Abstract
Invariant natural killer T (iNKT) cells are a distinct subset of human T cells, which expresses an invariant T cell receptor Vα24 Jα18 and recognizes glycolipid antigens in the context of CD1d molecules. iNKT cells exert pivotal regulatory roles in many immune responses, including antitumor immune responses. Alterations in iNKT cell frequency, phenotype, and activation state have been reported in cancer patients. No data are available on the iNKT cells in malignant pleural mesothelioma (MPM), a rare, but very aggressive, malignancy of the pleura with a very poor prognosis. Here, we studied the frequency, phenotype, and cytokine profile of circulating iNKT cells in MPM patients, and correlated results with tumor histological types (epithelioid, sarcomatoid, biphasic) and clinical stages (I, II, III). We found that the iNKT cell frequency was significantly increased in MPM patients with epithelioid and sarcomatoid types in comparison with healthy volunteers (HV); only three biphasic mesotheliomas were available in this study, thus no conclusions can be drawn for this MPM type. The increased frequency significantly correlates with the clinical stage of tumor with the highest value at the stage III in both epithelioid and sarcomatoid subtypes. According to the histological types, we measured changes in the frequencies of CD4+CD8+ (DP) and CD4-CD8- (DN), but not in the cytokine profiles (IFN-γ/IL-4 expression). These results demonstrate that the frequency of iNKT cells is increased in MPM patients and that this increase correlates with MPM type and stage.
Introduction
Malignant mesothelioma (MM) is a rare but very aggressive tumor that arises from mesothelial cells lining the pleural, peritoneal, and pericardial cavities. Malignant pleural mesothelioma (MPM) is the most common type, accounting for about 70% of all MM cases, of which approximately 80% can be attributed, albeit not exclusively, to asbestos fiber exposure.Citation1 MPM can be sub-typed into three forms according to the histological morphology: epithelioid, sarcomatoid and biphasic.Citation2 Despite the great improvement in diagnosis and treatment of this disease, MPM prognosis remains dramatically poor: the median survival time for the three types of tumor is 18, 8, and 11 mo respectively, leading the research in MPM management a current priority.Citation3-Citation5 In the last few years, immunomolecular studies showed the presence of several immunological alterations in asbestos-related diseases, such as MPM, even if the specific relationships between immune system and cancer are still unknown.Citation6,Citation7 Starting from the early studies of Leigh and Webster,Citation8 who demonstrated a close relationship between the presence of a lymphocyte infiltration into the tumor and a better prognosis, many evidences exist concerning pathological functional roles of different T cell populations [e. g., cytotoxic T lymphocytes (CTL), helper T lymphocytes (Th), T suppressor (Ts) cells, natural killers (NK) cells] in MPM.Citation9 The existence of an imbalance in the T-cell subsets, with a significant reduction in Th to Ts ratios, as well as the possibility that MPM patients can spontaneously mount a tumor-specific immune response, have been reported.Citation10-Citation13
Consequently, different immunotherapeutic approaches have been experimented in MPM patients, showing an antitumor activity of intra-pleural administration of cytokines, such as interleuchin (IL)-2, which was in clinical trials for MPM treatment.Citation14-Citation17 Several studies have also been performed with interferons (IFN) (α, β, γ), and, in particular, it has been demonstrated that patients treated with IFN- β have an increased survival, based on the dual action of interferon on both immune and tumor cells.Citation18 In addition, new and potentially effective treatments have been reported with either the active, where one or several antigens are used for triggering an immune response, or the passive immunotherapy, which relies on effector cells, isolated and activated in vitro before their re-injection in vivo.Citation19 The overall data suggest that immunotherapy could be highly promising also for MPM treatment.
A relatively recent discovery of a different T-cell subset, the invariant natural killer T cells (iNKT), with a pivotal regulatory function in many immune responses together with an immunoregulatory role in tumor responses, has opened new promising perspectives. iNKT cells are an unique autoreactive CD1d-restricted T-cell subpopulation, characterized by the surface expression of both an invariant T cell receptor (TCR) α chain, (Vα14-Jα18 in mice, and Vα24-Jα18 in humans) and receptors typical for NK cells, such as CD56 and/or CD161.Citation20-Citation23 iNKT cells recognize, through their semi-invariant TCR, glycolipid antigens, such as α-galactosylceramide (α-GalCer) and its analogs, presented by antigen presenting cells (APC) in the context of CD1d presenting molecules. Other CD1d-restricted NKT cells exist that use different TCR α- and β-chains and can recognize distinct lipid-based antigens presented by CD1d; these are often referred to as “diverse” or “type 2” NKT cells.Citation24 After TCR ligation, iNKT cells promptly produce a large amount of both Th1 (e.g., IFN-γ) and Th2 (e.g., IL-4) cytokines, and can bias adaptive immune responses toward Th1, Th2, Th17, or regulatory T (Treg) cell differentiation.Citation25 iNKT cells are also capable of extensive talks with a variety of other cell types, including dendritic cells (DC), NK cells, macrophages, neutrophils, conventional T and B cells, different subsets of Treg cells.Citation26
iNKT cells can be sub-divided into at least four different subsets: CD4+; CD8+; CD4-CD8- [double negative (DN)] and CD4+Cd8+ [double positive (DP)].Citation27-Citation29 Notably, CD4+ and DP iNKT cells produce both Th1 and Th2 cytokines, whereas the DN and CD8+ iNKT cell subsets primarily produce Th1 cytokines.Citation30-Citation32 Although iNKT cells constitute approximately only a 0.01–0.1% of the human peripheral blood T lymphocytes, these cells play important roles in a variety of immune responses, such as infection, autoimmunity, and cancer.Citation26 In the context of tumor immunity, iNKT cells have a primarily protective role, which has been demonstrated in transplantable, chemically-induced, and genetic tumor models.Citation33 In vitro, human iNKT cells can be expanded and skewed toward IFN-γ production by autologous DC pulsed with α-GalCer.Citation26 Expanded iNKT cells can induce either direct cytotoxicity against tumor cells in a CD1d-dependent manner or tumor lysis through the activation of NK cells.Citation34,Citation35 In vivo, a defect in iNKT cell number and function has been observed in progressive malignant multiple myeloma patients, in prostate cancer patients, and in patients with a number of solid tumors as well.Citation36-Citation38 The loss of iNKT cell function to produce IFN-γ appears to correlate with the clinical stage of tumors and may be, at least in part, reversible. Several clinical trials have been performed demonstrating that iNKT cell-based immunotherapy, with α-GalCer itself or autologous α-GalCer-loaded DC, is well tolerated and effective in mediating iNKT cell expansion and activation in cancer patients.Citation39,Citation40 This therapy led to prolonged disease stabilization or extended time to tumor progression in some patients.Citation41
No investigations examine the role of iNKT cells in MPM; here we, first, compared the frequency of iNKT cells, isolated from peripheral blood of MPM patients, with that from age- and gender-matched healthy volunteers (HV). Then, we evaluated whether a correlation exists between the tumor clinical stage and the frequency of iNKT cells. The cytokine profile was also analyzed by intracellular examining IFN-γ and IL-4 cytokine production.
