Abstract
The overall 5 year survival rate for pancreatic ductal adenocarcinoma (i.e., PDAC) is a dismal 5%, although patients that have undergone surgical resection have a somewhat better survival rate of up to 20%. Very long-term survivors of PDAC (defined as patients with ≥ 10 year survival following apparently curative resection), on the other hand, are considerably less frequent. The molecular characteristics of very long-term survivors (VLTS) are poorly understood, but might provide novel insights into prognostication for this disease. In this study, a panel of five VLTS and stage-matched short-term survivors (STS, defined as disease-specific mortality within 14 months of resection) were identified, and quantitative proteomics was applied to comparatively profile tumor tissues from both cohorts. Differentially expressed proteins were identified in cancers from VLTS vs. STS patients. Specifically, the expression of galectin-1 was 2-fold lower in VLTS compared with STS tumors. Validation studies were performed by immunohistochemistry (IHC) in two additional cohorts of resected PDAC, including: 1) an independent cohort of VLTS and 2) a panel of sporadic PDAC with a considerable range of overall survival following surgery. Immunolabeling analysis confirmed that significantly lower expression of stromal galectin-1 was associated with VLTS (p = 0.02) and also correlated with longer survival in sporadic, surgically-treated PDAC cases (hazard ratio = 4.9, p = 0.002). The results from this study provide new insights to better understand the role of galectin-1 in PDAC survival, and might be useful for rendering prognostic information, and developing more effective therapeutic strategies aimed at improving survival.
Introduction
Invasive pancreatic ductal adenocarcinoma (PDAC), is the fourth leading cause of cancer-related deaths in the USA, with over 37,000 individuals estimated to die from this disease in 2012.Citation1 The overall 5 year survival rate of pancreatic cancer is approximately 5%, which is due in large part to the fact that the vast majority of patients (~80%) are diagnosed with surgically unresectable, locally advanced or distant metastatic disease.Citation1 Chemo- and/or radiation therapy are only marginally effective, increasing survival by only a few months for most patients.Citation2 The best reported median survival outcomes with multimodality regimens like FOLFIRINOX are still in the range of 12 months for advanced disease.Citation3
In contrast to advanced disease, the outcome for patients that undergo surgical resection is considerably better, with 5 year survival rates of up to 20% reported in numerous studies.Citation4-Citation13 Clinicopathological features associated with improved survival at 5 year post-resection include small tumor size (< 2 cm), negative lymph node status, negative resection margin (i.e., R0), absence of angiolymphatic and perineural invasion, and low grade of differentiation. Relatively longer (i.e., ≥ 5-y) survival after surgery for PDAC has been increasing for the last two decades, largely due to centralization of surgery to high-volume (i.e., expert) centers.Citation14,Citation15 Moreover, several clinicopathological characteristics have been demonstrated to have significant effect on long-term survival, such as small tumor size (< 2 cm), negative lymph node status, negative resection margin (i.e., R0) and differentiation grade.Citation15 In addition, the relative levels of expression of certain drug transporters responsible for gemcitabine influx, such as the human equilibrative nucleoside transporter-1 (hENT-1) have been implicated in improved survival in the adjuvant setting.Citation16
Although survival to five years would be considered a “cure” in some cancers, this is unfortunately not true in the case of PDAC, with additional attrition of survivors occurring between five and ten years post-surgery.Citation5 Thus, “very long-term survivors” (VLTS) of PDAC (defined as patients with ≥ 10-year survival following resection) are quite uncommon, and even their very existence has been questioned in some studies, following careful re-evaluation of histopathology in the corresponding tumors.Citation17,Citation18 The most common reason for misclassification has been the inclusion of either ampullary cancers, or invasive cancers arising in the setting of a cystic neoplasm of the pancreas (intraductal papillary mucinous neoplasm, or IPMN), both of which have significantly better prognosis than conventional PDAC.Citation11,Citation19-Citation21 Nonetheless, there are a handful of published studies which have identified ≥ 10-y survivors of histologically validated PDAC, usually at a frequency less than 5% in most series.Citation4,Citation5,Citation12,Citation22 Of note, these cohorts of VLTS patients not only include those with small T1, node-negative (N0) tumors, but also patients with more aggressive features, including T2-T4 sized lesions, and lymph node involvement.Citation5 This would suggest that the basis of VLTS in PDAC extends beyond simply resecting node-negative localized cases, but might indicate an intrinsic molecular phenotype of such tumors.
