Abstract
Pancreatic cancer is one of the most malignant tumors with high mortality and poor prognosis even with the aggressive conventional therapies. Biotherapy based on the understanding of tumorigenesis mechanism is ongoing to improve the outcomes of cancer patients. We sought here to evaluate the therapeutic potential of a proapoptotic gene, PUMA, in pancreatic cancer. We found that PUMA was differently expressed in a series of pancreatic ductal adenocarcinoma cancer cell lines, and adenovirus-mediated expression of PUMA (Ad-PUMA) in these cells resulted in massive apoptosis. PUMA was more potent than p53 in suppressing growth of cancer cells. RT-PCR and Western Blot revealed that exogenous PUMA was expressed 6 h after Ad-PUMA infection. Furthermore, we assessed the efficacy of Ad-PUMA combining anticancer drugs (5-fluorouracil, cisplatin, gemcitabine hydrochloride, respectively) in these pancreatic cancer cell lines. Data revealed that PUMA significantly sensitized pancreatic carcinoma cell lines to chemotherapeutics, which may be resulted from abundant apoptosis induction. In nude mice with PANC-1 xenografts, Ad-PUMA treatment significantly inhibited the tumor growth. These results suggest that PUMA is a potent molecular tool in suppressing tumor growth sensitizing pancreatic carcinoma cells to chemical drugs. PUMA plays roles in negatively regulating cancer cell growth and may be a promising tool for cancer biotherapy, with or without combination with chemotherapeutic agents.
Introduction
Cancer is the most notorious disease that seriously threatens patients’ lives, which is now considered to be a genetic disease involving multiple factors, multiple steps and multiple genes. The causes of pancreatic cancer remain unclear. Several environmental factors have been implicated, a causative role of molecular imbalance may exist.Citation1 As for the multi-step carcinogenesis of pancreatic cancer, alterations in the K-ras proto-oncogene and the p16INK4a, p53, FHIT, and DPC4 tumor suppressor genes occur in a high percentage of this tumor.Citation2
Because of its insidious onset, aggressive behaviors and lack of effective early diagnostic methods, pancreatic cancer is usually not diagnosed until distal metastasis occurred. Conventional cancer treatments, including radiotherapy, chemotherapy and surgery, have made great progress, but they are still unable to remove the tumor from the root and prevent the metastasis and recurrence in many cases. The median survival time of patients after diagnosis of pancreatic cancer is generally less than 6 mo. The extremely poor prognosis of patients with pancreatic carcinoma calls for the development of novel therapeutic approaches.
Cancer gene therapy is one of the novel treatment approaches potentially improving the treatment of cancer disease. P53 gene therapy, which was carried with retroviral vector, was first attempted in patients with non-small cell lung carcinoma in 1996.Citation3 Later, adenoviral vector expressing human full coding sequence of wild-type p53 was widely evaluated for the treatment of cancers at advanced stages. As so far, Gendicine, a commercial name of Ad-p53 which has been approved for clinical cancer practice in China, and Advexin, the US counterpart of Ad-p53, have shown activity in a number of clinical trials.Citation4 Advanced head and neck cancers are the mostly evaluated indications for cancer gene therapy.Citation5-Citation7 To date, there are at least 12 HNSCC gene therapy clinical protocols that have been registered officially in the clinicaltrials.gov registry.Citation5 Some of these clinical trials produced remarkable tumor remission, with more data coming. It was also suggested that gene therapy combining conventional therapies may come up with extra benefits.Citation5 When it comes to pancreatic cancer, gene therapy also brings hope for the development of new treatment strategies.
Key molecules involved in apoptosis are often chosen as candidates in cancer gene therapy strategies, especially for the cancer types with defect apoptosis pathways.Citation8,Citation9 Some publications have reported encouraging results from the experimental evaluation of cancer therapy using pro-apoptotic molecules. BH3-only proteins, a subfamily of pro-apoptotic proteins belonging to Bcl-2 family, are essential initiators of apoptosis and are required for chemo-drugs induced apoptosis. The only functional domain known in these proteins is Bcl-2 homology domain 3 (Bcl-2 homology region 3, BH3). Without the BH3 domain, BH3-only proteins will lose their apoptosis-manipulating function.Citation10 They initiate the apoptosis cascade by inactivating the protective bcl-2 family members and activating the pro-apoptotic bcl-2 members.Citation11 The BH3-only proapoptotic subfamily of Bcl-2 family includes PUMA, Noxa, Bim, Bid and Blk, etc.Citation11
Among these BH3-only Bcl-2 proteins, PUMA (p53 upregulated modulator of apoptosis) was first reported by Yu in 2001, and plays an important role in inducing cancer cell apoptosis through p53-dependent and p53-independent pathways.Citation12 Exogenous PUMA expression in colon cancer cells caused extensive and rapid apoptosis. Subsequently, a number of in vitro and in vivo studies showed that Ad-PUMA can inhibit cell growth and promote apoptosis in a panel of cancer cells, including lung cancer,Citation13 malignant glioma cells,Citation14 esophageal cancer cellsCitation15 and choriocarcinoma cells,Citation16 etc. PUMA is more powerful than the classical tumor suppressor gene P53 in inducing apoptosis, guaranteeing its potential as an ideal effecter for cancer gene therapy. Here, we report the data on the experimental efficacy of Ad-PUMA on pancreatic carcinoma which is most notorious for its malignancy and poor outcome.