Results
Characteristics of the study population
summarizes the baseline characteristics of the MPM patients and HV. The eligibility criteria for MPM patients were: histological diagnosis of pleural MM, assessed through thoracoscopy or thoracotomy, confirmed by an immunohistochemical profile; evaluable disease, surgically not resectable; symptomatic pleural effusion requiring thoracentesis. Patients were excluded in case of inadequate cardiopulmonary functions, active infections, and concomitant chemo/immunotherapies or for the presence of another neoplasm. On the basis of histological features, MPM were classified as epithelioid in 22 patients (73%), biphasic in 3 patients (10%) and sarcomatoid in 5 patients (17%).Citation45 Histological findings were reviewed internally in the “Ospedale S. Spirito” by a team of pathologists experienced in MPM immunohistochemistry. Patients were divided on the basis of the tumor clinical stage (I, II, III, and IV), according to the “US/Canadian Mesothelioma Panel.”Citation42 Of all MPM patients, 23 were men and 7 women; the median age was 62.5 y (range 41–80 y). All patients came from a geographic area with increased risk for asbestos pollution (Casale Monferrato, Italy). Occupational exposure to asbestos was documented in 8 patients, while indirect exposure was in 19 patients. For 3 patients no evidence of asbestos exposure was reported. At the moment of diagnosis, 9 patients were still smoking, 10 patients had never smoked, and 10 stopped smoking before diagnosis. In only one case smoking data were not available. There were not differences in average age and gender ratios between MPM patients and HV.
Table 1. Characteristics of MPM patients and healthy volunteers
Circulating iNKT cells in MPM patients
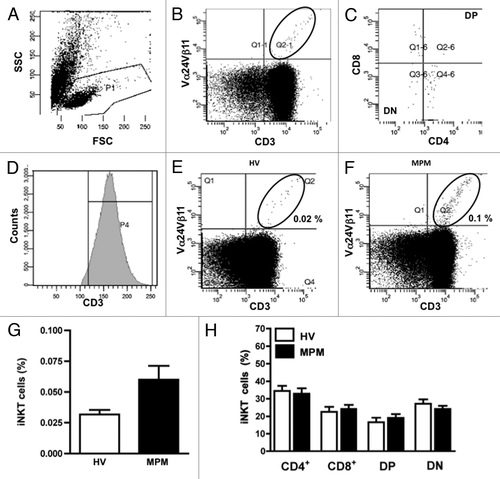
Circulating iNKT cells are a relatively rare population, ranging in frequency among total T cells from 0.01% to approximately 3%.Citation28 In our studies iNKT cells were identified as CD3+, Vα24+ and Vβ11+ cells among total T lymphocytes using two-color flow cytometric analysis. The flow cytometry gating strategy is shown in and C, while a representative flow cytometric dot plot, illustrating the expression of iNKT cells in HV and MPM patient, is shown in and F. A trend, but not statistically significant, toward an increased frequency of iNKT cells was observed in MPM patients in comparison to HV (0.02 vs.0.1%) (). The result was similar when the frequency of iNKT cells was expressed as absolute number of iNKT cells per ml blood rather than percentage of CD3+ cells (data not shown). Among total iNKT cells four iNKT cell subsets were identified on the basis of their CD4 and CD8 expression (). Analysis of the CD4/CD8 phenotype of iNKT cells revealed no differences in the percentage of cell populations in MPM patients in comparison to HV.
Figure 1. Flow cytometric analysis of iNKT cells and subsets from MPM patients and HV. PBMC were stained with anti-CD3 and anti-Vα24Vβ11 mAb. (A) Lymphocytes were gated on a side scatter (SSC)-forward scatter (FSC) dot plot. (B) In the lymphocyte gate, iNKT cells were identified as CD3+Vα24Vβ11+ double-positive cells. (C) Phenotypic analysis of iNKT cells was performed by CD4 and CD8 staining with anti-CD4 and anti-CD8 mAb. (D) A representative mean fluorescence intensity (MFI) histogram of CD3 expression on gate of lymphocytes. Representative flow cytometric dot plots of a HV (E) and a MPM patient (F); iNKT cell percentages are also shown in each panel. (G) The frequency of iNKT cells detected in the lymphocyte gate of MPM patients (n = 30; black bar) and HV (n = 22; unfilled bar). (H) Analysis of the frequency of iNKT cell subsets of MPM patients (black bars) and HV (unfilled bars). Results are expressed as mean ± SEM.