The objective of this study was to perform proteomic profiling of histologically validated PDACs from VLTS compared with stage-matched short-term survivors (STS, defined as patients with disease specific mortality within 14 months post-resection), in order to identify molecular networks that might be contributing to improved survival in this otherwise lethal disease. Our experiments have identified several candidates as being associated with long survival, and in particular, low expression of stromal galectin-1, was further validated in two independent cohorts of PDAC patients. These studies provide some of the first insights into molecular mechanisms underlying prolonged survival in PDAC.
Results
Identification of differentially expressed proteins in cancers from VLTS vs. STS patients
Stable isotope-labeled based quantitative proteomics was performed on tumor tissues to reveal proteins associated with VLTS in PDAC. Equal amounts of proteins extracted from PDAC tissue from 5 VLTS and 5 STS samples were pooled to create a very long-term and short-term survivor reference sample, respectively. The two pooled samples were labeled with iTRAQ stable isotope tags individually, then combined, subjected to subsequent fractionation and purification, followed by mass spectrometry analysis as described previously.Citation24 Quantitative proteomics based on a LC MALDI TOF/TOF analysis revealed 26 differentially expressed proteins at ≥ 1.5-fold: 11 were overexpressed, and 15 were under-expressed in VLTS ().
Table 1. Differentially expressed proteins associated with long-term survivorship in pancreatic ductal adenocarcinoma
The differentially expressed proteins were entered into the online DAVID Bioinformatics database for functional annotation.Citation25,Citation26 Seven of the proteins are involved in defense response (AZU1, DEFA3 RNASE3, C1QC, KRT1, THBS1 and FN1), all of which, except KRT1, were under-expressed in tumors from the VLTS cohort. Four proteins (AZU1, LGALS1, TXNDC5, and THBS1) are involved in regulation of apoptosis, and all four were under-expressed in VLTS. Some of these differentially expressed proteins, namely galectin 1 (LGALS1), thrombospondin 1 (THBS1), and S100A4 have previously been reported to be overexpressed in PDAC tissues.Citation24,Citation27,Citation28 Among these differentially expressed proteins, the expression of galectin-1 (LGALS1) was almost 2–fold higher in the STS pool compared with matched VLTS pool (). Related to this observation, we have previously identified increased galectin-1 expression in the stroma of PanIN lesions and stroma of PDAC.Citation24 Previous studies have reported the association between galectin-1 upregulation and advanced histological grade, systemic metastases, and immune evasion in the tumor microenvironment.Citation29-Citation31 Base on these observations, we chose to further investigate galectin-1 in the context of PDAC patient survival.
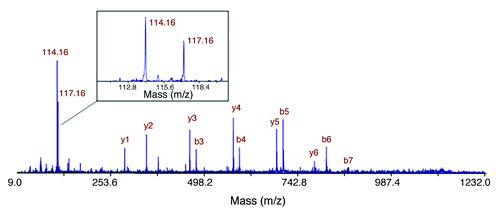
Figure 1. Identification of overexpression of galectin-1 in PDAC from STS in comparison to VLTS. The MS/MS spectrum leading to identification of a unique peptide (SFVLNLGK) from galectin-1 is displayed. The insert shows the iTRAQ reporting peaks of 114 and 117, representing STS and VLTS samples, respectively. The ratio of galectin-1 expression in STS relative to VLTS was calculated to be 1.91.
