Abstract
Pancreatic adenocarcinoma is mostly fatal and generally resistant to conventional treatments, such that effective therapies with tolerable side effects are desperately needed. Ion channels including the transient receptor potential (TRP) channels have been implicated in human malignancies, but their roles in pancreatic cancer were mostly unknown. Recent identification of the melastatin-subfamily members of the TRP family of ion channels, and their functions in pancreatic epithelia and adenocarcinoma, is expected to provide a new perspective to understanding the mechanism underlying pancreatic tumorigenesis. In this report, we present the clinical and pathological features of a mini-series of patients with pancreatic adenocarcinoma, which aberrantly exhibits immunoreactivity against the TRPM8 channel. We have recently demonstrated the proliferative role of TRPM8 channel in pancreatic cancer cells. Here, we present evidence that RNA interference-mediated silencing of TRPM8 induces replicative senescence in pancreatic adenocarcinoma cells. This suggests that the aberrantly expressed TRPM8 channel may contribute to pancreatic tumorigenesis by preventing oncogene-induced senescence, and targeted inhibition of TRPM8 may enhance tumor sensitivity to therapeutics. Based on these observations, we hypothesize that the TRPM8 ion channel plays a crucial role in the growth and progression of pancreatic neoplasia during tumorigenesis. We propose that TRPM8 can be exploited as a clinical biomarker and as a therapeutic target for developing personalized therapy in pancreatic adenocarcinoma.
Introduction
Pancreatic adenocarcinoma is a highly lethal disease; its incidence is rising, and afflicted individuals generally have poor prognosis.Citation1 Most of the patients die from metastatic disease that is typically resistant to conventionally used chemotherapeutic agents. Novel approaches targeting the genetic alterations involved in pancreatic carcinogenesis are expected to facilitate design of effective and patient-tailored therapy. Novel biomarkers and molecular targets are emerging, and this area of research continues to be a focus of active investigation with the goal of improving treatment response in patients with pancreatic adenocarcinoma.
Molecular studies have revealed the genetics and biology of multi-step pancreatic carcinogenesis. Development of pancreatic neoplasia involves pre-senescence of pancreatic epithelia and deregulation of developmental pathways, oncogenes, and tumor suppressors. Pancreatic intra-epithelial neoplasia (PanINs) are pre-malignant lesions, and their progression to invasive adenocarcinoma is associated with aberrant activation of hedgehog signaling, overexpression of epidermal growth factor receptor (EGFR), oncogenic mutations in K-RAS, and inactivation or loss of the tumor suppressors WRN, p16INK4A, SMAD4, LKB1, and p53.Citation2-Citation6 Besides, accumulating evidence has revealed important roles of ion channels in human cancers, but very little is known about their identity and functional roles in pancreatic cancer.
The transient receptor potential (TRP) family of ion channels play diverse physiological roles by sensing physical stimuli in the cellular environment and responding with modulation of intracellular ionic currents and signaling pathways.Citation7,Citation8 The TRP members possess common features, including a trans-plasma membrane channel that exhibits relative ionic selectivity, and they exert regulatory effects on cellular proliferation, survival, differentiation, and migration. Consistent with these cellular functions, aberrantly expressed TRP channels play important roles in the pathogenesis of many human diseases, including cancer. We have recently shown that the TRP melastatin-subfamily members, TRPM7 and TRPM8, are required for proliferation of pancreatic adenocarcinoma cells.Citation9,Citation10 Here, we present the clinical and pathologic features of a mini-series of patients with pancreatic adenocarcinoma, which exhibits aberrant immunoreactivity against TRPM8. Moreover, we show that targeted silencing of TRPM8 with small interfering RNA (siRNA) induces replicative senescence in pancreatic adenocarcinoma cells. A working model is proposed for TRPM8-mediated signaling that modulates EGF-induced cellular proliferation and survival in pancreatic adenocarcinoma. We hypothesize that TRPM8 contributes to development and progression of pancreatic neoplasia, and we suggest that TRPM8 can be developed as a clinical biomarker and a therapeutic target in pancreatic adenocarcinoma.
Clinicopathological Features of Pancreatic Adenocarcinoma that Aberrantly Expresses TRPM8
Among the patients included in this clinical case series, one of them is presented in detail with the medical history, clinical course, radiological images, and histopathology. The clinicopathological features and outcomes of all of these five patients are summarized in . The first patient is a 67 y-old Caucasian man, who initially presented with painless jaundice, dark urine, acholic stools, and weight loss of 60 lb in one year. Computed tomographic scan of the abdomen revealed a mass in the head and neck region of the pancreas, with compression of the bile duct (). Endoscopic retrograde cholangiopancreatography was performed with placement of a biliary stent, and the signs and symptoms associated with biliary obstruction resolved. He was further evaluated using endoscopic ultrasonography () with fine needle aspiration of the pancreatic mass; the cytology was consistent with adenocarcinoma.
