Abstract
Background: Primary brain tumors have always been associated with high morbidity and mortality. Glioma is the most common type of malignant brain tumors,with a high probability of recurrence after surgical excision and with poor prognosis.The purpose of this study was to compare the therapeutic efficacy of computed tomography (CT)-guided interstitial 125I seed implantation with traditional radiochemotherapy for treatment of recurrent gliomas.
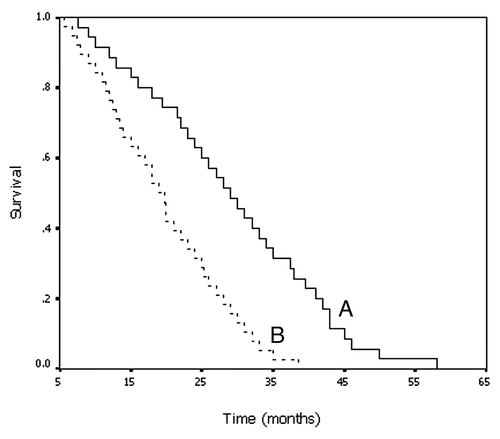
Results: The response rate at 1, 3, 6 and 12 months after 125I seed implantation was 68.6, 74.3, 77.1 and 62.8% respectively, which was significantly higher than the group treated with the conventional chemoradiation protocol (p < 0.05). Patients exposed to 125I seed implantation had a median survival of 29.0 months, whereas the median survival of those treated with traditional radiochemotherapy was 19.0 months. The difference observed between the two groups was significant. There were no severe complications or mortality associated with either treatment, except for one case of intracerebral hemorrhage around the tumor area in the 125I seed implants group.
Methods: From November 2002 to May 2010, 73 consecutive patients with recurrent gliomas were treated with CT-guided 125I seed implantation (35 cases) or traditional radiochemotherapy (38 cases). Patients were followed up after treatment and the therapeutic effect was evaluated by comparing the response and survival rates of the two groups. In particular, patients treated with 125I seed implantation were monitored for adverse side effects.
Conclusions: CT-guided 125I seed implantation is safe and well-tolerated and more importantly, shows superior efficacy compared with conventional radiochemotherapy. This suggests that CT-guided 125I seed implantation could be an alternative approach for recurrent gliomas.
Introduction
Malignant gliomas, which typically arise in the lobar white matter or in the deep gray matter of the brain, are characterized by a diffusely infiltrating spread and are the most common and aggressive primary brain tumors in adults.Citation1 The duration of survival of patients with malignant gliomas has remained essentially the same in the last three to four decades, despite striking progress achieved in medical and surgical technology and in the understanding of the biological basis as well as the molecular genetics of glioma cells.Citation2,Citation3
The current standard therapy for newly diagnosed malignant gliomas consists of surgery, preferably a gross total resection of contrast-enhanced areas, subsequent fractionated external-beam radiotherapy, adjuvant chemotherapy in some cases, or combinations of these three approaches.Citation4,Citation5 Despite optimal treatment with surgery, radiotherapy and temozolomide, and subsequent beneficial outcomes, tumor recurrences is frequently observed. Treatment of recurrent malignant gliomas is challenging, because tumor infiltration and the potentiation of neurologic toxicity often makes repeated surgery unfeasible. Facing limited treatment options, patients are usually enrolled into chemotherapy trials. As a result, an increasing number of studies have been focused on developing effective approaches for the treatment of recurrent malignant gliomas.