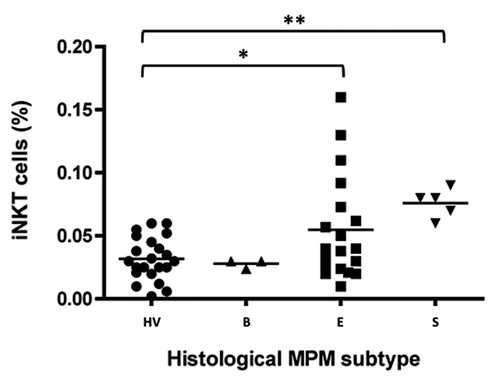
The frequencies of iNKT cells according to histological MPM subtypes
We, then, analyzed the frequency of iNKT cells in different groups of MPM patients, divided according to the histological tumor subtyping, and compared the results with those from HV.Citation45 A significant (p < 0.05) increase in the iNKT cell frequency was observed in epithelioid MPM subtype (+ 66 ± 9%), and this result reached a higher significance level (p < 0.01) in sarcomatoid (+78 ± 7%) MPM subtype. On the contrary, no difference was observed between biphasic MPM type and HV ().
Analysis of iNKT cell subsets in MPM patients
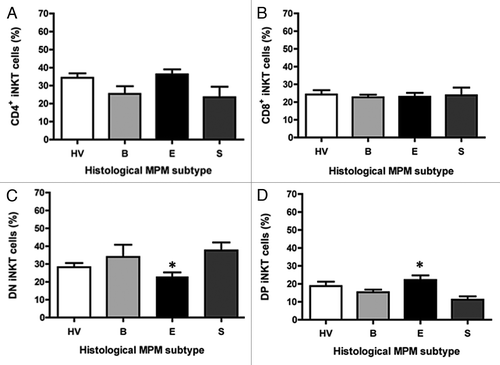
We, then, determined whether the difference in the iNKT cell frequency in MPM patients was correlated with similar alterations in the composition of iNKT cell subsets. For each MPM histological type the iNKT cell population was broken into subsets, using CD4 and CD8 as phenotypic markers. The frequency of CD4+ or CD8+ iNKT cells in all groups was equivalent, with no significant difference among the groups (). shows, on the contrary, a significant (p < 0.05) reduction (20 vs. 25%) in average percentage of iNKT cells expressing a CD4−CD8− phenotype (i.e., double negative or DN) in epithelioid patients compared with HV. The decrease in the proportion of DN iNKT cells in this group was associated with proportional statistically significant increases (28 vs. 18%) in CD4+CD8+ (i.e., double positive or DP) iNKT cells. No significant difference was observed when biphasic or sarcomatoid MPM were compared with HV. Presumably, the very low number of sarcomatoid and biphasic patients may account for the absence of relationships.
Figure 3. The frequency of iNKT cell subsets according to histological MPM subtypes. Analysis of the frequency of iNKT cell subsets was performed by CD4 and CD8 staining with anti-CD4 and anti-CD8 mAb. The values are expressed as percentage of iNKT cells expressing the molecules over the total iNKT cells. Data are the mean ± SEM; *p < 0.05.
Correlation between clinical stage and frequency/phenotype of iNKT cells
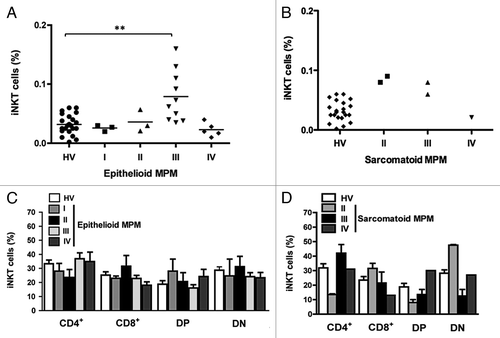
Patients were reclassified on the basis of the tumor clinical stage (I, II, III, and IV) using the “US/Canadian Mesothelioma Panel.”Citation42 shows the frequency of iNKT cells in epithelioid and sarcomatoid MPM patients at a certain clinical stage. A significant (p < 0.01) increase in the iNKT cell frequency was measured in epithelioid (+88 ± 13%) MPM at stage III. Conversely, patients with the same tumor type at stage IV of exhibit a lower, but not significant, frequency of total iNKT cells. It was not possible to perform any statistical analysis on data from sarcomatoid patients, because of the low number of cases with this tumor enrolled. No significant correlation between the clinical stage and the phenotype of iNKT cells was observed ().
Figure 4. The frequency of iNKT cells and iNKT cell subsets in epithelioid and sarcomatoid MPM patients belonging to a certain clinical stage. Scatter plot of the iNKT cell frequency in HV and patients with epithelioid (A) or sarcomatoid (B) MPM at different clinical stages. Horizontal bar is the median value for each group. **p ≤ 0.01. (C and D) Analysis of the frequencies of iNKT cell subsets was performed by CD4 and CD8 staining with anti-CD4 and anti-CD8 mAb. The values are expressed as percentage of iNKT cells expressing the molecules over the total iNKT cells. Data are the mean ± SEM.