Table 1. Clinicopathological features and anti-TRPM8 immunoreactivity of pancreatic adenocarcinoma in a mini-series of five patients
Figure 1. Radiographic and pathological features of pancreatic adenocarcinoma with metastasis to regional lymph nodes. (A) computed tomograph showing heterogeneous attenuation in the head and neck region of the pancreas (green arrow) with abrupt termination of common bile duct and pancreatic duct consistent with pancreatic cancer. (B) endoscopic ultrasonograph showing a large, hypoechoic mass (indicated diagonally by yellow and blue dotted lines) in the head of the pancreas with irregular outer margins. (C–F) histology of resected pancreatic adenocarcinoma stained with hematoxylin and eosin. (C) pancreatic tumor that contains atypical glands infiltrating a markedly desmoplastic stroma consistent with well-differentiated adenocarcinoma (20x magnification). (D) pancreatic adeno-carcinoma, in which atypical glandular epithelia contain large, pleomorphic nuclei with prominent nucleoli (200x magnification). (E and F) peri-pancreatic lymph nodes containing metastatic adenocarcinoma (20x magnification).
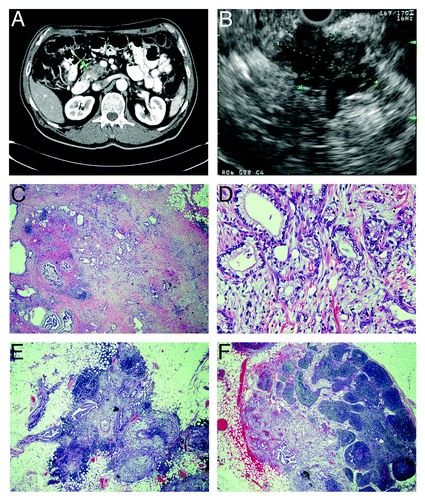
His past medical history was significant for previously treated prostate cancer and non-insulin-dependent diabetes mellitus. He denied any family history of cancer and any tobacco use. The patient appeared cachectic, and physical examination was otherwise unremarkable. Laboratory tests showed elevated serum levels of the tumor marker CA 19–9 (449 U/ml, normal range 0–37 U/ml), glucose 197 mg/dl (normal range 65–99 mg/dl), and alkaline phosphatase 128 U/l (normal range 35–104 U/l). Hepatic transaminases and total bilirubin were within the normal limits.
The patient underwent pancreaticoduodenectomy (Whipple’s procedure) for resection of the localized pancreatic tumor. Pathological evaluation revealed a 3.5-cm pancreatic adenocarcinoma extending beyond the pancreas, but showed no involvement of celiac axis and superior mesenteric artery, and no tumor was detected in the surgical margins (). However, metastatic tumor cells were present in four peri-pancreatic lymph nodes (). Therefore, the pathological stage of his pancreatic adenocarcinoma is IIb (T3 N1 M0). Following surgical resection of the pancreatic tumor, he received adjuvant chemotherapy (5-fluorouracil) and radiation therapy. He tolerated the adjuvant therapy fairly well, except for febrile neutropenia. Subsequently, he was diagnosed with bilateral pulmonary emboli and treated with enoxaparin for anti-coagulation. He then developed hematochezia, after which his condition began to deteriorate, and he eventually died. Autopsy was offered for investigation of his death but declined by his family.
The other four patients in this mini-series were also diagnosed with stage II pancreatic adenocarcinoma, and they were treated by surgical resection. One of them received adjuvant chemoradiation therapy and subsequently died from sepsis. The other died from sepsis following the operation. One of the patients received adjuvant chemoradiation therapy, and tumor recurrence has not been detected so far. The last patient on the list was treated with adjuvant chemoradiation therapy; she then developed tumor recurrence in the remaining pancreas, and she underwent total pancreatectomy, and received further adjuvant chemotherapy ().
The clinicopathological features of these patients are somewhat representative of the majority of cases of pancreatic adenocarcinoma.Citation1,Citation2,Citation11 Consistent with the previous studies showing that cigarette smoking accounts for one third of the cases of pancreatic cancer, 60% of the patients described here did not have a history of smoking. The clinical presentations of these patients resulted from biliary obstruction caused by the tumors located in the pancreatic head, which is a common location for the majority of this tumor. CA19-9 is a commonly used clinical biomarker of pancreatic adenocarcinoma, and its lack of good correlation with tumor status can be observed in this mini-case series. In general, the median survival of patients with stage II pancreatic adenocarcinoma is 12–24 mo, and adjuvant therapy has been shown to prolong survival by approximately 8 mo. In the patients discussed above, post-operative complications and possibly recurrence of pancreatic cancer contribute to mortality. These data further support the urgent need of identifying and validating clinically useful biomarkers, therapeutic targets, and agents to facilitate early detection and improve treatment response of this malignant disease.