Interstitial brachytherapy is a form of radiotherapy in which radioactive seeds or sources are placed in or near local tumor sites. One of the advantages of brachytherapy compared with regular radiation therapy is that it delivers a high dose of radiation to the tumor but reduced radiation exposure to the surrounding healthy tissue.Citation6 Radioactive iodine-125 (125I) and palladium-103 (103Pd) are two commonly used seeds and have been applied in different circumstances. 125I is a low-energy radioisotope with a relatively long half-life (59.6 d). It emits gamma-rays with maximum energies of 35 keV, which causes DNA double-strand breaks in tumor cells and eventually suppresses cell proliferation or leads to cell death.Citation7 Brachytherapy with 125I seeds has been used as a first-line choice for early prostate cancer.Citation8 In addition, it provides an alternative approach for the treatment of various other cancers, including colon, cervical, prostate, breast, liver and skin cancers.Citation9-Citation13
Despite its wide range of application and beneficial outcomes in various types of cancer therapy, the application of brachytherapy in central nervous system (CNS) tumors, such as recurrent malignant gliomas, has been greatly debated.Citation14 In the current study, we used CT (CT)-guided precise interstitial 125I implants in the treatment of recurrent gliomas. Unlike previous studies, we used skull drilling instead of skull opening for the 125I seed implantation so as to reduce cerebral damage. A parallel treatment group received traditional chemoradiation therapy. The therapeutic efficacy was assessed and compared between the two treatment options. In addition, patients were followed up and complications due to treatment were monitored. The study found that characterized CT-guided 125I seed implantation was more effective and better tolerated compared with conventional chemoradiation therapy. Therefore this report reveals its potential in the treatment of recurrent gliomas.
Results
Response rate comparison
To evaluate the therapeutic effectiveness of the 125I seed implantation, we begun by comparing the patients’ responses at different time-points after the 125I seed implantation with those of the patients given conventional chemoradiation therapy (Table 2). At 1, 3, 6 or 12 mo after 125I seed implantation, 20.0%, 22.9%, 20.0% and 17.1% of patients respectively showed CR and 48.6%, 51.4%, 57.1%, 45.7% of patients partially responded. By comparison, the RRs(which reflect overall therapeutic efficacy)of the patients in the chemoradiation group at these time points were significantly lower, RRs at 1, 3, 6 and 12 mo after 125I seed implantation was 68.6%, 74.3%, 77.1% and 62.8% respectively, which was significantly higher than the group treated with conventional chemoradiation (p < 0.05).
These data together support the superior efficacy of 125I seed implantation relative to the conventional treatment options for patients with recurrent gliomas,and indicate optimal efficacy of 125I seed implantation treatment in recurrent gliomas.( and )
Figure 1. A 54-y-old female patient with recurrent gliomas. (A) CT images before 125I seed implantation treatment showed that brain tumor of left posterior parietal region was 30 mm × 40 mm × 50 mm. (B) outlined the planning target volume (PTV) with computerized treatment planning system (TPS) before 125I seed implantation to determine the needle insertion and dose distributions. (C) needle insertion during 125I seed implantation operation. (D) seed distribution after 125I seed implantation treatment. E: verification of TPS immediately after 125I seed implantation treatment. (F) dose volume histogram of PTV of pre-operation TPS. (G) dose volume histogram of PTV of post-operation TPS verification.
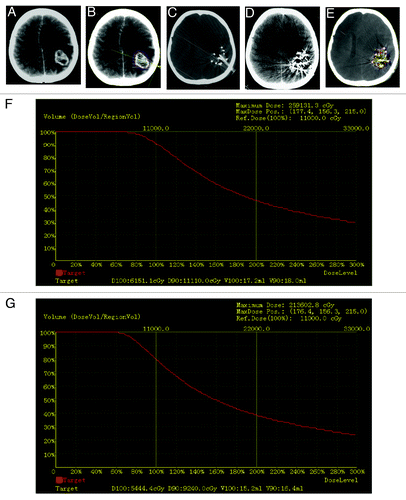
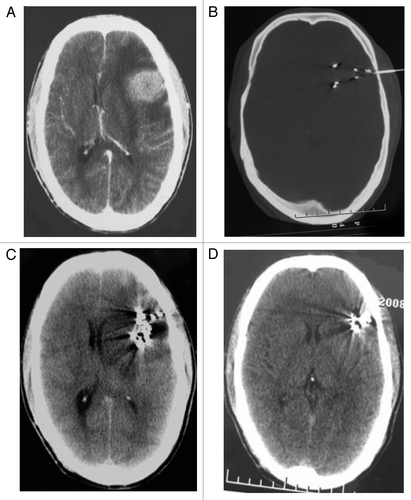
Figure 2. A 68-y-old male patient with recurrent gliomas. (A) CT images before 125I seed implantation treatment showed that brain tumor of left anterior parietal region was 25 mm × 30 mm × 40 mm. (B) needle insertion during 125I seed implantation operation. C:two months after 125I seed implantation treatment. (D) one year after 125I seed implantation treatment.