Functional analysis of iNKT cells in MPM patients
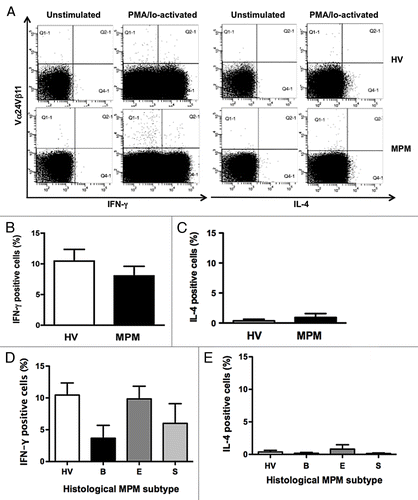
Since iNKT cells produce either Th1 (i.e., IFN-γ) or Th2 (i.e., IL-4) cytokines we compared the percentage of IFN-γ- and IL-4-secreting cells in the total iNKT cells from MPM patients and HV. shows representative dot plots displaying IFN-γ- or IL-4-positive iNKT cells. No difference was determined between the two subject groups () and this result was confirmed when data relative to each particular histological subtype were considered ().
Figure 5. Intracellular cytokine expressions in iNKT cells. After stimulation of PBMC from HV and MPM patients with PMA/Io for 4 h, the cells were stained with anti-Vα24Vβ11 mAb followed by intracellular IFN-γ or IL-4 staining. (A) Representative dot plots displaying iNKT cell gates of unstimualted and PMA/Io-activated samples from a HV and a MPM patient. Percentages of IFN-γ and IL-4 positive cells in MPM patients (B and C) and in different MPM subtypes (D and E) compared with HV. Data are the mean ± SEM.
Discussion
In our study, we found that the iNKT cell frequency differs in MPM patients in comparison to HV, according to the histological type of tumor. Particularly, patients with epithelioid MPM showed a significant increase in iNKT cells, while no conclusions can be drawn for biphasic (n = 3) and sarcomatoid (n = 5) mesotheliomas. In the same epithelioid patient group, we also observed that the increased iNKT cell frequency significantly correlates with the clinical stage of tumor, being higher at the later stages (III) than at the lower ones (I, II). To the best of our knowledge this is the first study showing a direct correlation between iNKT cell frequency and MPM type/stage.
A large bulk of literature shows that circulating iNKT cell number significantly decreases in patients with different cancers in comparison to HV (see Introduction). Reduced iNKT cell numbers seem to be independent from tumor type or tumor load, and iNKT cells seem to represent a risk factor for the development of tumors rather than a result of the tumor presence.Citation46 No data have been so far published on iNKT cells and MPM.
Our results need to be confirmed in a larger number of MPM patients; we cannot, indeed, rule out the possibility that differences in protocols for iNKT cell gating strategies are responsible for a iNKT cell overestimation and, consequently, for controversial results. In this regard, we have planned to expand the analysis on a large MPM patient number using, more specific reagents for iNKT cell determination (CD1d/α-GalCer tetramers) (experiments in progress).Citation47 However, present results have been always compared with those obtained in HV, in the same experimental conditions, and this should support the objectivity of our observations.
Data might suggest that in MPM the chronic stimuli for iNKT cell activation become more potent together with the increased aggressiveness of the tumor. In the stage III of both epithelioid and sarcomatoid MPM, which are characterized by tumor mass spreading to other organs on the same side, a higher frequency of circulating iNKT cells was, indeed, measured.Citation48 Surprisingly, similar results were not determined in the stage IV of the same tumor types. The number of patients with tumor stage IV in our series was limited, and results should be interpreted with caution. However, we cannot exclude the possibility that when the tumor mass spreads to lymph nodes or extends to other organs on the opposite side (stage IV) the proportion between circulating and tissue-infiltrating iNKT cells may change. More accurate and representative data will be obtained by examining the pleural biopsies from the same MPM patients and by comparing circulating and tissue-specific iNKT cell frequencies (experiments in progress). These findings will help to clarify whether changes in the peripheral iNKT cells are secondary to decrease/increase in the number of iNKT cells penetrating into tumor site.
The mechanism underlying the increased frequency of iNKT cells in MPM is not clear. We speculate that the peculiar characteristics of this tumor at the cellular/molecular level might be responsible for differences with other tumors, where decreased iNKT cells numbers were reported.Citation46 Several epidemiologic investigations demonstrated that exposure to several chemical/physical agents in the external environment greatly contribute to the development of neoplasia. Asbestos, which is categorized by the International Agency for Research on Cancer (IARC) as group I carcinogen, represents one of the most important environmental carcinogens, causing high malignant lung cancer or mesothelioma.Citation49 The peculiarity of asbestos is that every fiber in a mass is different in length, diameter, crystallinity, and contaminant metals, and has different carcinogenic power. Previous studies demonstrated that asbestos fibers longer than 8–20 µm and thinner than 0.25 µm induce MPM more readily.Citation50 The effect of these fibers on epithelial/mesothelial cells is unique, since they are not fully phagocytosed by phagocytic cells, but remain uncovered, so that they can stimulate the activation of other immune cells, such as DC and iNKT cells, and induce pulmonary localized chronic inflammation.Citation51
It is interesting to note that iNKT cells are also increased during Mycobacterium tuberculosis infection, another pathology characterized by pulmonary localized chronic inflammation. In the bronchoalveolar lavage of patients with tuberculosis the number of iNKT cells resulted higher than HV and their activation may specifically contribute to host defense against infection.Citation52,Citation53 During this infection, activated macrophages ingest microrganisms and generate phagolysosomes, which operate the destruction of the pathogen.Citation54 The failure of generation of phagolysosomes enables the mycobacteria to reside safely in vacuoles, where they survive and replicate, initiating local granulomatous inflammation. Granulomatous inflammation, that chronically persists, can in turn stimulate immune cell proliferation (e.g., iNKT cells), contributing to the prevention of the pathology widespread.