Results
Expression of Ad-PUMA in pancreatic cancer cell lines
Coxsackie virus and Adenovirus Receptor (CAR) on the cell surface is an important factor for the attachment of adenovirus to the cell membrane and is the primary receptor for adenovirus infection which is related to the adenoviral infection efficiency. We compared CAR expression levels among the four PDAC cell lines. In comparison, as shown in , all of the cell lines showed certain levels of CAR expression, guaranteeing the adoption of adenoviral vector to evaluate the gene therapy efficacy in pancreatic cancers. The highest level of CAR expression was observed in P3 cell line and intermediate level of CAR expression was seen in PANC-1 cell line. This facilitated the adenoviral vector-delivered gene therapy in this study.
Figure 1. Expression of Ad-PUMA in pancreatic cancer cell line. (A) Protein expression of CAR in four pancreatic cancer cell lines was analyzed by western blotting. (B) The four pancreatic cancer cell lines were infected with Ad-GFP at the indicated MOI for 24 h before they were checked using the inverted fluorescence microscope. (C) PUMA and p53 mRNA levels after Ad-PUMA infection in MIA PaCa-2 cells at different time points. (D) Protein expression of PUMA and p53 after Ad-PUMA infection in MIA PaCa-2 cells at different time points.
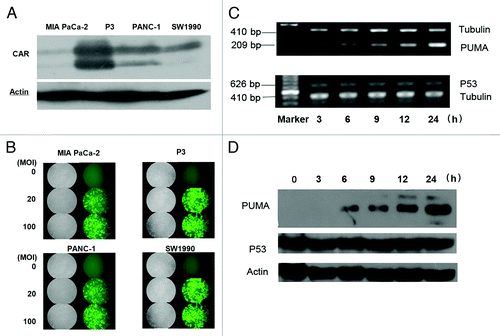
In order to analyze the ability of adenovirus to infect pancreatic cancer cells, we infected the four PDAC cell lines with Ad-GFP (0, 20, 100 MOI). Twenty-four hours after infection, the infection efficiency was observed under fluorescence microscope. As shown in , GFP was expressed in all the tested pancreatic cancer cell lines and almost 100% of the cells infected with 20 MOI or 100 MOI of Ad-GFP were GFP positive, which indicated that adenovirus vector was able to infect pancreatic cancer cells efficiently, and adenovirus vector-mediated gene expression could be used for studies on cell biology and animal experiment in pancreatic cancer cells. Infection of pancreatic cancer cell lines with Ad-PUMA dramatically increased the abundance of PUMA mRNA and protein in cell lines. As shown in , the level of PUMA increased significantly 6h after Ad-PUMA infection. The increase became even more impressive at 24 h. However, p53 expression was not altered during Ad-PUMA infection, supporting the fact that p53 is an up-stream regulator of PUMA.
Ad-PUMA induced the apoptosis in pancreatic cancer cells
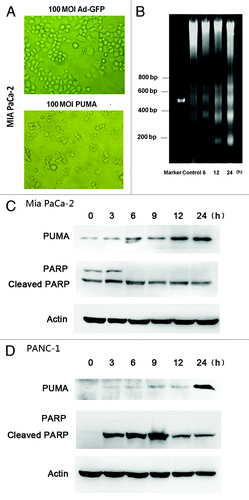
MIA PaCa-2 cells were infected with Ad-PUMA or Ad-GFP at 100 MOI for 12 h. As showed in , Ad-GFP has no significant effects on cell morphology and biological behaviors of MIA PaCa-2 cells, including the shape and adherence of the cells. On the contrary, Ad-PUMA infection caused prominent morphological change as well as many died or apoptotic cells floating in the medium. The Ad-PUMA infected-cells were characterized by shrinking cells losing normal shape when observed under microscope. Next, the apoptosis induced by Ad-PUMA was evaluated first by DNA ladder electrophoresis. Twenty MOI of Ad-PUMA infection for 6 h could cause obvious DNA fragmentation in MIA PaCa-2 cell (). At molecular level, as shown in , cleaved PARP gradually increased with time after Ad-PUMA's infection in MIA PaCa-2 cells and PANC-1 cells by western blotting assay. In conclusion, this result demonstrates that Ad-PUMA is able to induce the apoptosis in pancreatic cells rapidly.