Survival rate comparison
The survival of the patients following each treatment were compared (Table 3). Consistent with the RR, the median survival of patients treated with 125I seed implantation was significantly longer that those exposed to chemoradiation therapy. Two-year survival after brachytherapy was 77.1%, which was significantly higher than that of patients treated with chemoradiation therapy (52.6%). Although the one-year survival was also greater for the brachytherapy group, the difference was not statistically significant ().These data together demonstrate that CT-guided 125I seed implantation has the curative advantage compared with the conventional approach.
Safety and tolerance
To evaluate CT-guided 125I seed implantation more fully as an alternative approach for the treatment of recurrent glioma, after the implantation patients were monitored for adverse side effects and associated complications. During the follow-up period, no hemorrhagic inflammation, cerebrospinal fluid fistula, or radiation encephalopathy was detected in any patient treated with implantation, except one case of intracerebral hemorrhage around the tumor area. The patient recovered from the bleeding after emergency cerebral surgery. No treatment-related death occurred in either the 125I seed implanted or chemoradiation-treated groups.Side effects due to chemoradiation therapy included bone marrow suppression, gastrointestinal reactions and alopecia.
Discussion
Implantation of radioactive 125I seeds has long been recognized for its superiority to other radiotherapies. As low-energy radioactive seed interstitial implantation results in positive clinical treatment of many tumors previously resistant to high doses of radiation. The advantages of internal implantation are, first, due to the shorter distance to local tumor sites, thereby enabling a higher cumulative dose, up to 160 Gy, to the tumor.Citation15 Second, with the substantial attenuation of radiation potency over short distance, implantation of radioactive seeds within or near the tumor avoids the serious complications caused by radiation exposure to non-target tissues, which frequently occur with traditional radiotherapy. Third, the inhomogeneous radiation absorption observed in traditional radiotherapy, results in attenuated therapeutic potency, which is not the case in internal radiation. In addition, previous study has shown that external radiotherapy is only effective in cells at certain phases of the cell cycle while 125I seeds can kill tumor cells at any time by causing them to stay in the resting period and by inducing apoptosis in tumor stem cells. Finally, it is also noted that the implantation of low-energy radioactive seeds is able to suppress tumor metastasis by changing the immunophenotype of tumor cells.Citation16
Given all these advantages, it is of no surprise that high expectations have also been placed on the efficacy of internal implantation to improve the outcome for patients with CNS tumors. However, various studies have shown conflicting results,Citation17-Citation20 with some reports of no significant survival advantage. This has raised a question about the potential of applying brachytherapy in the treatment of CNS tumors, including malignant gliomas.Citation21 In the present study, the question was addressed by comparing 125I implantation and conventional chemoradiation in the treatment of recurrent gliomas. Our data indicate that brachytherapy is a more effective and better tolerated alternative to traditional chemoradiation. We speculate that differences in implants, optimal dose rate, target volume, timing of brachytherapy and patient selection might account for the controversial outcomes reported so far. Our data could provide guidance on using brachytherapy in the treatment of CNS tumors. Pseudo-progression: has been known to be relevant to treatment of high-grade gliomas. However, we did not observe the incidence of radiation-induced brain edema or early pseudo-progression.Citation22 The reasons might be: 1) low dose rate radiation we applied causes mild side-effects and the brain edema did not last longCitation23; 2) by the time of assesment, which is usually 4–6 weeks following implantation, the paitents likely have recovered from acute edema caused by surgeryCitation24; 3) the intratumoral 125I implantation indeed caused very mild affect on the normal tissue regions and thereofore the radiation-induced edema was less servere compared with external radiation,Citation25,Citation26
Several prognostic factors, such as age, histology and extent of surgery have been established for patients newly diagnosed with malignant gliomas. However, the prognostic factors for patients with recurrent malignant gliomas remain unclear. A previous study of 375 patients from eight phase-II trials which examined outcomes and prognostic factors associated with recurrent gliomas, indicated that histology was a dominant factor in determining outcome in this group of patients.Citation27 A more recent phase-II study of 51 adults with recurrent malignant gliomas suggested that histology, myelotoxicity and prior chemotherapy were significantly correlated with both time to tumor progression and survival.Citation28 All these factors should be considered when designing future trials for recurrent gliomas.