Correlation of galectin-1 with pancreatic cancer survival
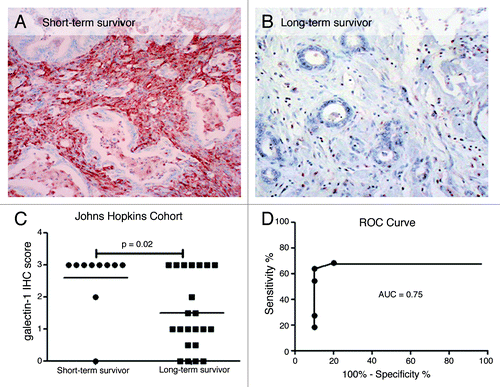
To verify the association of galectin-1 expression with survival and to localize the sub-compartment within which this expression was occurring, IHC was performed on an independent survivor validation cohort, including 22 VLTS and 10 STS (). The expression of galectin-1 was essentially restricted to the peritumoral stromal compartment, with no expression within the neoplastic epithelium per se; therefore, only stromal labeling was evaluated. Using our previously described semi-quantitative scoring approach,Citation32 galectin-1 immunolabeling was strong in the stromal cells in STS cancers, and had significantly less or minimal expression in VLTS samples (p = 0.02, Mann-Whitney U test). Eighty percent of STS showed strong immunolabeling (3+) for galectin-1 compared with 30% of VLTS. As seen in , ROC curve analysis to determine the performance of stromal galectin-1 immunolabeling in predicting very long-term survival showed an AUC of 0.75 (p = 0.026). Moreover, using negative or weak immunolabeling (IHC score ≤ 1) as cutoff, stromal galectin-1 achieved 64% sensitivity and 90% specificity in predicting very long-term survival.
Figure 2. Galectin-1 staining in VLTS and STS in validation cohort 1 (Johns Hopkins cohort). Galectin-1 IHC of invasive pancreatic cancer displaying strong staining (3+, brown color) in desmoplatic stromal cells from a patient with short-term survival (A) and absent immunostaining in a VLTS patient (B). Distribution of galectin-1 staining from short-term survivors and very long-term survivors (C). ROC curve analysis displaying an ROC of 0.75, and 64% sensitivity and 90% specificity in predicting very long-term survival (D).
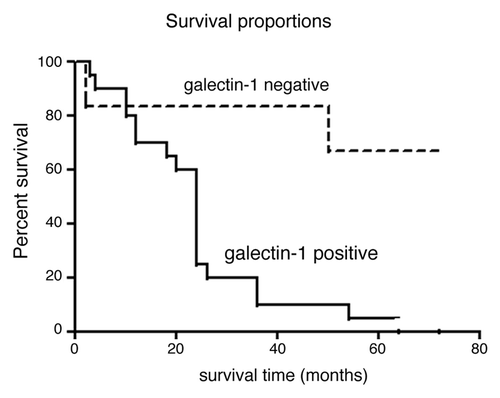
To further validate the correlation of stromal galectin-1 expression with survival, a second independent validation set was used for galectin-1 IHC. After scoring of the TMA (with blinding of survival data) of 43 PDAC cases, 26 had confirmed survival time ranging from 2 to > 72 mo. Survival curve analysis revealed that negative galectin-1 expression (IHC score 0) had significantly longer survival compared with cases with positive stromal galectin-1 labeling (IHC score ≥ 1+) (hazard ratio = 4.9, p = 0.002, ). The median survival time was 24 mo for galectin-1 positive cases, and 72 mo for galectin-1 negative patients. Of interest, of the four cases that showed absent stromal galectin-1 immunolabeling, all had at least 72 mo (6 y) survival post-resection.
Figure 3. Survival analysis of galectin-1 immunostaining and patient survival in validation cohort 2 (Cleveland Clinic cohort). Patients with negative galectin-1 staining (IHC score 0) had significantly longer survival than the patients with positive galectin-1 staining (IHC score ≥ 1+) with hazard ratio 4.9 (p = 0.002).