Figure 2. Ad-PUMA induced apoptosis in pancreatic cancer cell lines. (A) Morphological changes of MIA PaCa-2 cells infected with 100 MOI of Ad-GFP or Ad-PUMA for 12 h. (B) DNA fragmentation was detected in MIA PaCa-2 cells treated with Ad-PUMA at 20 MOI for designed time. (C) Activation of PARP was induced in MIA PaCa-2 cells infected with 10 MOI Ad-PUMA. (D) PARP was activated in PANC-1 cells infected with Ad-PUMA at 50 MOI.
Ad-PUMA exerted more powerful growth inhibition than Ad-P53 in MIA Paca-2 and PANC-1 cell lines
To investigate the effect of Ad-PUMA and Ad-p53 on the growth inhibition of pancreatic cancer cells, Ad-PUMA, Ad-p53 and Ad-GFP at the indicated MOI were used to infect MIA PaCa-2 and PANC-1 cell lines. Infection of Ad-PUMA and Ad-p53 vectors resulted in a dose-dependent decrease in cell viability. By contrast, infection of Ad-GFP had only a marginal effect on cell viability even at the highest MOI used in the study. Moreover, comparing with Ad-p53, Ad-PUMA is more powerful in suppressing pancreatic cancer cell growth (), reducing the IC50 low to about one sixth of its original value (as shown in ). To verify the efficient transfer of Ad-p53, the expression of p53 and its relevant targets was detected in two cell lines infected with Ad-p53 .
Figure 3. Ad-PUMA inhibited the growth of MIA PaCa-2 and PANC-1. Adenoviral vectors (Ad-PUMA, Ad-p53, Ad-GFP) infected MIA PaCa-2 (A) and PANC-1 (B) cells at various MOI (5, 10, 20, 50, 100, 400) for 72 h and then cell viability was assayed by CCK-8 assay. (C) The expression of P53 and its relevant targets was detected in MIA PaCa-2 and PANC-1 cells treated with Ad-p53 for 48 h. *indicating significant p value for Ad-PUMA vs. Ad-P53 (p < 0.05). #Significant p value for Ad-PUMA vs. Ad-GFP (p < 0.05). *Significant p value for Ad-P53 vs. Ad-GFP (p < 0.05).
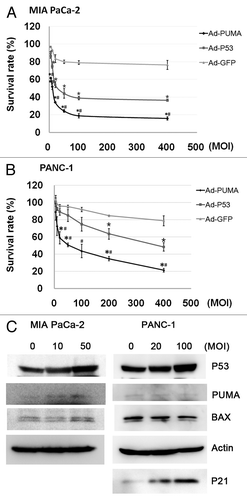
Table 1. IC50 of Ad-PUMA and Ad-p53 in pancreatic cancer cells
Ad-PUMA sensitized pancreatic carcinoma cells to chemotherapeutic agents
To evaluate whether Ad-PUMA is able to enhance the cytotoxicity of chemotherapeutic agents to cancer cells, we investigated the re-sensitization of cancer cells by Ad-PUMA to chemotherapeutics via cell viability assay. Cell proliferation was inhibited more significantly in both cell lines when treated with anticancer drugs (cDDP, 5-FU, Gemcitabine) combined with Ad-PUMA, compared with when combined with Ad-GFP. Significantly, exogenous expression of PUMA decreased the IC50 of the pancreatic carcinoma cell to chemotherapeutic agents by 4.9- to 64.1-fold, compared with that when chemotherapeutic agent was used alone (). The Ad-PUMA titers used in the combination treatment was 2 and 10 MOI, respectively, in MIA PaCa-2 and PANC-1 cells, which was considered not enough to change the cell viability.
Table 2. Ad-PUMA sensitized MIA PaCa-2 and PANC1 to anticancer drugs
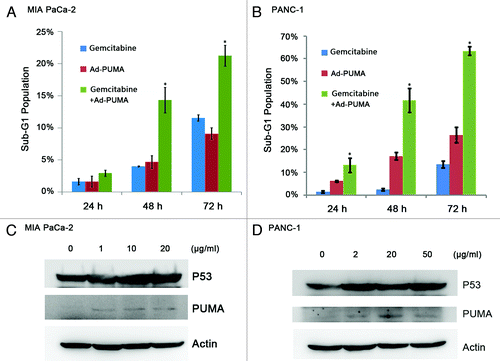
Compared with Gemcitabine or Ad-PUMA alone, combinational treatment using both for 24, 48 and 72 h significantly increased the percentage of cancers in Sub-G1 phase (p < 0.05). As shown in , Gemcitabine-combined Ad-PUMA treatment significantly increased the proportion of apoptotic cells in MIA PaCa-2 and PANC-1 cells (3.0, 14.4 and 21.3% vs. 13.2, 41.7 and 63.5%), compared with those when Gemcitabine (1.7, 4.0 and 11.6% vs, 1.5, 2.5 and 13.6%) or Ad-PUMA was used alone (1.7, 4.7 and 9.1% vs. 6.2, 17.2 and 26.5%). Gemcitabine could induce expression of P53 and PUMA in both MIA PaCa-2 and PANC-1 cells (shown in ).