While many studies have focused on demonstrating the therapeutic effect of radioactive seed interstitial implantation, few have concentrated on delineating the molecular mechanisms that underlie outcome differences in traditional radiotherapy vs. interstitial implanted radiation. Fundamental research leading to a better understanding of the biological mechanisms may allow therapeutic modifications in radiotherapies, including brachytherapy, which could be evaluated in future clinical studies. Given the fact that most patients enrolled in this study had recurrent cancer following chemotherapy, the standard VM-26 (teniposide 200 mg/m2) regimen was replaced with elemene or temozolomide for patients in severe conditions, such as those with grade III + IV tumors or even worse. Elemene is an antitumor composite derived from the traditional Chinese Medicines, which was characterized by the ability to pass the blood-brain barrier and minor toxicity . In spite of such modification in regimen, the clinical outcome of our study was in agreement with the previous reports.Citation29,Citation30
In spite of the small number of cases reviewed in the current study, the data strongly suggest that for treatment of recurrent gliomas CT-guided precise 125I interstitial implantation holds advantages over conventional options. These advantages include precision of radiation location, less cerebral damage, and fewer complications. The use of CT-guided precise 125I interstitial implantation should therefore be considered a viable alternative for the treatment of recurrent gliomas.
Patients and Methods
Patients
This study was approved by the local ethics committee and Institutional Review Board (Cancer Centre of Sun Yat-Sen University and Pingyi branch of Qilu Hospital). From November 2002 to May 2010, 73 consecutive patients with diagnosed recurrent brain gliomas were enrolled. Patients were informed of the potential risks, and written consent was obtained from the patients or their guardians before beginning the study. The enrolled patients were admitted to either of two independent hospitals that were working collaboratively. All the patients met the following inclusion criteria: the presence of post-surgical recurrences of glioma confirmed by pathological diagnosis, tumor diameter below 3 cm, tumor with clear borders on CT imaging, no metastasis observed in other organs, and suitable for the treatment approaches used in this study, i.e., the level of blood platelets was not less than 10 × 1010, coagulation time was less than 4 sec, and no intracranial vascular malformations, intracranial hemorrhage or thrombotic disorders detected. In the cases where tumor borders were hard to identify, the critical functional regions, such as those around brainstem, temporal lobe and optic nerve, were avoided during implantation. The patients enrolled in this study were divided into two groups. The detailed information of each group is presented in . No significant difference (p > 0.05) was observed between the two groups.
Table 1. Characteristics of 73 patients treated with recurrent gliomas
Instruments
A High Speed Advantage Genesis CT scanner (GE Healthcare) was used. A treatment planning system was developed by Beijing University of Aeronautics and Astronautics. Instruments for seed implantation included 18-G implantation needles and a turntable implantation gun. 125I seeds were obtained from Jun’an Pharmaceuticals Co. Ltd. The diameter and length of each seed was 0.8 mm and 4.5 mm respectively. The thickness of the wall of the titanium capsule was 0.05 mm. 125I emits gamma rays (5% of 35 keV, 95% of 28 keV) with a half life of 59.6 d, a half value thickness of 0.025 mm of lead, penetration of 17 mm and incipient rate of 7 cGy/h.
Treatment planning system
A computerized treatment planning system was used to determine the dose distributions so as to maximize tumor control and minimize complications in normal tissue. Treatment planning and applied radiation dosages were based on three-dimensional (3D) models representing patient anatomy and tumor targets. Detailed information about the targeted tumor, including tumor size, status and location, as well as tumor connected vessels was collected using images and histological information provided by CT, magnetic resonance imaging and 3D models. Based on this information, decisions regarding the insertion site, path and direction of needles were decided and an isodose curve distribution was plotted.