Correlation of stromal galectin-1 expression with neoplastic progression
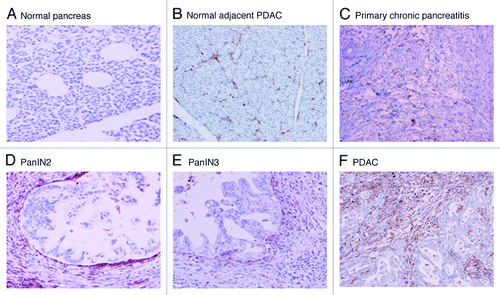
Analysis of IHC revealed that galectin-1 expression was absent in all pancreatic epithelial cells. This was true for all three of the primary diagnostic categories, including normal pancreas, benign chronic pancreatitis and PDAC. However, the immunolabeling of stromal cells varied widely between underlying pathology (e.g., normal pancreas vs. pancreatitis vs. PDAC), and dramatically increased with the development of neoplasia. There was virtually no or mild labeling (0–1+) in benign controls (n = 33), to limited labeling (1+) in primary chronic pancreatitis not associated with malignancy (n = 30), to the highest level (2+ to 3+) immunolabeling in stroma of PDAC (n = 43) ( and ). Normal pancreas adjacent to PDAC displayed increased galectin-1 labeling compared with completely benign pancreatic tissue ( vs. ). Secondary chronic pancreatitis (chronic pancreatitis associated with PDAC) also displayed increased galectin-1 labeling compared with the primary chronic pancreatitis. Intermediate labeling was observed in the stroma surrounding intermediate- and high-grade pancreatic intraepithelial neoplasia (PanIN-2 and PanIN-3, respectively).
Table 2. Galectin-1 staining in Pancreatic ductal adenocarcinoma TMA
Figure 4. Representative galectin-1 staining of pancreas tissue microarrays. All ductal epithelial cells and neoplastic cells were negative for galectin-1 staining. The positive staining was detected in the stromal cells for galectin-1 positive specimens. A. Normal pancreas (no staining); B. Normal pancreas adjacent to PDAC (1+); C. Primary benign chronic pancreatitis (1+); D. PanIN 2 (2+); E. PanIN3 (2+); F. PDAC (3+).
Discussion
Very long-term survival (i.e., ≥ 10 y) in PDAC is extremely rare; it occurs only in very few patients after resection of their primary tumor. Several tumor characteristics have been identified to be of benefit to (very) long-term survival; however, its molecular characteristics remain unidentified. In this study, quantitative proteomics was applied to compare protein expression in PDAC tumor tissues in VLTS with stage -matched STS, followed by expression validation analysis using IHC.
Galectin-1, a member of β-galactoside-binding proteins implicated in modulating cell-cell and cell-matrix interactions, was found to be underexpressed in the VLTS pool, and its significance to survival was further studied by immunolabeling of tumor tissues using two independent patient cohorts with annotated survival data. First, galectin-1 expression was analyzed in surgical samples from 22 VLTS and 10 STS patients, confirming the significantly reduced stromal expression in VLTS. ROC curve analysis for galectin-1 expression as predictor of very-long-term survival showed 64% sensitivity and 90% specificity. Second, galectin-1 immunolabeling using an existing TMA showed that PDAC patients with negative stromal galectin-1 staining had significantly longer survival than the patients with positive staining, with 4 of the PDAC patients with absent galectin-1 labeling alive at 72 mo or greater post follow-up. These results suggest that increased/strong galectin-1 expression is a negative predictor of pancreatic cancer survival, or conversely, that absence or minimal stroma galectin-1 might be a harbinger of improved survival. Overexpression of galectin-1 has been well documented in many different tumor types, including breast adenocarcinoma, hepatocellular carcinoma, and oral squamous cell cancer, among others; and is generally localized to the stroma, and usually correlated with tumor aggressiveness and metastatic phenotypes.Citation29-Citation31 Our current study provides evidence that expression of galectin-1 correlates negatively with survival; in line with earlier findings that overexpression is an adverse prognostic factor.
