19102331
- Lu SM, Yu L, Tian JJ, Ma JK, Li JF, Xu W, et al. Twist modulates lymphangiogenesis and correlates with lymph node metastasis in supraglottic carcinoma. Chin Med J (Engl) 2011; 124:1483 - 7; PMID: 21740802
- Tang S, Yang G, Meng Y, Du R, Li X, Fan R, et al. Overexpression of a novel gene gankyrin correlates with the malignant phenotype of colorectal cancer. Cancer Biol Ther 2010; 9:88 - 95; http://dx.doi.org/10.4161/cbt.9.2.10283; PMID: 19901563
- Huang D, Gao Q, Guo L, Zhang C, Jiang W, Li H, et al. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem Cells Dev 2009; 18:465 - 73; http://dx.doi.org/10.1089/scd.2008.0033; PMID: 18680391
- Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet 2010; 6:e1000879; http://dx.doi.org/10.1371/journal.pgen.1000879; PMID: 20300657
- Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han S, et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int 2009; 75:1278 - 87; http://dx.doi.org/10.1038/ki.2009.62; PMID: 19279556