Conversely, in other lung diseases, such as sarcoidosis (a multisystem granulomatous disorder), where the granulomatous inflammation is not localized and cannot be properly managed by immune cells, iNKT cells were found absent or greatly reduced.Citation53
When other chemical carcinogens, such as those present in the cigarette smoke, are considered, the clearance from respiratory tract appears to be completely different compared with asbestos. First of all, the main size of smoke particles ranges from a nanometer to few micrometers, an overall magnitude lower than asbestos fibers; smoke particles are, therefore, capable of reaching small airways, where they can be completely ingested by phagocytes.Citation55,Citation56 Second, smoking also induces a chronic inflammatory process in the airways, but here the antigen expression and the metabolic activity of macrophages are high, leading to a more pronounced phagocytosis and a less active APC activity.Citation57 Consequently, smoke-induced immune response is completely different from that observed for asbestos or Mycobacterium tuberculosis infection, where a persistent APC activity exists and iNKT cells can be continuously activated. Data from Hogan et al. support this hypothesis.Citation58 These Authors, investigating the effects of cigarette smoke on the circulating iNKT cell number and function, measured a significant reduction in both iNKT cell frequency and cytokine secretion in smoking subjects. The analysis of data from smoking/non-smoking subjects, enrolled for this study, did not reveal any difference in the iNKT cell frequency (data not shown), confirming previously published data.Citation59
On the other hand, the increased frequency of iNKT cells did not functionally correlate with an increased release of Th 1(IFN-γ) or Th 2 (IL-4) cytokines, suggesting that the persistent stimulation of these cells by long asbestos fibers may lead to cell anergy.
iNKT cell anergy has several features in common with conventional T-cell anergy: anergic cells are hyporesponsive in terms of their capacity to proliferate, produce cytokines, transactivate other cell types, and to prevent tumor metastasis formation. α-GalCer administration to mice induces iNKT cell long-term anergy, which is reminiscent of the response of conventional T cells to strong stimuli, such as superantigens.Citation60 Asbestos may act on peripheral T cells as a superantigen, enhancing multiple, but not clonal, TCRVβ expression.Citation61,Citation62 Of note, various TCRVβ were overexpressed in MPM patients, suggesting that long-term and low dose exposure to asbestos can lead to iNKT cell resistance to apoptosis.Citation6 In particular, it has been observed that asbestos fibers induce a cascade of intracellular events, such as the activation of Src family kinases, the enhancement of IL-10 transcription and secretion, the auto/paracrine activation of IL-10 receptors, and the IL-10-induced signal transducers and activators of transcription protein (STAT)-3 and B-cell lymphoma (bcl)-2 activation, all leading to resistance to apoptosis. Consistently, CD4+ T lymphocytes from MM patients exhibited significant upregulation of bcl-2 expression; similar results could be obtained for iNKT cells, a population with a high frequency of CD4+ T lymphocytes.Citation6
In conclusion, this study demonstrates, for the first time, a significant increase in the frequency of circulating iNKT cells in MPM patients. This increase seems to be correlated with the type and severity of disease.
Material and Methods
Study populations
The study population was composed of 30 patients (mean age 62.5 ± 1.8; range 41 – 80 y; 23 males and 7 females) and 22 HV matched for sex and age (mean age 58.5 ± 1.9; range 44 – 76 y; 19 males and 3 females). Study population characteristics are summarized in . MPM was histological diagnosed by RECIST (response evaluation criteria in solid tumors) criteria and classified into four clinical stages (I, II, III, and IV), according to the “US/Canadian Mesothelioma Panel.”Citation42 Patients were excluded in case of active infection, recent or concomitant chemo-and/or immune-therapy, corticosteroid therapy, or for the presence of a previous or concomitant tumor. HV had no past or present history of malignant diseases, did not live in the same area of patients, and they were not exposed to asbestos. The protocol of this study was approved by the Research Ethic Committee of Human Experimentation at the“A.S.O.SS. Antonio e Biagio e C. Arrigo of Alessandria (Italy). All subjects gave their informed consent.
Antibodies
The following monoclonal antibodies (mAb) were used: phycoerythrin-labeled anti-human Vα24Vβ11 (Miltenyi Biotec), peridinin-chlorophyll protein-labeled anti-human CD3 (Becton Dickinson), fluorescein isothiocyanate-labeled anti-human CD4, phycoerythrin Cy7-labeled anti-human CD8, allophycocyanin anti-human IFN-γ and anti-human IL-4 (BioLegend).
Cell preparation and activation
Human peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation of peripheral heparinized whole blood from MPM patients and HV, and suspended in complete growth medium for immediate use.Citation43 PBMC were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1% non-essential amino acids, 1 mM sodium pyruvate, and 100 µg/ml kanamycin (EuroClone), hereafter referred to as “complete medium.” Viability of cells was determined by trypan blue exclusion test. Viability of all samples was always higher than 95%. PBMC were diluted with 1 ml of complete medium in flow cytometry (FACS) tubes and activated with 3 ng/ml phorbol 12-myristate 13-acetate (PMA), 1 mg/ml ionomycin and 5 U/ml IL-2 (Sigma-Aldrich). These cells were incubated for 4 h (optimal incubation time to observe simultaneous peak expressions of IFN-γ and IL-4) at 37°C and 5% CO2 in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich), a transport inhibitor that prevents cytokine release from cells.Citation44 Samples incubated with brefeldin A alone were considered as unstimulated controls.