In an attempt to gain insight into the clinical significance of TRPM8 in pancreatic cancer, we analyzed its expression in the surgical specimens of pancreatic adenocarcinoma by immuno-histochemistry. In contrast to normal pancreatic ducts and acini,Citation10 aberrant expression of TRPM8 is consistently observed in the pancreatic adenocarcinoma ( and ; ). In agreement with the proliferative role of TRPM8 in pancreatic cancer cells,Citation10 these findings further support our hypothesis for a contributory role of TRPM8 in pancreatic tumorigenesis. Although the mechanisms that mediate the functional role of TRPM8 in pancreatic cancer cells are unknown, biochemical and molecular studies of TRPM8 in other types of cells have begun to elucidate the regulation of its ionic channel activity and its cellular functions.
Figure 2. TRPM8 is aberrantly expressed in pancreatic adenocarcinoma. The paraffin-embedded tumor sections from the surgically resected specimen were analyzed by immunohistochemistry using rabbit anti-human TRPM8 antibodies (Lifespan Biosciences) at 1:50 dilution, followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (EnVision+ System, Dako). The signals were detected by color reaction using 3,3′-diaminobenzidine (Dako), counterstained with hematoxylin (Richard-Allan Scientific), and mounted using Permount (Sigma). The brown color indicates the presence of TRPM8 protein. Strong immunoreactivity against TRPM8 can be observed in the ductal epithelia of the pancreatic adenocarcinoma, with staining in both plasma membrane and cytoplasm. There is heterogeneity for expression of TRPM8 in the tumor. (A) 40x magnification. (B) 200x magnification. (C) 400x magnification. Tissue sections incubated in the absence of anti-TRPM8 antibodies were processed in parallel as control, and they did not exhibit any detectable immunoreactivity against TRPM8 (data not shown).
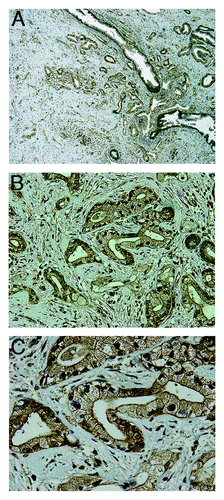
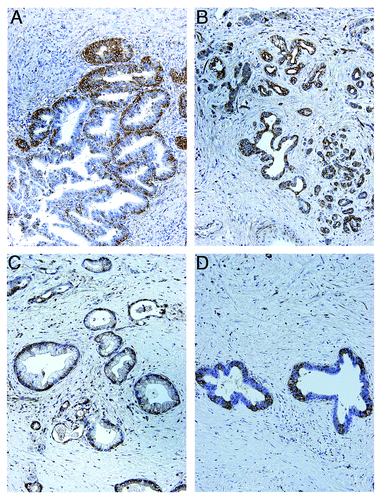
Figure 3. Expression of TRPM8 in pancreatic adenocarcinoma tissues. Surgically resected specimens were embedded in paraffin and processed for immunohistochemistry using rabbit anti-human TRPM8 antibodies (Lifespan Biosciences) followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (EnVision+ System, Dako). The signals were detected by color reaction using 3,3′-diaminobenzidine (Dako), counterstained with hematoxylin (Richard-Allan Scientific), and mounted using Permount (Sigma). The brown color indicates the presence of TRPM8 protein. Note the heterogeneity for expression of TRPM8 in the tumors. Controls using the consecutive tissue sections for each paraffin block were processed in the absence of anti-TRPM8 antibodies, and no immunoreactivity against TRPM8 was detected (data not shown). The panels A though D correspond to the patients as described in .
Biochemical Functions and Molecular Genetics of TRPM8
Structure and channel activity of TRPM8
TPRM8 is a Ca2+ permeable, non-selective, and voltage-gated ion channel. It consists of 1,104 amino acid residues, with a relative molecular mass of 128,000.Citation12,Citation13 TRPM8 contains six transmembrane domains located between amino acids 695–1,024.Citation12 The S1-S4 transmembrane domains demonstrate weak voltage-sensing properties, and the channel pore is located between the S5 and S6 domains.Citation12,Citation13 The ion channel of TRPM8 can be activated by cold temperature (between 25°C and 15°C) and cooling compounds (such as menthol, eucalyptol, and icilin), resulting in increased intracellular Ca2+ levels.Citation12,Citation13 Indeed, TRPM8 is permeable to multiple cations—monovalent ions such as Na+ and K+ as well as divalent ions including Ca2+ and Ba2+. Opening of the channel is voltage-dependent and the probability of opening increases with membrane depolarization.Citation14 Furthermore, experiments in Chinese hamster oocytes and trigeminal neurons showed that TRPM8 had a large outward rectifying current with poor cationic selectivity.Citation12,Citation13 However, in the prostatic cancer cell line LNCaP (lymph node carcinoma of the prostate), TRPM8 shows a strong inward rectifying current with high Ca2+ selectivity. Such difference in channel characteristics of TRPM8 may be related to its localization to both endoplasmic reticulum (ER) membrane and plasma membrane of LNCaP cells.Citation15 It has been demonstrated that, in LNCaP cells, TRPM8 mediates Ca2+ release from the ER as induced by cold temperature or menthol and also from the plasma membrane store-operated channels.Citation16 Thus, TRPM8 functions as a non-selective cation channel that is active at depolarized membrane potentials, and its channel activity appears to depend on the cellular context.