Figure 4. Ad-PUMA further enhanced the Gemcitabine-induced apoptosis. (A) MIA PaCa-2 cells were treated with 1 μg/ml Gemcitabine alone or in combination with 5 MOI Ad-PUMA for the indicated time and then subjected to FACS analysis. (B) PANC-1 cells were treated with 2 μg/ml Gemcitabine alone or in combination with 10 MOI Ad-PUMA for the indicated time and then subjected to FACS analysis. PUMA and p53 expression was detected in MIA PaCa-2 (C) and PANC-1 (D) cells following 48 h Gemcitabine treatment.
Ad-PUMA slowed down the growth of tumors xenografts in nude mice
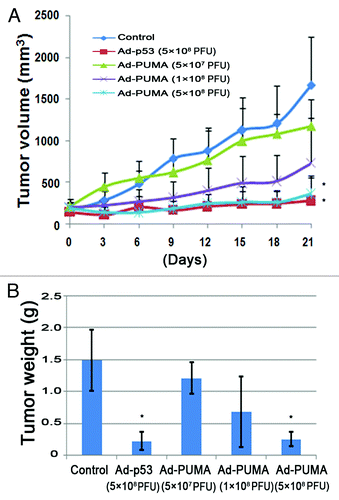
To investigate the effects of Ad-PUMA on tumor growth in vivo, we established nude mouse model bearing PANC-1 tumor. The mice lived in a normal healthy condition during Ad-PUMA treatment by introtumoral injection. As showed in , Ad-PUMA produced a dose-dependent growth inhibition on PANC-1 tumor model. In various Ad-PUMA-treated groups, the tumor volumes were smaller than that of control group. In the group treated by Ad-PUMA at high dose (5 × 108 PFU), Ad-PUMA produced almost the equal growth inhibition to that of Ad-p53. Compared with the control, even five times reduction in the titer of the Ad-PUMA can still be effective to suppress tumor growth (1 × 108 PFU).
Figure 5. Ad-PUMA suppressed tumor growth in vivo. (A) The growth curve of PANC-1 tumors (n = 10 per group) subjected to Ad-PUMA and Ad-p53 treatments as described in Materials and Methods. Treatments were administered on days 0, 3, 6, 9, 12 and 15, respectively. Note: * = the differences between treat groups and control group were significant (p < 0.05). (B) Tumors from different treatment groups were weighted at the end of the experiment. Note: * = the differences between treat groups and control group were significant (p < 0.05)
Discussion
In this study, we evaluated the potential of adenoviral vector carrying PUMA as a tool for the gene therapy against pancreatic cancer. We found that Ad-PUMA was a potent cytotoxic molecule and held promise for pancreatic cancer gene therapy in combination with chemotherapeutic agents or alone.
Morbidity and mortality of pancreatic cancer are listed within the top 10 among all the cancer types with even increasing incidence worldwide. The 5 year survival rate of this disease is only 0.4–4.0%.Citation17 Though early surgical resection of pancreatic cancer gives the only chance to patients to survive, a majority of the patients, if not all, were at their advanced disease stages when they were first diagnosed. For patients with pancreatic cancer not feasible to surgery treatment, gemcitabine-based chemotherapy and radiotherapy are the main techniques. However, optimistic outcome could never be expected with these patients, since adjuvant chemotherapy leads a large proportion of patients (> 20%) to drug resistance when they almost cannot effectively response to any treatments.Citation18,Citation19 Acquired resistance of pancreatic cancer cells toward 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes.Citation20 There is urgent demand to develop novel therapy strategies to re-establish or enhance the functional apoptosis pathways to improve the efficacy in pancreatic cancer treatment.
Biotherapy has been developed as a novel adjuvant therapeutic strategy for cancer treatment. Concerning deregulation of apoptosis is one of the main mechanisms in carcinogenesis and subsequent chemoresistant property, apoptosis promotion is the focus of anti-cancer drug development. This strategy is also applied to pancreatic cancer. PUMA was cloned in 2001, and soon demonstrated as one of the most powerful apoptosis-promoting molecules. PUMA, a member of the BH3-only proteins, was identified as a p53 down-stream gene and plays a critical role in p53-dependent apoptosis. Though PUMA locates downstream to P53, it has higher activity than p53 in promoting apoptosis. The protein encoded by PUMA gene was exclusively localized to mitochondria where it interacted with Bcl-2 and Bcl-XL through its BH3 domain.Citation12 As the sensors to discrete apoptotic stimuli, BH3-only proteins interact with the multidomain Bcl-2 family proteins to either antagonize or activate their functions.21As a result, a cascade of downstream events is triggered, including collapse of mitochondrial membrane potential, release of the apoptogenic mitochondrial proteins cytochrome c and SMAC, and activation of caspase cascade. On the other hand, PUMA is closer to the terminal apoptosis effecters than p53; therefore it takes effects more rapidly than p53. In this study, we demonstrated that PUMA is more potent than p53 in growth suppression and chemosensitization in pancreatic carcinoma cells. PUMA-mediated growth suppression and apoptosis may be accomplished by cleaving PARP. Collectively, our results suggest that PUMA may play negative roles in cancer cell growth and may be a promising molecule to be used for the cancer gene therapy in combination with chemotherapeutic agents. We found that 6 h after infection the expression of PUMA has significantly increased and continued to goes up, still staying at a high level 24 h after adenovirus infection, demonstrating that adenovirus-mediated PUMA expression was quick and efficient.