Fluorescent labeling and flow-cytometric analysis
After activation PBMC were centrifugated at 1,300 rpm for 5 min at room temperature (RT), resuspended in 50 µl phosphate-buffered saline (PBS), and incubated with the respective mAb for T-cell surface antigen determination at RT in the dark for 10 min. Then, 200 µl fixation buffer (1% paraformaldehyde in PBS, pH 7.4) was added and incubated for 8 min to stabilize the mAb surface antigen complex. Cells were washed and resuspended in 500 µl permeabilizing solution (0.5% saponin) and incubated for 10 min at RT in the dark. Following, cytokine specific mAb were added to the cells for 30 min, at RT in the dark. After one final wash with 2 ml FACS buffer (PBS, 2% bovine serum albumin (BSA), 0.1% sodium azide), pellets were resuspended in 200 µl FACS buffer 1% paraformaldehyde and acquired using FACSVantage-SE® flow cytometry (Becton Dickinson). Data were analyzed by BD Facs Diva software (Becton-Dickinson Bioscences). A minimum of 50,000 viable lymphocytes were acquired for each sample (patients and HV). The lymphocytes were gated using forward/sideward scatter analysis and iNKT cell frequency evaluated with the specific Vα24Vβ11 mAb.
Reproducibility of data
To examine the stability of iNKT cells over time, two samples were taken from 20 HV. Reproducibility over time was determined by measuring the mean difference between repeated observations in an individual and the SE of these differences (Bland-Altman analysis). A subset of MPM patients (n = 10) also underwent repeated sampling. (Data not shown).
Statistical analysis
All statistical analysis was performed using Prism version 4.0 software (GraphPad). Data sets were analyzed using two ways ANOVA, and p values for comparisons between groups were determined using Student’s t-test for paired varieties. Differences were considered statistically significant when p ≤ 0.05.
| Abbreviations: | ||
| APC | = | antigen presenting cells |
| bcl | = | B-cell lymphoma |
| BSA | = | bovine serum albumin |
| CTL | = | cytotoxic T lymphocytes |
| DC | = | dendritic cells |
| DN | = | double negative |
| DP | = | double positive |
| FACS | = | flow cytometry |
| FBS | = | fetal bovine serum |
| HV | = | healthy volunteers |
| IFN | = | interferon |
| IL | = | interleukin |
| iNKT | = | invariant natural killer T-cells |
| mAb | = | monoclonal antibodies |
| MM | = | malignant mesothelioma |
| MPM | = | malignant pleural mesothelioma |
| NK | = | natural killers cells |
| PBMC | = | peripheral blood mononuclear cells |
| PBS | = | phosphate-buffered saline |
| PMA | = | phorbol 12-myristate 13-acetate |
| RT | = | room temperature |
| STAT | = | transcription protein |
| TCR | = | T cell receptor |
| Th | = | T helper lymphocytes |
| Treg | = | regulatory T cells |
| Ts | = | suppressor T cells |
| α-GalCer | = | α-galactosylceramide |
Acknowledgments
This work was supported by grants to G.L. from Piedmont County Grants for Research (prot. no. 36529, 2009, Italy).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Carbone M, Ly BH, Dodson RF, Pagano I, Morris PT, Dogan UA, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol 2012; 227:44 - 58; http://dx.doi.org/10.1002/jcp.22724; PMID: 21412769
- Bueno R, Reblando J, Glickman J, Jaklitsch MT, Lukanich JM, Sugarbaker DJ. Pleural biopsy: a reliable method for determining the diagnosis but not subtype in mesothelioma. Ann Thorac Surg 2004; 78:1774 - 6; http://dx.doi.org/10.1016/j.athoracsur.2004.05.007; PMID: 15511473
- Pistolesi M, Rusthoven J. Malignant pleural mesothelioma: update, current management, and newer therapeutic strategies. Chest 2004; 126:1318 - 29; http://dx.doi.org/10.1378/chest.126.4.1318; PMID: 15486399
- Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol 2008; 9:147 - 57; http://dx.doi.org/10.1007/s11864-008-0067-z; PMID: 18709470
- Ak G, Metintas S, Metintas M, Yildirim H, Erginel S, Kurt E, et al. Prognostic factors according to the treatment schedule in malignant pleural mesothelioma. J Thorac Oncol 2009; 4:1425 - 30; http://dx.doi.org/10.1097/JTO.0b013e3181ba2033; PMID: 19752758
- Miura Y, Nishimura Y, Maeda M, Murakami S, Hayashi H, Fukuoka K, et al. Immunological alterations found in mesothelioma patients and supporting experimental evidence. Environ Health Prev Med 2008; 13:55 - 9; http://dx.doi.org/10.1007/s12199-007-0012-y; PMID: 19568881
- Murakami S, Nishimura Y, Maeda M, Kumagai N, Hayashi H, Chen Y, et al. Cytokine alteration and speculated immunological pathophysiology in silicosis and asbestos-related diseases. Environ Health Prev Med 2009; 14:216 - 22; http://dx.doi.org/10.1007/s12199-008-0063-8; PMID: 19568841
- Leigh RA, Webster I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S Afr Med J 1982; 61:1007 - 9; PMID: 7089768
- Lew F, Tsang P, Holland JF, Warner N, Selikoff IJ, Bekesi JG. High frequency of immune dysfunctions in asbestos workers and in patients with malignant mesothelioma. J Clin Immunol 1986; 6:225 - 33; http://dx.doi.org/10.1007/BF00918702; PMID: 2424930