The channel activity of TRPM8 can be modulated by chemical and physical stimuli in the cellular environment as well as intracellular signaling molecules. Thermal cooling decreases the depolarization potential required to activate the channel.Citation17 Menthol and cold temperature can directly activate TRPM8, whereas icilin activates TRPM8 only in the presence of extracellular Ca2+ ions.Citation13,Citation18 Acidic pH produces an inhibitory effect whereas alkaline pH exerts a potentiating effect on the channel activity.Citation19 Besides, the lipid signaling molecule phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) also plays a regulatory role in the channel activity of TRPM8. PtdIns(4,5)P2 is necessary for the activation of TRPM8 by external stimuli including cold temperatures and cooling agents.Citation20,Citation21 Addition of exogenous PtdIns(4,5)P2 to cells with desensitized TRPM8 partially restores the response of TRPM8 to cold stimuli and cooling agents as well as directly activates the channel. Phosphoinositide (PI) 4-kinase is involved in PtdIns(4,5)P2 synthesis, and addition of PI 4-kinase inhibitors to whole cells reduced TRPM8 activity confirming the importance of PtdIns(4,5)P2 in its channel function.Citation20 Evidence indicates that PtdIns(4,5)P2 interacts with the positive carboxy terminus residues in TRPM8, and the affinity of PtdIns(4,5)P2 for TRPM8 is increased by cold or cooling agents. On the other hand, the influx of Ca2+ activates a Ca2+-sensitive phospholipase C that deplete PtdIns(4,5)P2, causing desensitization of TRPM8.Citation21 Taken together, a variety of environmental cues contribute to the regulation of TRPM8 channel activity, whereas PtdIns(4,5)P2 can act as a messenger regulating the function of TRPM8 in response to environmental stimuli, including cold temperature and alkaline pH.
Expression and cellular functions of TRPM8
In adult humans, TRPM8 is expressed in a tissue-selective pattern, and hormones play a regulatory role. The prostate gland expresses relatively high level of TRPM8 mRNA.Citation22 It is also expressed at a discernable level in the liver. In dorsal root ganglion (DRG) and trigeminal ganglion (TG) neurons, TRPM8 is expressed in the C- and Ad- fibers that sense cold temperature and pain.Citation12,Citation23 In prostatic carcinoma, expression of TRPM8 is upregulated in apical epithelial cells and androgen-dependent. Putative androgen response elements have been found upstream and downstream of the coding sequence of TRPM8 in prostate carcinoma cell lines. As the epithelia become de-differentiated and androgen-independent, TRPM8 expression decreases.Citation15,Citation24,Citation25 TRPM8 has been shown to localize in both plasma membrane and ER of prostate cancer cells.Citation15 Androgen appears to play a role in the localization and function of TPRM8 on the plasma membrane, whereas localization to the ER is androgen-independent.Citation26 In breast cancer cell lines, expression of TRPM8 also seems to be hormone-dependent, since estrogen upregulates TRPM8 levels in estrogen receptor-positive cells.Citation27
TRPM8 plays a role in cellular proliferation and survival as well as detecting and mediating thermosensation,Citation15,Citation23 yet how these cellular functions are inter-related and mediated remain to be determined. PtdIns(4,5)P2 is necessary for the activation of TRPM8 by cold temperature, and it may be important in regulating TRPM8-mediated proliferation.Citation20,Citation21 Geraniol is an intermediate in the biosynthesis of cholesterol; it enhances cell proliferation in prostatic epithelia, possibly by acting as an intracellular messenger that interacts with TRPM8.Citation15,Citation28 It has been demonstrated that TRPM8 mediates depletion of intracellular Ca2+ store in prostatic cancer cells.Citation16 Hypothetically, external stimuli that influence phosphatidylinositol metabolism and cholesterol homeostasis regulate the production of intra-cellular messengers such as PtdIns(4,5)P2 and geraniol, which in turn modulate the function of TRPM8 and intracellular calcium levels, and thus cellular proliferation and survival.
Role of TRPM8 in Pancreatic Cancer
Aberrant expression of TRPM8 in pancreatic adenocarcinoma
Until recently, the role of TRP ion channels in pancreatic adenocarcinoma had been under-investigated. Of the eight mammalian TRPM channels, only the expression of TRPM8 is consistently elevated in the seven human pancreatic adenocarcinoma cell lines being examined.Citation10 This is confirmed by analysis of TRPM8 immunoreactivity in surgically resected specimens of pancreatic adenocarcinoma (reference Citation10 and this study). The mechanism underlying the role of TRPM8 in cellular proliferation and survival of pancreatic epithelia and neoplasia is unclear, and it has become an area of active investigation in our laboratory.