Though less effective than in other cancer types, chemotherapy is routinely adopted in pancreatic cancer treatment. The limiting necks in the chemotherapy are aberrant apoptosis pathway-caused insensitivity of pancreatic cancer to chemotherapeutics and the systematic toxicity from high doses of chemical drugs. Here we found that Ad-PUMA promotes pancreatic cancer cells growth inhibition, as well as sensitize them to chemical drugs. By sensitizing pancreatic cancer cells to the chemotherapeutics, Ad-PUMA produces higher efficacy at the same dose of chemotherapeutic drugs or reduces the dosage of chemotherapeutic drugs without impairing their efficacy. We verified that Ad-PUMA inhibited the growth of MIA PaCa-2 and PANC-1 and the inhibitory effect became more potent with the increase of virus titers. Ad-PUMA exerted its effects no matter whether the cells are drug-resistant or not, suggesting that both cell lines were sensitive to Ad-PUMA. The cell proliferation was inhibited more significantly in both cell lines when treated with Ad-PUMA combined with anticancer drugs (cDDP, 5-Fu, Gemcitabine) than when treated with anticancer drugs alone. More significantly, exogenous expression of PUMA decreased the IC50 of the pancreatic carcinoma cell to chemotherapeutic agents by 4.9- to 64.1-fold compared with that when chemotherapeutic agents were used alone. So far, this is the first report that adenovirus-mediated PUMA transfer is able to sensitize the pancreatic cancer cells to chemotherapeutics.
Besides pancreatic cancer, other cancer types also have the possibility to benefit from Ad-PUMA treatment. For example, analysis of tissue biopsies from breast cancer patients showed that PUMA mRNA was induced within 6 h after chemotherapy.Citation22 PUMA was induced by dexamethasone in leukemia cells isolated from patients who were sensitive to the treatment, but not from those who were resistant.Citation23 Wang et al. showed that Ad-PUMA inhibited growth of KYSE150 esophageal cancer cells more powerful and faster than Ad-p53.Citation16 Sun et al. also showed that Ad-PUMA inhibited cancer cell growth and induced apoptosis in head and neck squamous cell carcinoma cell line 1483 and PCI-15B, taking advantages over Ad-p53.Citation24 In a series of cell culture and xenograft studies, elevated PUMA expression, either alone or in combination with chemotherapy or irradiation, induced profound toxicity to cancer cells. A variety of cancer cells have been analyzed, including those from the lung,Citation13 head and neck,Citation24 esophagus,Citation15 drug-resistant choriocarcinoma,Citation16 melanoma,Citation25 malignant gliomagastric glands,Citation26 breastCitation27 and prostate.Citation28 A recent study also showed that PUMA, by activating mitochondria-mediated apoptosis pathway, can induce the cell death, which also provides a new way for the treatment of epithelial carcinoma. Therefore, PUMA exogenous transfer may be a promising cancer treatment strategy.Citation29
In the current study, Ad-PUMA induced massive apoptosis and growth inhibition in pancreatic cancer cells. PUMA is a p53-downstream pro-apoptotic effecter in the apoptosis pathway. Therefore, its pro-apoptotic effects are independent of p53 status, which endows PUMA an advantage over p53 in killing cancer cells with dysfunctional p53 pathway. Among the four cell lines used in this study, MIAPaCa-2, PANC-1 and SW1990 were known as p53 mutant cells. It was reported that p53 mutations were common in pancreatic cancers.Citation30,Citation31 Hence, pancreatic cancers are proper indications for Ad-PUMA treatment. In vivo experiments also confirmed the synergistic effects of Ad-PUMA and chemical drugs on pancreatic cancer cell model. p53 has been known as a classical and most potent tumor suppressor. It is the mostly evaluated molecule in the cancer gene therapy protocols. In China, it has been developed as a therapeutic agent used in clinical cancer practice. In this study, we set up Ad-p53 as a treatment control and data showed that Ad-PUMA exerted the same cancer growth inhibition effect as Ad-p53 did, confirming an effective and promising feature of PUMA as an anticancer molecule. This provides novel potential strategy for the treatment of the most malignant cancer type. We prospect that Ad-PUMA is worthy of further development and application in the field of pancreatic cancer gene therapy. The next step should be to evaluate the Ad-PUMA efficacy through the venous system administration and the improvement of PUMA treatment by the oncolytic adenoviral vectors.