Seeds were implanted 0.5–1 cm apart. For patients undergoing radiation treatment for the first time, the matched peripheral dose (MPD) was 90–110 Gy while that for patients who had been exposed to radiation therapy before the MPD was 80–90 Gy. The planning target volume margin was 0.5 cm greater than the clinical target volume. Dosage delivered to affected organs and tumors was measured using a dose-volume histogram. 125I treatment plan is not based on tumor grade, but it is based on the size of tumor volume to obtain effective treatment dose. The specific effect of 125I radiation on tumor cells can emits gamma-rays with maximum energies of 35 keV, which causes DNA double-strand breaks in tumor cells and eventually suppresses cell proliferation or leads to cell death, which have been reflected in a broad range of literature. CT scanning was performed immediately after seed implantation to confirm that the treatment plan was being followed and modification was made in case of inaccuracy ( and ).
125I seeds implantation
Implantation was guided by CT according to our treatment planning system. The volume and shape of each tumor was assessed with CT scanning and used to calculate the MPD and to determine the position and number of seeds needed by simulating the approximation of dose distribution in any given tumor ( and ). The patients had their heads shaved and they were treated with diazepam the day before the surgery. General anesthesia was applied during the surgery. The drilling holes in the skull were located closest to the tumor foci and avoided critical cerebral areas, such as the major vessels and cerebral venous sinus. Needle insertion followed a fan-shaped path so as to minimize cerebral damage. In the fan-shaped area, the needle was gradually retrieved after reaching the furthest point. 125I seeds were implanted into the cancerous embolus 5–10 mm apart along the length and diameter of the tumor. CT scanning was performed immediately after implantation to monitor potential complications, in particular, cerebral hemorrhage. The needle was inserted and retrieved with minor adjustment of angles as needed and this process was repeated two to five times to achieve the correct implant location. After the procedure, the puncture site was bandaged and compressed for homeostasis.
Treatment
In the conventional chemoradiation treated group, all the patients were irradiated 30–35 times at a dose of 60–70 Gy in a cycle of 40–60 d. The dosages were set prior to treatment according to the maximum accumulative dosages in different brain regions. The brainstem cumulative dose could not exceed 54 Gy, while those for regions close to temporal lobe, spinal cord, optic nerve and crystalline lens were below 60 Gy, 45Gy, 50 Gy and 9 Gy respectively, which is suitable for both external radiation and internal implantation. However, the low-energy photon 125I applied in this study has been know to lose over 80% genery at a distance of 2 cm from the source center and practically all seeds were all implanted in the non-functional area in this case. It might not be necesseary to concider the dosage contraits.Citation25,Citation26 Simultaneously, VM-26 (teniposide, 200 mg/m2) was given for three consecutive days followed by a two-week break in each cycle. The cycle was repeated four times. For patients with more severe conditions, such as those with grade III + IV tumors or larger, additional treatments with elemene injection (600 mg/day for 14 consecutive days each cycle) or temozolomide (250 mg/day for seven consecutive days each cycle) were included.
Evaluation of therapeutic effect
The assessment of therapeutic effect followed the Response Evaluation Criteria in Solid Tumors guidelines published by the World Health Organization. Tumor volume was examined by CT scanning. Two-dimensional (2D) tumor measurements (the product of the longest diameter and the longest perpendicular diameter for each tumor) were performed. The assessment criteria were: complete response (CR), tumor disappeared; partial response (PR), at least 50% decrease in values obtained in the 2D tumor measurements; no change (NC), no more than 50% decrease and less than 25% increase in the product of the two largest diameters; progressive disease (PD), more than 25% increase in the product of the longest diameter and its longest perpendicular diameter or appearance of a new tumor. The response rate (RR) was calculated as the sum of the CR and PR.Citation24
Statistical analyses
Data were represented as mean ± SD. Statistical analyses were performed using SPSS 11.5 software (SPSS Inc. Chicago, IL, USA). Cumulative survival curves were constructed using the Kaplan-Meier method and compared with the log-rank test. Results were considered significant at p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Notes
† These authors contributed equally to this work.