- Tsang PH, Chu FN, Fischbein A, Bekesi JG. Impairments in functional subsets of T-suppressor (CD8) lymphocytes, monocytes, and natural killer cells among asbestos-exposed workers. Clin Immunol Immunopathol 1988; 47:323 - 32; http://dx.doi.org/10.1016/S0090-1229(88)80009-6; PMID: 2967138
- Mutti L, Valle MT, Balbi B, Orengo AM, Lazzaro A, Alciato P, et al. Primary human mesothelioma cells express class II MHC, ICAM-1 and B7-2 and can present recall antigens to autologous blood lymphocytes. Int J Cancer 1998; 78:740 - 9; http://dx.doi.org/10.1002/(SICI)1097-0215(19981209)78:6<740::AID-IJC12>3.0.CO;2-5; PMID: 9833768
- Robinson C, Robinson BW, Lake RA. Sera from patients with malignant mesothelioma can contain autoantibodies. Lung Cancer 1998; 20:175 - 84; http://dx.doi.org/10.1016/S0169-5002(98)00014-2; PMID: 9733052
- Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res 2005; 11:3814 - 20; http://dx.doi.org/10.1158/1078-0432.CCR-04-2304; PMID: 15897581
- Castagneto B, Zai S, Mutti L, Lazzaro A, Ridolfi R, Piccolini E, et al. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: Results of a phase II study on 31 consecutive patients. Lung Cancer 2001; 31:303 - 10; http://dx.doi.org/10.1016/S0169-5002(00)00192-6; PMID: 11165411
- Powell A, Creaney J, Broomfield S, Van Bruggen I, Robinson B. Recombinant GM-CSF plus autologous tumor cells as a vaccine for patients with mesothelioma. Lung Cancer 2006; 52:189 - 97; http://dx.doi.org/10.1016/j.lungcan.2006.01.007; PMID: 16563560
- Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer 2008; 44:46 - 53; http://dx.doi.org/10.1016/j.ejca.2007.08.028; PMID: 17945478
- Alì G, Boldrini L, Lucchi M, Picchi A, Dell’Omodarme M, Prati MC, et al. Treatment with interleukin-2 in malignant pleural mesothelioma: immunological and angiogenetic assessment and prognostic impact. Br J Cancer 2009; 101:1869 - 75; http://dx.doi.org/10.1038/sj.bjc.6605438; PMID: 19935800
- Monnet I, Breau JL, Moro D, Lena H, Eymard JC, Ménard O, et al. Intrapleural infusion of activated macrophages and gamma-interferon in malignant pleural mesothelioma: a phase II study. Chest 2002; 121:1921 - 7; http://dx.doi.org/10.1378/chest.121.6.1921; PMID: 12065358
- Grégoire M. What’s the place of immunotherapy in malignant mesothelioma treatments?. Cell Adh Migr 2010; 4:153 - 61; http://dx.doi.org/10.4161/cam.4.1.11361; PMID: 20179421
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A 1998; 95:5690 - 3; http://dx.doi.org/10.1073/pnas.95.10.5690; PMID: 9576945
- Molling JW, Moreno M, de Groot J, van der Vliet HJ, von Blomberg BM, van den Eertwegh AJ, et al. Chronically stimulated mouse invariant NKT cell lines have a preserved capacity to enhance protection against experimental tumor metastases. Immunol Lett 2008; 118:36 - 43; http://dx.doi.org/10.1016/j.imlet.2008.02.007; PMID: 18405982
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 2005; 23:877 - 900; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115742; PMID: 15771592
- Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol 1999; 60:20 - 31; http://dx.doi.org/10.1016/S0198-8859(98)00098-6; PMID: 9952024
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name?. Nat Rev Immunol 2004; 4:231 - 7; http://dx.doi.org/10.1038/nri1309; PMID: 15039760
- Taniguchi M, Tashiro T, Dashtsoodol N, Hongo N, Watarai H. The specialized iNKT cell system recognizes glycolipid antigens and bridges the innate and acquired immune systems with potential applications for cancer therapy. Int Immunol 2010; 22:1 - 6; http://dx.doi.org/10.1093/intimm/dxp104; PMID: 19858073
- van der Vliet HJ, Molling JW, von Blomberg BM, Nishi N, Kölgen W, van den Eertwegh AJ, et al. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin Immunol 2004; 112:8 - 23; http://dx.doi.org/10.1016/j.clim.2004.03.003; PMID: 15207777
- Das R, Sant’Angelo DB, Nichols KE. Transcriptional control of invariant NKT cell development. Immunol Rev 2010; 238:195 - 215; http://dx.doi.org/10.1111/j.1600-065X.2010.00962.x; PMID: 20969594
- Chan AC, Serwecinska L, Cochrane A, Harrison LC, Godfrey DI, Berzins SP. Immune characterization of an individual with an exceptionally high natural killer T cell frequency and her immediate family. Clin Exp Immunol 2009; 156:238 - 45; http://dx.doi.org/10.1111/j.1365-2249.2009.03888.x; PMID: 19250277
- Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 2007; 122:1 - 14; http://dx.doi.org/10.1111/j.1365-2567.2007.02647.x; PMID: 17662044
- Lin H, Nieda M, Rozenkov V, Nicol AJ. Analysis of the effect of different NKT cell subpopulations on the activation of CD4 and CD8 T cells, NK cells, and B cells. Exp Hematol 2006; 34:289 - 95; http://dx.doi.org/10.1016/j.exphem.2005.12.008; PMID: 16543063
- Kim CH, Butcher EC, Johnston B. Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol 2002; 23:516 - 9; http://dx.doi.org/10.1016/S1471-4906(02)02323-2; PMID: 12401396
- Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, et al. Distinct homeostatic requirements of CD4+ and CD4- subsets of Valpha24-invariant natural killer T cells in humans. Blood 2004; 104:4150 - 6; http://dx.doi.org/10.1182/blood-2004-04-1629; PMID: 15328159
- Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res 2008; 101:277 - 348; http://dx.doi.org/10.1016/S0065-230X(08)00408-9; PMID: 19055947
- Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol 2009; 39:1046 - 55; http://dx.doi.org/10.1002/eji.200838575; PMID: 19266498
- Ishihara S, Nieda M, Kitayama J, Osada T, Yabe T, Kikuchi A, et al. Alpha-glycosylceramides enhance the antitumor cytotoxicity of hepatic lymphocytes obtained from cancer patients by activating CD3-CD56+ NK cells in vitro. J Immunol 2000; 165:1659 - 64; PMID: 10903777
- Dhodapkar MV, Richter J. Harnessing natural killer T (NKT) cells in human myeloma: progress and challenges. Clin Immunol 2011; 140:160 - 6; http://dx.doi.org/10.1016/j.clim.2010.12.010; PMID: 21233022
- Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol 2001; 167:4046 - 50; PMID: 11564825
- Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, Nicol AJ. Modulation of human Valpha24(+)Vbeta11(+) NKT cells by age, malignancy and conventional anticancer therapies. Br J Cancer 2004; 91:1880 - 6; http://dx.doi.org/10.1038/sj.bjc.6602218; PMID: 15520823
- Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res 2002; 8:3702 - 9; PMID: 12473579
- Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol 2009; 182:2492 - 501; http://dx.doi.org/10.4049/jimmunol.0800126; PMID: 19201905
- Fujii S, Takayama T, Asakura M, Aki K, Fujimoto K, Shimizu K. Dendritic cell-based cancer immunotherapies. Arch Immunol Ther Exp (Warsz) 2009; 57:189 - 98; http://dx.doi.org/10.1007/s00005-009-0025-x; PMID: 19479202
- McCaughey WT, Colby TV, Battifora H, Churg A, Corson JM, Greenberg SD, et al. Diagnosis of diffuse malignant mesothelioma: experience of a US/Canadian Mesothelioma Panel. Mod Pathol 1991; 4:342 - 53; PMID: 2068061
- Fallarini S, Paoletti T, Panza L, Lombardi G. Alpha-galactosylceramide modulates the induction of indoleamine 2,3-dioxygenase in antigen presenting cells. Biochem Pharmacol 2008; 76:738 - 50; http://dx.doi.org/10.1016/j.bcp.2008.07.001; PMID: 18671950
- Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem 1988; 263:18545 - 52; PMID: 3192548
- Chirieac LR, Corson JM. Pathologic evaluation of malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2009; 21:121 - 4; http://dx.doi.org/10.1053/j.semtcvs.2009.06.005; PMID: 19822283
- Molling JW, Kölgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer 2005; 116:87 - 93; http://dx.doi.org/10.1002/ijc.20998; PMID: 15756674
- Metelitsa LS. Flow cytometry for natural killer T cells: multi-parameter methods for multifunctional cells. Clin Immunol 2004; 110:267 - 76; http://dx.doi.org/10.1016/j.clim.2003.11.005; PMID: 15047204
- Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med 2010; 134:1645 - 58; PMID: 21043818
- Rom WN, Palmer PE. The spectrum of asbestos-related diseases. West J Med 1974; 121:10 - 21; PMID: 4601063
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 2006; 92:5 - 22; http://dx.doi.org/10.1093/toxsci/kfj130; PMID: 16484287
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol 2010; 7:5; http://dx.doi.org/10.1186/1743-8977-7-5; PMID: 20307263
- Gansert JL, Kiessler V, Engele M, Wittke F, Röllinghoff M, Krensky AM, et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol 2003; 170:3154 - 61; PMID: 12626573
- Ho LP, Urban BC, Thickett DR, Davies RJ, McMichael AJ. Deficiency of a subset of T-cells with immunoregulatory properties in sarcoidosis. Lancet 2005; 365:1062 - 72; PMID: 15781102
- Kusner DJ. Mechanisms of mycobacterial persistence in tuberculosis. Clin Immunol 2005; 114:239 - 47; http://dx.doi.org/10.1016/j.clim.2004.07.016; PMID: 15721834
- Becquemin MH, Bertholon JF, Attoui M, Roy F, Roy M, Dautzenberg B. [Particle size in the smoke produced by six different types of cigarette]. Rev Mal Respir 2007; 24:845 - 52; http://dx.doi.org/10.1016/S0761-8425(07)91386-8; PMID: 17925666
- Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol 2008; 59:Suppl 6 19 - 34; PMID: 19218630
- Sköld CM, Blaschke E, Eklund A. Transient increases in albumin and hyaluronan in bronchoalveolar lavage fluid after quitting smoking: possible signs of reparative mechanisms. Respir Med 1996; 90:523 - 9; http://dx.doi.org/10.1016/S0954-6111(96)90144-4; PMID: 8984526
- Hogan AE, Corrigan MA, O’Reilly V, Gaoatswe G, O’Connell J, Doherty DG, et al. Cigarette smoke alters the invariant natural killer T cell function and may inhibit anti-tumor responses. Clin Immunol 2011; 140:229 - 35; http://dx.doi.org/10.1016/j.clim.2011.01.011; PMID: 21684213
- Ilavská S, Jahnová E, Tulinská J, Horváthová M, Dusinská M, Wsolová L, et al. Immunological monitoring in workers occupationally exposed to asbestos. Toxicology 2005; 206:299 - 308; http://dx.doi.org/10.1016/j.tox.2004.09.004; PMID: 15588921
- Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest 2005; 115:2572 - 83; http://dx.doi.org/10.1172/JCI24762; PMID: 16138194
- Otsuki T, Maeda M, Murakami S, Hayashi H, Miura Y, Kusaka M, et al. Immunological effects of silica and asbestos. Cell Mol Immunol 2007; 4:261 - 8; PMID: 17764616
- Maeda M, Miura Y, Nishimura Y, Murakami S, Hayashi H, Kumagai N, et al. Immunological changes in mesothelioma patients and their experimental detection. Clin Med Circ Respirat Pulm Med 2008; 2:11 - 7; PMID: 21157517