The development of pancreatic cancer is a complex process and it is still one of the most malignant cancer types till now. Here we first report that adenoviral vector-mediated PUMA delivery kills pancreatic cancer cells by itself and sensitizes pancreatic cancer cells to chemotherapeutics. With the joint strategy to treat cancers is a new trend up to date in clinics, we may adopt a variety of ways to regulate endogenous expression of PUMA or, through the role of multiple targets, more effective regulation of exogenous PUMA expression. This gives us the hint to flexibly use PUMA as a target of gene therapy to fight against cancer.Citation32 It is reasonable to expect a considerable progress in cancer treatment in the future with the development of combined regimens including PUMA manipulating techniques.
Materials and Methods
Cell lines and cell culture
Four human PDAC (pancreatic ductal adenocarcinoma cancer) cell lines (MIA PaCa-2, PANC-1, P3, SW1990) and HEK293 cells were from State Key Laboratory of Molecular Oncology, Chinese Academy of Medicine Science. Pancreatic carcinoma cells were cultured in RPMI 1640 (GIBCO, Invitrogen) medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin in humidified air at 37°C with 5% CO2. HEK293 cells were maintained in DMEM (GIBCO, Invitrogen) with 10% FBS.
Recombinant adenoviruses
The recombinant adenovirus Ad-PUMA was a gift from Dr Jian Yu (University of Pittsburgh), which was propagated in HEK293 cells and purified by CsCl2 gradient centrifugation.Citation33 Ad-GFP was purchased from Sinogenomax, Chinese National Human Genome Center (Beijing, China). Ad-p53 was obtained from Sibiono GeneTech Co., Ltd. Viral titers were determined by serial 2-fold dilution infection method.
Infection and efficacy of Ad-PUMA on pancreatic carcinoma cells
MIA PaCa-2 and PANC-1 were plated at 5 × 103 cells/well in a 96-well plate. The next day, the growth medium was removed and replaced with 100 µl virus-containing medium [diluted in growth medium without serum at the desired multiplicity of infection (MOI)]. Plates were tapped to spread the virus evenly and placed back to the incubator at 37°C. Ninety minutes later, 100 µl fresh medium completed with serum was added to each well. The effects of viral infection on cell viability and proliferation were analyzed 72 h after infection.
RT-PCR
Total RNA was isolated from MIA PaCa-2 cells with TRIzol reagent (Life technologies) according to the manufacturer's instructions. Sequences of the RT-PCR primers for PUMA were: forward, 5′-GTCCTCAGCCCTCGCTCT-3; reverse, 5-CTGCTGCTCCTCTTGTCTCC-3. The product was 209 bp in length. Sequences of the RT-PCR primers for p53 were: forward, 5′-TACCAGGGCAGCTACGGTTT-3′; reverse, 5′-CCTTTCTTGCGGAGATTCTCT-3′. The product is 572 bp in length. Tubulin was amplified as internal control.
Western blotting
Whole-cell protein extracted from diversely treated cells were prepared and quantified by the BCA Protein Assay Kit. (Pierce). Proteins (80 µg/lane) were denatured, resolved on 12% SDS-PAGE gels and semi-dry transferred (Bio-Rad) at 12 V for 3 h onto nitrocellulose membranes (Bio-Rad). The membrane was incubated with primary antibodies, followed by incubation of secondary goat anti-rabbit or anti-mouse IgG conjugated by horseradish peroxidase and detection using SuperSignal ECL (Applygen Technologic, Inc.). To control protein loading, membranes were stripped and reprobed with anti-actin (1:5,000). The following antibodies were used in the experiments: CAR (sc-15405), p53 (sc-126), p21(zs-6246), Bax(zs-7480)_ and actin (sc-8432) were commercially achieved from Santa Cruz Biotechnology; PUMA (#4976) and PARP (#9542) was purchased from Cell Signaling Technology. HRP-labeled anti-mouse IgG secondary antibody (ZB-2305) and anti-rabbit IgG secondary antibody (ZB-2301) was from Beijing Zhongshan Golden Bridge Biotechnology.
CCK-8 assay
Cells were seeded into 96-well plates and exposed to the indicated concentrations of drugs or virus. After 72 h, the cells were incubated with CCK-8 (CK04–13, Dojindo Molecular Technologies) for 1 h. OD value was measured at 450 nm with reference wavelength of 630 nm.
FACS analysis
To assess the apoptosis, after various treatments, cells were collected (both attached and floating cells) and mixed with 70% ethanol at 4°C overnight. After two washes with PBS, they were incubated in RNase A/PBS (100 μg/ml) at 37°C for 30 min. Intracellular DNA was labeled with propidium iodide (PI 50 μg/ml) and analyzed by FACS. The percentages of sub-G1 in each population were determined from at least 1 × 104 cells.