In comparison with the minimal, if any, expression in normal pancreatic ducts,Citation10 TPRM8 is aberrantly expressed in pancreatic adenocarcinoma ( and ). The mechanism underlying the elevated expression remains to be determined. Hypothetically, amplification of the TRPM8 gene, or epigenetic mechanisms such as histone modifications or DNA hypomethylation of the TRPM8 promoter region, can lead to elevated expression of the TRPM8 gene. Androgen receptor is variably expressed in human pancreatic adenocarcinoma cell lines,Citation29 and it is possible that androgen may upregulate expression of TRPM8 in pancreatic adenocarcinoma in a similar manner as in prostatic carcinoma. We are currently examining a large number of malignant pancreatic tissues for expression of TRPM8 and correlating it with the clinicopathological features and molecular alterations. These studies are expected to help shed light on the clinical significance of TRPM8 in pancreatic cancer. Furthermore, we plan to test our hypothesis that TRPM8 is functionally expressed in pancreatic cancer stem cells or tumor-initiating cells using flow cytometry with stem cell-specific markers.
Proliferative role of TRPM8 in pancreatic adenocarcinoma
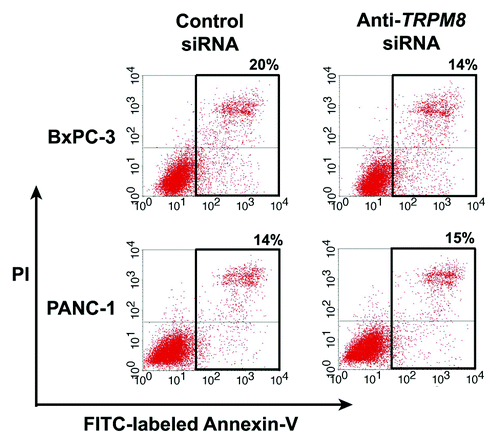
Expression of TRPM8 is essential for maintaining cellular proliferation in pancreatic adenocarcinoma. This is supported by our recent evidence that RNA interference-mediated silencing of TRPM8 in pancreatic adenocarcinoma cell lines impaired cellular proliferation and impeded cell cycle progression.Citation10 As examined by phase contrast microscopy and DAPI staining of nuclei, the TRPM8-deficient cells are large and flattened, and they contain multiple nuclei and cytoplasmic vacuoles.Citation10 These features are suggestive of non-apoptotic cell death through mitotic catastrophe and cellular senescence.Citation30 In agreement with these findings, flow cytometric analysis indicated that targeted knockdown of TRPM8 expression did not increase the proportion of apoptotic cells, as compared with the control siRNA-transfected cells ().
Figure 4. Anti-TRPM8 siRNA did not induce apoptotic cell death. The BxPC-3 and PANC-1 cells were transfected with anti-TRPM8 siRNA or non-targeting control siRNA by using Nucleofector® (Amaxa/Lonza) as described.Citation10 Following transfection, the cells were seeded at 2 x 105 cells / 3 ml in each well of a 6-well cell culture cluster (Corning Inc., costar) and incubated at 37°C for 72 h. The cells were then washed with phosphate buffered saline (PBS, pH 7.4), incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin V (Invitrogen) and propidium iodide (PI, Invitrogen), and analyzed for apoptosis by flow cytometry as described.Citation4 The proportion of cells undergoing early apoptosis (lower right quadrant) and late apoptosis (upper right quadrant) is indicated. The baseline level of apoptotic cells in the control siRNA-transfected group may be attributed to electrical pulsation during Nucleofection®, detachment of cells by trypsinization, and disaggregation of cells by filtration prior to flow cytometric analysis.
Next, we analyzed TRPM8-deficient pancreatic cancer cells for cellular senescence by determining the activity of senescence-associated β-galactosidase (SA β-gal) as a marker. Results of the SA β-gal assay indicate that RNA interference-mediated silencing of TRPM8 induced senescence in the pancreatic cancer cell lines BxPC-3 and PANC-1 (). These data suggest that TRPM8 is required to prevent non-apoptotic cell death that involves replicative senescence. In addition, molecular analysis revealed that the impaired cell cycle progression in the TRPM8-deficient cells is associated with increased mRNA levels of the cyclin-dependent kinase inhibitors, p21CDKN1A and p27CDKN1B.Citation10 On the basis of these data, we hypothesize that aberrantly upregulated expression of TRPM8 in pancreatic adenocarcinoma contributes to the uncontrolled proliferation of the cancer cells by facilitating cell cycle progression and by preventing non-apoptotic cell death through replicative senescence. We propose a working model that will form the basis for testing our hypotheses regarding the signaling mechanisms that mediate the proliferative and pro-survival effects of TRPM8 in pancreatic adenocarcinoma cells ().