Galectin-1, protein product of the LGASL1 gene, participates in diverse cell signaling pathways, including regulation of cell growth, cell migration, interaction with Ras signaling and modulation of innate and adaptive immune response.Citation33 On the other hand, mucins are high molecular weight glycoproteins that play important roles in carcinogenesis and tumor invasion. It is well known that several members of mucins and their glycosylation forms are overexpressed in PDAC. In fact, the current pancreatic cancer biomarker CA19–9 detects the glycosylations associated with a group of mucins. A recent study suggested that galectin-1 could directly interact with the Thomsen-Friedenreich (TF or T) antigen (Galβ1-3GalNAcα1-O-Ser/Thr), which is the core 1 structure of O-linked mucin type glycans appearing in tumor-associated glycosylation.Citation34 It would be interesting to further investigate whether and how the interactions of galectin-1 and mucins confer functional significance in promoting tumor progression and invasion in pancreatic cancer.
The mechanism by which galectin-1 influences tumor properties remains to be elucidated for PDAC. A role has been suggested for galectin-1 in its contribution to tumor immune response escape through induction of apoptosis of activated T cells,Citation35 and may provide an immunosuppressive environment at the tumor site in favor of tumor-immune escape.Citation29 More recently, galectin-1 has been shown to participate in angiogenesis; increased expression of galectin-1 was observed in the vasculature of several human malignant tumors.Citation36 More specifically, the uptake of galectin-1 by cultured endothelial cells promotes H-Ras signaling and stimulates endothelial cell proliferation and migration.Citation36 Conversely, knockdown of galectin-1 expression in endothelial cells was shown to inhibit proliferation and migration, and galectin-1-null mice displayed hampered tumor growth due to decreased angiogenic activity.Citation37 These results suggest that the active role galectin-1 plays in the proliferation and migration of tumors is via angiogenesis. Last, but perhaps most significant, galectin-1 is upregulated in the initial stages of inflammation and wound repair signaling.Citation38 This is pertinent to cancer progression in that activated myofibroblasts are unable to partner with pancreatic cancer cells in assisting invasion and escape, unless a inflammatory trigger or wounding signal is present. This supports a paradigm of how some cancers can remain indolent, despite having the standard poor prognostic features such as poor differentiation, large size or more advanced staging. Galectin-1 may be one of the key inflammatory triggers that promote aggression and the lack of galectin-1 in the VLTS identifies the indolent tumor.
Our finding that galectin-1 expression was observed in the stroma surrounding non-invasive precursor lesions of the pancreas, and increases in intensity with neoplastic progression suggests that it plays a role early in tumorigenesis and that there appears to be a “dose response,” as the stroma interacts with increasingly malignant cells. The finding of galectin-1 in the cancer-associated stroma, and not in the cancer epithelial cells, is particularly interesting in light of recent studies that identify stromal cells as key collaborators in cancer invasion and metastasis.Citation39-Citation42 Galactin-1 was not present in stromal cells in normal pancreas and only marginally elevated in the stromal cells associated with pancreatitis. Targeted inhibition of galectin-1 expression has been proposed.Citation37 Anti-LGALS1 compounds are currently being investigated to target migrating cancer cells.Citation33 Rather than knocking-down the LGALS1 gene in migrating tumor cells as has been previously suggested,Citation37 it may be more effective inhibiting tumor invasion by blocking expression of LGALS1 in the stromal cells. In such a setting it may be possible to turn an aggressive tumor into an indolent one.
In addition to galectin-1, our study identified several other aberrantly expressed proteins that could be further investigated in relation to pancreatic cancer survival. Two of the proteins overexpressed in the VLTS “pool” compared with the STS cohort by proteomic analysis were osteoglycin (OGN) (3-fold) and prolargin (PRELP) (2.3-fold), respectively. Osteoglycan belongs to the family of the small leucine-rich proteoglycans, which are involved in the regulation of cellular matrix and cellular growth. More specifically, this proteoglycan family of proteins are tissue organizers involved in orienting and ordering collagen fibrils during ontogeny and in pathological processes such as wound healing, tissue repair, and tumor stroma formation.Citation43 A recent study suggested that abundant expression of osteoglycan suppresses lymph node metastasis in highly metastatic hepatocellular cancer.Citation44 Overexpression of osteoglycan in VLTS might suggest that it suppresses metastasis in PDAC and/or regulates the tumor stroma to modulate tumor cell invasion. On a different note, prolargin (proline/arginine-rich end leucine-rich repeat protein) is a glycosaminoglycan and collagen-binding anchor protein that is abundantly expressed in cartilage, basement membranes, and developing bone.Citation45 Previous studies have suggested that prolargin acts as a cell-type specific inhibitor of the NFκB pathway that impairs osteoclastogenesis.Citation45 In addition, increased NFκB activity has been shown to promote angiogenesis, invasion, metastasis, as well as chemoresistance in pancreatic cancer.Citation46 Thus, in VLTS, increased prolargin could potentially inhibit or diminish the NFκB signaling and thereby blocking tumorigenesis, invasion, angiogenesis, and metastasis. Of note, comparable to galectin-1, these two overexpressed proteins are also localized to the stromal compartment, underscoring the critical role the microenvironment is playing in modulating the natural history of PDAC. These potential targets will be investigated in future studies.