Effects of PUMA expression in vivo
PANC-1 cells (1.0 × 107cells in 1 ml 0.9% NaCl) in 200 μl solution were inoculated subcutaneously into the right flank of mice. When the tumor diameter reached about 15–20 mm, the mice were killed. The tumors were cut into about 3–5 mm diameter tumor blocks, and were implanted subcutaneously into 50 nude mice. The nude mice were randomly divided into five groups (n = 10) for saline control, Ad-P53 (5 × 108PFU/ml), Ad-PUMA (5 × 107PFU/ml), Ad-PUMA (1 × 108PFU/ml), Ad-PUMA (5 × 108PFU/ml). Administration was given once every 3 d and was given five times in total.
Statistical analysis
Statistical analysis was performed using SPSS 11.0 Software (SPSS Inc.). P < 0.05 was considered statistically significant. Data are presented as the mean ± SD.
| Abbreviations: | ||
| PUMA | = | p53 up-regulated modulator of apoptosis |
| 5-FU | = | 5-fluorouracil |
| Ad | = | adenovirus |
| Bcl-2 | = | B cell lymphoma/leukemia-2 |
| CAR | = | Coxsackie and Adenovirus Receptor |
| Caspase | = | Cysteine aspartic acid specific protease |
| CCK-8 | = | Cell Counting Kit-8 |
| cDDP | = | Cisplatin |
| FBS | = | fetal bovine serum |
| GFP | = | green fluorescent protein |
| IC50 | = | inhibitory concentration of 50% |
| MOI | = | multiplicity of infection |
| PARP | = | poly ADP-ribose polymerase |
| PDAC | = | Pancreatic Ductal Adenocarcinoma Cancer |
| PFU | = | Plaque Formation Unit |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the the National High Technology Research and Development Program of China (2007AA021001), (2009CB521807), Major State Basic Research Development Program (973) (2009CB521807), National Natural Science Foundation of China (30572150, 30772527), and Beijing Natural Science Foundation (7062043).
Notes
† These authors contributed equally to this work.
References
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362:1605 - 17; http://dx.doi.org/10.1056/NEJMra0901557; PMID: 20427809
- Koliopanos A, Avgerinos C, Paraskeva C, Touloumis Z, Kelgiorgi D, Dervenis C. Molecular aspects of carcinogenesis in pancreatic cancer. Hepatobiliary Pancreat Dis Int 2008; 7:345 - 56; PMID: 18693168
- Roth JA, Nguyen D, Lawrence DD, Kemp BL, Carrasco CH, Ferson DZ, et al. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat Med 1996; 2:985 - 91; http://dx.doi.org/10.1038/nm0996-985; PMID: 8782455
- Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol 2010; 2:a001222; http://dx.doi.org/10.1101/cshperspect.a001222; PMID: 20463003
- Thomas SM, Grandis JR. The current state of head and neck cancer gene therapy. Hum Gene Ther 2009; 20:1565 - 75; http://dx.doi.org/10.1089/hum.2009.163; PMID: 19747066
- Vattemi E, Claudio PP. The feasibility of gene therapy in the treatment of head and neck cancer. Head Neck Oncol 2009; 1:3; http://dx.doi.org/10.1186/1758-3284-1-3; PMID: 19284676
- Abuzeid WM, Li D, O’Malley BW Jr.. Gene therapy for head and neck cancer. Adv Otorhinolaryngol 2011; 70:141 - 51; http://dx.doi.org/10.1159/000322490; PMID: 21358197
- Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui YX, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett 2010; 294:204 - 10; http://dx.doi.org/10.1016/j.canlet.2010.02.001; PMID: 20207475
- Chen K, Luo Z, Li Z, Liu Y, Zhao Q. PERP gene therapy attenuates lung cancer xenograft via inducing apoptosis and suppressing VEGF. Cancer Biol Ther 2011; 12:12; PMID: 22236877
- Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A 2001; 98:11318 - 23; http://dx.doi.org/10.1073/pnas.201208798; PMID: 11572983
- Bouillet P, Strasser A. BH3-only proteins - evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci 2002; 115:1567 - 74; PMID: 11950875
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001; 7:673 - 82; http://dx.doi.org/10.1016/S1097-2765(01)00213-1; PMID: 11463391
- Yu J, Yue W, Wu B, Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin Cancer Res 2006; 12:2928 - 36; http://dx.doi.org/10.1158/1078-0432.CCR-05-2429; PMID: 16675590