Figure 5. RNA interference-mediated silencing of TRPM8 induced replicative senescence. The BxPC-3 and PANC-1 cells were transfected with either anti-TRPM8 siRNA or non-targeting control siRNA and then seeded at 4 x 104 cells / 2 ml medium in each well of a 12-well cell culture cluster (Corning Inc., costar). The transfected cells were incubated at 37°C for 72 h, and then analyzed for activity of SA β-gal using the Senescence Detection Kit (Biovision Medical Products) according to the manufacturer’s instructions. Images were acquired under an inverted light microscope with phase contrast (Nikon TE-300) and processed using Adobe Photoshop CS3 extended. Appearance of blue color indicates SA β-gal activity (arrows), which is detected in the anti-TRPM8 siRNA-transfected cells but not in those transfected with control siRNA.
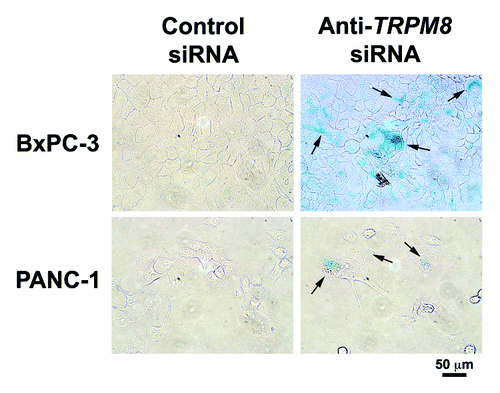
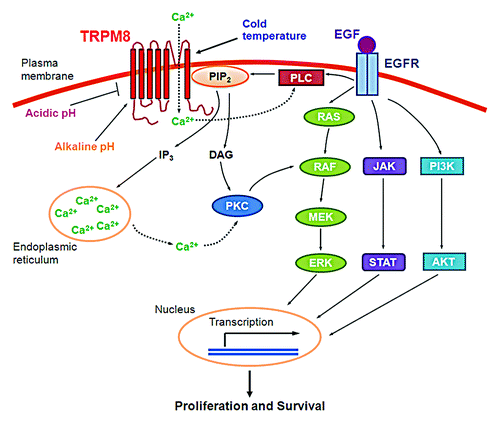
Figure 6. A working model for the proliferative and pro-survival roles of TRPM8 in pancreatic adenocarcinoma cells. Cold temperature, menthol, alkaline pH, or phosphatidylinositol-4,5-bis-phosphate (PtdIns(4,5)P2) activates the ion channel of TRPM8. Conversely, acidic pH inhibits the channel activity of TRPM8. Once activated, TRPM8 allows inflow of Ca2+ from the extracellular medium into the cytosol, leading to activation of Ca2+-sensitive phospholipase C (PLC) and hydrolysis of PtdIns(4,5)P2, thus providing negative feedback inhibition of the TRPM8 channel activity. Hydrolysis of PtdIns(4,5)P2 produces inositol 1,4,5-triphosphate (Ins(1,4,5)P3), which causes release of Ca2+ from intracellular stores and generates diacylglycerol (DAG). The increased Ca2+ or DAG activates protein kinase C (PKC). PKC in turn activates RAF in the RAS/ERK pathway,Citation34 leading to transcription of a variety of genes and resulting in cellular proliferation and survival.Citation35
Potential of TRPM8 as a diagnostic biomarker and a therapeutic target
Considering the functional expression of TRPM8 in pancreatic adenocarcinoma, we intend to explore the potential utility of TRPM8 as a biomarker for early detection and monitoring therapeutic response as well as a target for therapy. Assay for the expression level of TRPM8 along with other biomarkers in pancreatic tissues and patients’ blood with clinicopathological correlation may help establish TRPM8 as part of the molecular phenotype of pancreatic adenocarcinoma. The molecular signature thus established may be developed into a clinical tool for improving diagnosis and early detection of this disease, predicting and monitoring response to therapy, and determining prognosis.
The purported role of TRPM8 as a therapeutic target of pancreatic adenocarcinoma may be facilitated by the studies using small molecule inhibitors to probe the biophysical functions of TRPM8. For instance, SKF96365, 4-(3-chloro-pyridin-2-yl)-piperazine-1-carboxylic acid (4-tert-butyl-phenyl)-amide (BCTC), and 1,10-phenanthroline have been shown to be negative allosteric modulators of TRPM8 channel function.Citation33 Each acts to shift the voltage for activation of TRPM8 to more depolarized membrane potentials. The utility of these small molecules suggests the possibility of developing novel therapy through chemical modulation of TRPM8 channel activity. Moreover, targeted inhibition of TRPM8 expression and/or activity may enhance tumor sensitivity to the cytotoxic effects of chemotherapeutics or radiation therapy through induction of replication senescence. Thus, application of high-throughput screening of small molecule modulators of TRPM8 channel activity in combination with automated patch-clamp assays of ionic currents may help expedite discovery of anti-tumor therapies in pancreatic cancer. To prevent any effect on non-tumor target organs such as the prostate gland and sensory neurons, nanoparticles mediated delivery of the small molecule modulators of TRPM8 or siRNA directed against TRPM8 are expected to provide specific targeting of pancreatic cancer. Besides, TRPM8 may be utilized as a target for ligand-directed nanoparticles for pancreatic cancer-specific delivery of anti-tumor agents.