Our study has explored the protein expression pattern of very long-term survivors vs. short-term survivors of surgically resected PDAC, and identified several aberrantly expressed candidates. In this study, the stromal galectin-1 was closely associated with pancreatic cancer survival, with a significantly decreased expression observed in true VLTS survivors, as well as those at the higher end of the survival spectrum following resection. Its specific function and mechanism involved in PDAC survival warrants further investigation using functional studies in bona fide cancer associated fibroblasts (CAFs) and in vivo disease models. In addition to galectin-1, several other differentially expressed proteins were identified that could provide valuable insights into the molecular basis of prolonged survival in PDAC.
Materials and Methods
Specimens
This study was approved by the Human Subjects Division of the University of Washington, the Internal Review Board of the Cleveland Clinic, and the Internal Review Board of the Johns Hopkins Medical Institutions. Very long-term survivors (VLTS) and short-term survivors (STS) were identified as survivors of PDAC ≥ 10 y and < 14 mo, respectively, following surgical resection. Before inclusion, the histology was reviewed for all identified VLTS and STS cases from Johns Hopkins by two experts in pancreatic pathology (RHH and AM), in order to confirm the diagnosis of PDAC and exclude the possibility of confounding lesions like ampullary cancers or IPMNs. Finally, five VLTS and five STS were matched by tumor stage and grade in order to perform unbiased proteomic analysis (the changing standards for adjuvant chemotherapy over the past 15 y precluded us from matching cases by type of treatment received) (Table S1). For validation studies, two additional cohorts were formed of patients from two institutions (Johns Hopkins and Cleveland Clinic). The first cohort consisted of 22 VLTS and 10 stage-matched STS, all obtained from the Hopkins archives. The second validation cohort included 106 patients, including PDAC (n = 43), primary chronic pancreatitis (n = 30), and benign normal pancreatic controls (n = 33), which were used to construct a tissue microarray (TMA), subsequent to verification of the underlying pathology by an expert (MPB). Of note, the latter validation cohort was selected from patients undergoing pancreatic surgery without knowledge of the cancer survivor-status.
Protein extraction and sample preparation
Protein extraction from formalin fixed paraffin embedded (FFPE) tissue was performed as previously described.Citation23 Briefly, slides holding 15µm, non-H&E stained sections of paraffinized tissue were heated and deparaffinized. One hematoxylin and eosin (H&E) stained reference section was examined under the microscope to delineate the areas with highest neoplastic cellularity (including both PDAC epithelium and associated stroma), while excluding obvious areas of non-neoplastic pancreatic acinar tissues and inflammatory infiltrates. Thereafter, ten unstained and deparaffinized serial sections of 10µm thickness each were used to obtain the aforementioned delineated area of cancerous tissue by manual dissection using a surgical knife and needle tip. The dissected cancerous tissue samples were collected and transferred into 300µl of solution with 70% 50mM ammonium bicarbonate and 30% acetonitrile. The lysates were then incubated at 90°C for 30 min followed by 60°C for 120 min to rehydrate the proteins and hydrolyze the crosslinks. The samples were sonicated for 2 min followed by 1 min incubation on ice. The process was repeated two more times. The homogenized samples were incubated at 60°C for 1 h, followed by a second sonication step.