- Ito H, Kanzawa T, Miyoshi T, Hirohata S, Kyo S, Iwamaru A, et al. Therapeutic efficacy of PUMA for malignant glioma cells regardless of p53 status. Hum Gene Ther 2005; 16:685 - 98; http://dx.doi.org/10.1089/hum.2005.16.685; PMID: 15960600
- Wang H, Qian H, Yu J, Zhang X, Zhang L, Fu M, et al. Administration of PUMA adenovirus increases the sensitivity of esophageal cancer cells to anticancer drugs. Cancer Biol Ther 2006; 5:380 - 5; http://dx.doi.org/10.4161/cbt.5.4.2477; PMID: 16481741
- Chen Y, Qian H, Wang H, Zhang X, Fu M, Liang X, et al. Ad-PUMA sensitizes drug-resistant choriocarcinoma cells to chemotherapeutic agents. Gynecol Oncol 2007; 107:505 - 12; http://dx.doi.org/10.1016/j.ygyno.2007.08.007; PMID: 17884151
- Takhar AS, Palaniappan P, Dhingsa R, Lobo DN. Recent developments in diagnosis of pancreatic cancer. BMJ 2004; 329:668 - 73; http://dx.doi.org/10.1136/bmj.329.7467.668; PMID: 15374918
- Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol 2008; 26:3511 - 6; http://dx.doi.org/10.1200/JCO.2007.15.8782; PMID: 18640932
- Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008; 26:3503 - 10; http://dx.doi.org/10.1200/JCO.2007.15.8469; PMID: 18640931
- Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology 2002; 62:354 - 62; http://dx.doi.org/10.1159/000065068; PMID: 12138244
- Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ 2006; 13:1351 - 9; http://dx.doi.org/10.1038/sj.cdd.4401987; PMID: 16763616
- Middelburg R, de Haas RR, Dekker H, Kerkhoven RM, Pohlmann PR, Fuentes-Alburo A, et al. Induction of p53 up-regulated modulator of apoptosis messenger RNA by chemotherapeutic treatment of locally advanced breast cancer. Clin Cancer Res 2005; 11:1863 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-04-1372; PMID: 15756011
- Xu B, Wang BJ, Li AM, Lock R. [Effect of glucocorticoid on the expression of Puma in acute lymphoblastic leukemia]. Zhongguo Dang Dai Er Ke Za Zhi 2006; 8:151 - 4; PMID: 16613713
- Sun Q, Sakaida T, Yue W, Gollin SM, Yu J. Chemosensitization of head and neck cancer cells by PUMA. Mol Cancer Ther 2007; 6:3180 - 8; http://dx.doi.org/10.1158/1535-7163.MCT-07-0265; PMID: 18089712
- Karst AM, Dai DL, Cheng JQ, Li G. Role of p53 up-regulated modulator of apoptosis and phosphorylated Akt in melanoma cell growth, apoptosis, and patient survival. Cancer Res 2006; 66:9221 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-05-3633; PMID: 16982766
- Dvory-Sobol H, Sagiv E, Liberman E, Kazanov D, Arber N. Suppression of gastric cancer cell growth by targeting the beta-catenin/T-cell factor pathway. Cancer 2007; 109:188 - 97; http://dx.doi.org/10.1002/cncr.22416; PMID: 17149756
- Wang R, Wang X, Li B, Lin F, Dong K, Gao P, et al. Tumor-specific adenovirus-mediated PUMA gene transfer using the survivin promoter enhances radiosensitivity of breast cancer cells in vitro and in vivo. Breast Cancer Res Treat 2009; 117:45 - 54; http://dx.doi.org/10.1007/s10549-008-0163-6; PMID: 18791823
- Giladi N, Dvory-Sobol H, Sagiv E, Kazanov D, Liberman E, Arber N. Gene therapy approach in prostate cancer cells using an active Wnt signal. Biomed Pharmacother 2007; 61:527 - 30; http://dx.doi.org/10.1016/j.biopha.2007.08.010; PMID: 17904788
- Zhang C, Zhang J, Zhang A, Wang Y, Han L, You Y, et al. PUMA is a novel target of miR-221/222 in human epithelial cancers. Int J Oncol 2010; 37:1621 - 6; PMID: 21042732
- Casey G, Yamanaka Y, Friess H, Kobrin MS, Lopez ME, Buchler M, et al. p53 mutations are common in pancreatic cancer and are absent in chronic pancreatitis. Cancer Lett 1993; 69:151 - 60; http://dx.doi.org/10.1016/0304-3835(93)90168-9; PMID: 8513440
- Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res 1994; 54:3025 - 33; PMID: 8187092
- Zhang K, Chen D, Wang X, Zhang S, Wang J, Gao Y, et al. RNA Interference Targeting Slug Increases Cholangiocarcinoma Cell Sensitivity to Cisplatin via Upregulating PUMA. Int J Mol Sci 2011; 12:385 - 400; http://dx.doi.org/10.3390/ijms12010385; PMID: 21339993
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 1998; 95:2509 - 14; http://dx.doi.org/10.1073/pnas.95.5.2509; PMID: 9482916