Conclusion and Prospective
Ion channels are functionally expressed in various types of cancer and they have the potential for development into clinical biomarkers and therapeutic targets. Pancreatic adenocarcinoma aberrantly expresses the TRPM8 ion channel, which is required for proliferation of the cancer cells by promoting cell cycle progression and by suppressing non-apoptotic cell death through replicative senescence. These data suggest that TRPM8 contributes to the uncontrolled growth of pancreatic neoplasia during the multi-step carcinogenesis, and provide a novel link between pancreatic cancer and cellular sensation. In ongoing studies, we aim to determine the mechanisms that mediate the proliferative and pro-survival role of TRPM8 by investigating TRPM8-mediated signal transduction in pancreatic adenocarcinoma cells and potentially in pancreatic cancer stem cells. By determining the expression levels of TRPM8 and other molecular markers in pancreatic adenocarcinoma and in patients’ blood, we aim to evaluate TRPM8 as a clinical biomarker for early detection, predicting and monitoring therapeutic response, and prognostic indication. Using small molecule modulators of TRPM8 along with ligand-directed nanoparticles, we will test and validate its role as a molecular target for development of cancer-specific therapy in this devastating disease.
| Abbreviations: | ||
| BCTC | = | 4-(3-chloro-pyridin-2-yl)-piperazine-1-carboxylic acid (4-tert-butyl-phenyl)-amide |
| CBD | = | common bile duct |
| DAG | = | diacylglycerol |
| DAPI | = | 4’6 diamidino-2-phenylindole |
| DRG | = | dorsal root ganglion |
| EGFR | = | epidermal growth factor receptor |
| ER | = | endoplasmic reticulum |
| IHC | = | immunohistochemistry |
| IP3 | = | inositol 1,4,5-triphosphate |
| PanIN | = | pancreatic intra-epithelial neoplasia |
| PCR | = | polymerase chain reaction |
| PI | = | phosphoinositide |
| PIP2 | = | phosphatidylinositol 4,5-bisphosphate |
| PKC | = | protein kinase C |
| PLC | = | phospholipase C |
| siRNA | = | small interfering RNA |
| TG | = | trigeminal ganglion |
| TRP | = | transient receptor potential |
| TRPM7 and TRPM8 | = | melastatin-subfamily member 7 and 8 of the TRP family |
Acknowledgments
N.S.Y. is supported by the Physician Scientist Stimulus Package from The Pennsylvania State University College of Medicine. R.D.B. is a recipient of the Medical Student Research Fellowship from The University of Iowa Carver College of Medicine. Funding support of this work was obtained from Penn State Hershey Cancer Institute (N.S.Y.) and Cancer Center Support Grant (P30 CA 086862 by National Cancer Institute to Holden Comprehensive Cancer Center at The University of Iowa (N.S.Y.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61:212 - 36; http://dx.doi.org/10.3322/caac.20121; PMID: 21685461
- Yee NS, Furth EE, Pack M. Clinicopathologic and molecular features of pancreatic adenocarcinoma associated with Peutz-Jeghers syndrome. Cancer Biol Ther 2003; 2:38 - 47; PMID: 12673116
- Yee NS, Pack M. Zebrafish as a model for pancreatic cancer research. Methods Mol Med 2005; 103:273 - 98; PMID: 15542913
- Chun SG, Zhou W, Yee NS. Combined targeting of histone deacetylases and hedgehog signaling enhances cytoxicity in pancreatic cancer. Cancer Biol Ther 2009; 8:1328 - 39; http://dx.doi.org/10.4161/cbt.8.14.8633; PMID: 19421011
- Yee NS. Zebrafish as a biological system for identifying and evaluating therapeutic targets and compounds. In: Han H, Grippo PJ, eds. Drug discovery in pancreatic cancer: models and techniques. New York:Springer, 2010:95-112.