References
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 2005; 64:479 - 89; PMID: 15977639
- DeAngelis LM. Brain tumors. N Engl J Med 2001; 344:114 - 23; http://dx.doi.org/10.1056/NEJM200101113440207; PMID: 11150363
- Mitchell P, Ellison DW, Mendelow AD. Surgery for malignant gliomas: mechanistic reasoning and slippery statistics. Lancet Neurol 2005; 4:413 - 22; http://dx.doi.org/10.1016/S1474-4422(05)70118-6; PMID: 15963444
- Burton EC, Prados MD. Malignant gliomas. Curr Treat Options Oncol 2000; 1:459 - 68; http://dx.doi.org/10.1007/s11864-000-0073-2; PMID: 12057153
- Carapella CM, Telera S, Oppido PA. Surgery of malignant gliomas: advances and perspectives. Curr Opin Oncol 2011; 23:624 - 9; http://dx.doi.org/10.1097/CCO.0b013e32834ace58; PMID: 21857513
- Ikushima H. Radiation therapy: state of the art and the future. J Med Invest 2010; 57:1 - 11; http://dx.doi.org/10.2152/jmi.57.1; PMID: 20299738
- Darakchiev BJ, Albright RE, Breneman JC, Warnick RE. Safety and efficacy of permanent iodine-125 seed implants and carmustine wafers in patients with recurrent glioblastoma multiforme. J Neurosurg 2008; 108:236 - 42; http://dx.doi.org/10.3171/JNS/2008/108/2/0236; PMID: 18240917
- Langley SE, Laing RW. Iodine seed prostate brachytherapy: an alternative first-line choice for early prostate cancer. Prostate Cancer Prostatic Dis 2004; 7:201 - 7; http://dx.doi.org/10.1038/sj.pcan.4500727; PMID: 15184864
- Zhang FJ, Li CX, Zhang L, Wu PH, Jiao DC, Duan GF. Short- to mid-term evaluation of CT-guided 125I brachytherapy on intra-hepatic recurrent tumors and/or extra-hepatic metastases after liver transplantation for hepatocellular carcinoma. Cancer Biol Ther 2009; 8:585 - 90; http://dx.doi.org/10.4161/cbt.8.7.7902; PMID: 19276683
- Tanderup K, Georg D, Pötter R, Kirisits C, Grau C, Lindegaard JC. Adaptive management of cervical cancer radiotherapy. Semin Radiat Oncol 2010; 20:121 - 9; http://dx.doi.org/10.1016/j.semradonc.2009.11.006; PMID: 20219550
- Mayadev J, Merrick GS, Reed JR, Butler WM, Galbreath RW, Allen ZA, et al. Permanent prostate brachytherapy in prostate glands <20 cm(3). Int J Radiat Oncol Biol Phys 2010; 76:1450 - 5; http://dx.doi.org/10.1016/j.ijrobp.2009.04.014; PMID: 20338476
- Sakurai H, Mitsuhashi N, Harashima K, Muramatsu H, Ishikawa H, Kitamoto Y, et al. CT-fluoroscopy guided interstitial brachytherapy with image-based treatment planning for unresectable locally recurrent rectal carcinoma. Brachytherapy 2004; 3:222 - 30; http://dx.doi.org/10.1016/j.brachy.2004.09.007; PMID: 15607154
- Van Limbergen E, Weltens C. New trends in radiotherapy for breast cancer. Curr Opin Oncol 2006; 18:555 - 62; http://dx.doi.org/10.1097/01.cco.0000245327.42281.9f; PMID: 16988575
- Liu BL, Cheng JX, Zhang X, Zhang W. Controversies concerning the application of brachytherapy in central nervous system tumors. J Cancer Res Clin Oncol 2010; 136:173 - 85; http://dx.doi.org/10.1007/s00432-009-0741-y; PMID: 19956971
- Ling CC. Permanent implants using Au-198, Pd-103 and I-125: radiobiological considerations based on the linear quadratic model. Int J Radiat Oncol Biol Phys 1992; 23:81 - 7; http://dx.doi.org/10.1016/0360-3016(92)90546-T; PMID: 1572833