- Chun SG, Yee NS. Werner syndrome as a hereditary risk factor for exocrine pancreatic cancer: potential role of WRN in pancreatic tumorigenesis and patient-tailored therapy. Cancer Biol Ther 2010; 10:430 - 7; http://dx.doi.org/10.4161/cbt.10.5.12763; PMID: 20657174
- Clapham DE. TRP channels as cellular sensors. Nature 2003; 426:517 - 24; http://dx.doi.org/10.1038/nature02196; PMID: 14654832
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 2007; 76:387 - 417; http://dx.doi.org/10.1146/annurev.biochem.75.103004.142819; PMID: 17579562
- Yee NS, Zhou W, Liang IC. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis Model Mech 2011; 4:240 - 54; http://dx.doi.org/10.1242/dmm.004564; PMID: 21183474
- Yee NS, Zhou W, Lee M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett 2010; 297:49 - 55; http://dx.doi.org/10.1016/j.canlet.2010.04.023; PMID: 20605675
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362:1605 - 17; http://dx.doi.org/10.1056/NEJMra0901557; PMID: 20427809
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108:705 - 15; http://dx.doi.org/10.1016/S0092-8674(02)00652-9; PMID: 11893340
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416:52 - 8; http://dx.doi.org/10.1038/nature719; PMID: 11882888
- Hui K, Guo Y, Feng ZP. Biophysical properties of menthol-activated cold receptor TRPM8 channels. Biochem Biophys Res Commun 2005; 333:374 - 82; http://dx.doi.org/10.1016/j.bbrc.2005.05.123; PMID: 15950184
- Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res 2004; 64:8365 - 73; http://dx.doi.org/10.1158/0008-5472.CAN-04-2146; PMID: 15548706
- Thebault S, Lemonnier L, Bidaux G, Flourakis M, Bavencoffe A, Gordienko D, et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J Biol Chem 2005; 280:39423 - 35; http://dx.doi.org/10.1074/jbc.M503544200; PMID: 16174775
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004; 430:748 - 54; http://dx.doi.org/10.1038/nature02732; PMID: 15306801
- Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 2004; 43:859 - 69; http://dx.doi.org/10.1016/j.neuron.2004.08.038; PMID: 15363396
- Andersson DA, Chase HW, Bevan S. TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J Neurosci 2004; 24:5364 - 9; http://dx.doi.org/10.1523/JNEUROSCI.0890-04.2004; PMID: 15190109
- Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci 2005; 25:1674 - 81; http://dx.doi.org/10.1523/JNEUROSCI.3632-04.2005; PMID: 15716403
- Rohács T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 2005; 8:626 - 34; http://dx.doi.org/10.1038/nn1451; PMID: 15852009
- Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 2006; 26:159 - 78; http://dx.doi.org/10.1080/10799890600637506; PMID: 16777713
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 2007; 27:14147 - 57; http://dx.doi.org/10.1523/JNEUROSCI.4578-07.2007; PMID: 18094254
- Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 2001; 61:3760 - 9; PMID: 11325849
- Bidaux G, Roudbaraki M, Merle C, Crépin A, Delcourt P, Slomianny C, et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr Relat Cancer 2005; 12:367 - 82; http://dx.doi.org/10.1677/erc.1.00969; PMID: 15947109
- Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest 2007; 117:1647 - 57; http://dx.doi.org/10.1172/JCI30168; PMID: 17510704
- Chodon D, Guilbert A, Dhennin-Duthille I, Gautier M, Telliez MS, Sevestre H, et al. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer 2010; 10:212; http://dx.doi.org/10.1186/1471-2407-10-212; PMID: 20482834
- Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function?. Endocr Relat Cancer 2006; 13:27 - 38; http://dx.doi.org/10.1677/erc.1.01093; PMID: 16601277
- Konduri S, Schwarz MA, Cafasso D, Schwarz RE. Androgen receptor blockade in experimental combination therapy of pancreatic cancer. J Surg Res 2007; 142:378 - 86; http://dx.doi.org/10.1016/j.jss.2006.09.034; PMID: 17559882
- Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 2004; 4:592 - 603; http://dx.doi.org/10.1038/nrc1412; PMID: 15286739
- Legrand G, Humez S, Slomianny C, Dewailly E, Vanden Abeele F, Mariot P, et al. Ca2+ pools and cell growth. Evidence for sarcoendoplasmic Ca2+-ATPases 2B involvement in human prostate cancer cell growth control. J Biol Chem 2001; 276:47608 - 14; http://dx.doi.org/10.1074/jbc.M107011200; PMID: 11606580
- Fraser SP, Pardo LA. Ion channels: functional expression and therapeutic potential in cancer. Colloquium on Ion Channels and Cancer. EMBO Rep 2008; 9:512 - 5; http://dx.doi.org/10.1038/embor.2008.75; PMID: 18451877
- Mälkiä A, Madrid R, Meseguer V, de la Peña E, Valero M, Belmonte C, et al. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol 2007; 581:155 - 74; http://dx.doi.org/10.1113/jphysiol.2006.123059; PMID: 17317754
- Marais R, Light Y, Mason C, Paterson H, Olson MF, Marshall CJ. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science 1998; 280:109 - 12; http://dx.doi.org/10.1126/science.280.5360.109; PMID: 9525855
- Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 2003; 17:1263 - 93; http://dx.doi.org/10.1038/sj.leu.2402945; PMID: 12835716