- Zhang FJ, Li CX, Jiao DC, Zhang NH, Wu PH, Duan GF, et al. CT guided 125iodine seed implantation for portal vein tumor thrombus in primary hepatocellular carcinoma. Chin Med J (Engl) 2008; 121:2410 - 4; PMID: 19102958
- Laperriere NJ, Leung PM, McKenzie S, Milosevic M, Wong S, Glen J, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys 1998; 41:1005 - 11; http://dx.doi.org/10.1016/S0360-3016(98)00159-X; PMID: 9719109
- Scharfen CO, Sneed PK, Wara WM, Larson DA, Phillips TL, Prados MD, et al. High activity iodine-125 interstitial implant for gliomas. Int J Radiat Oncol Biol Phys 1992; 24:583 - 91; http://dx.doi.org/10.1016/0360-3016(92)90702-J; PMID: 1429079
- Chamberlain MC, Barba D, Kormanik P, Berson AM, Saunders WM, Shea MC. Concurrent cisplatin therapy and iodine 125 brachytherapy for recurrent malignant brain tumors. Arch Neurol 1995; 52:162 - 7; http://dx.doi.org/10.1001/archneur.1995.00540260066018; PMID: 7848125
- Selker RG, Shapiro WR, Burger P, Blackwood MS, Arena VC, Gilder JC, et al, Brain Tumor Cooperative Group. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery 2002; 51:343 - 55, discussion 355-7; PMID: 12182772
- Nieder C, Andratschke N, Wiedenmann N, Busch R, Grosu AL, Molls M. Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome?. Strahlenther Onkol 2004; 180:401 - 7; PMID: 15241527
- Fink J, Born D, Chamberlain MC. Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol 2011; 12:240 - 52; http://dx.doi.org/10.1007/s11864-011-0157-1; PMID: 21594589
- Hygino da Cruz LC Jr., Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 2011; 32:1978 - 85; http://dx.doi.org/10.3174/ajnr.A2397; PMID: 21393407
- McDermott MW, Sneed PK, Gutin PH. Interstitial brachytherapy for malignant brain tumors. Semin Surg Oncol 1998; 14:79 - 87; http://dx.doi.org/10.1002/(SICI)1098-2388(199801/02)14:1<79::AID-SSU10>3.0.CO;2-4; PMID: 9407634
- Halligan JB, Stelzer KJ, Rostomily RC, Spence AM, Griffin TW, Berger MS. Operation and permanent low activity 125I brachytheraphy for recurrent high-grade astrocytomas. Int J Radiat Oncol Biol Phys 1996; 35:541 - 7; http://dx.doi.org/10.1016/S0360-3016(96)80017-4; PMID: 8655378
- Zamorano L, Li Q, Tekyi-Mensah S, Gaspar L, Fontanesi J, Levin K. Permanent iodine-125 interstitial radiation therapy in the treatment of non-glioblastoma multiforme high-grade gliomas. Stereotact Funct Neurosurg 2003; 81:10 - 7; http://dx.doi.org/10.1159/000075098; PMID: 14742958
- Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999; 17:2572 - 8; PMID: 10561324
- Gilbert MR, Friedman HS, Kuttesch JF, Prados MD, Olson JJ, Reaman GH, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol 2002; 4:261 - 7; PMID: 12356356
- Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y, et al. ß-Elemene inhibits proliferation of human glioblastoma cells and causes cell-cycle G0/G1 arrest via mutually compensatory activation of MKK3 and MKK6. Int J Oncol 2011; 38:419 - 26; PMID: 21132268
- Wu XS, Xie T, Lin J, Fan HZ, Huang-Fu HJ, Ni LF, et al. An investigation of the ability of elemene to pass through the blood-brain barrier and its effect on brain carcinomas. J Pharm Pharmacol 2009; 61:1653 - 6; http://dx.doi.org/10.1211/jpp.61.12.0010; PMID: 19958588