The protein lysates were then digested with sequencing-grade trypsin (Promega, catalog V5113) with a 1:50 ratio at 37°C for 18 h. The digested samples were centrifuged at 1500xg for 10 min and the supernatants were collected. Equal amounts of the digested pancreatic cancer samples from each group of the STS and VLTS patients were pooled together to generate the pooled short-term sample and the pooled long-term sample, respectively. The pooled samples were buffer-exchanged into iTRAQ (Applied Biosystems, catalog 4368879) dissolution buffer (0.5 M triethylammonium bicarbonate). The samples were reduced with 50 mM tris-(2 carboxyethyl)phosphine (TCEP) and blocked with 200 mM methyl methanethiosulfonate (MMTS). The short-term and long-term samples were labeled with iTRAQ reagents of 114 and 117, respectively, and then combined. The combined labeled sample was fractionated with a strong-cation-exchange (SCX) spin column into 3 fractions. The samples were then purified with C18 columns (UltraMicroSpin Column/Vydac C18 silica, The Nest Group, catalog SUM SS18V), dried down and stored in -20°C until mass spectrometric analysis.
Mass spectrometry and proteomics data analysis
Fractionated peptide samples were separated with reverse phase LC and spotted on a MALDI plate using a NanoLC 2-Dimensional HPLC system coupled with a Probot Micro Fraction collector (LC Packing/Dionex). The effluent from the capillary column was mixed with the re-crystallized α-cyano-4-hydroxycinnamic acid matrix solution with 1:1 ratio in a mixing tee then deposited onto a stainless MALDI plate with a 675-format (45 × 15). The spotted samples were calibrated and analyzed by an ABI 4800 MALDI TOF/TOF tandem mass spectrometer (Applied Biosystems) with reflector positive ion mode. Both MS and MS/MS data were acquired with an Nd:YAG laser with 200Hz repetition rate. For MS analysis, 800–4000 m/z mass range was used with 1000 acquisitions per spectrum. A maximum of 20 precursors per spot with minimum signal/noise ratio of 20 were selected for data-dependent MS/MS analysis. The CID (collision induced dissociation) was performed using air as the collision gas and 2500 acquisitions were accumulated for each MS/MS spectrum.
The MS/MS spectra were searched against the International Protein Index (IPI) human protein database (version 3.29) from the European Bioinformatics Institute using GPS software v3.6 (Applied Biosystems) running the Mascot search algorithm (Matrix Science) for peptide and protein identification. Data searches were performed with the following criteria: fixed modifications of iTRAQ labeling on the N terminus and lysine (144.10) and methyl methanethiosulfonate on cysteine (46.01); and differential modification of methionine oxidation (15.99). A 95% confidence interval was used for peptide identification. The iTRAQ quantification was performed using GPS software.
Immunohistochemistry (IHC)
IHC was performed on standard serial sections of the paraffin-embedded PDAC tissue sections and microarrays. Briefly, prior to incubation with the primary antibody, sections were pre-treated using a Borg antigen retrieval solution (Biocare Medical, catalog BD1000 S-250, MM, G1) in a pressure cooker for 15 min at 125°C, followed by cooling at room temperature for 15 min. Thereafter, primary antibody to galectin-1 polyclonal antibody (1:200 dilution; Fitzgerald Industries International, catalog 10R-G100a) was applied at 37°C followed by application of a diaminobenzidine detection kit (Ventana Medical Systems, catalog 760–091). Labeling was performed on an ES automatic immunohisto-chemical stainer (Ventana Medical Systems, catalog N750-BMKU-FS). Results were evaluated independently by two experienced GI-pathologists (MBP and ZL) without knowledge of patient survival information. As further detailed in the results section, galectin-1 expression was essentially restricted to the stromal compartment, with no expression within epithelial cells. Thus, labeling was assessed for the stromal compartment only. The number of stromal cells expressing galectin-1 varied widely between pathologic diagnoses and was scored semi-quantitatively from 0 to 3+. Specifically, cases with labeling of less than 5%, 5–32%, 33–67%, and greater than 67% of stromal cells were scored as 0, 1+, 2+, and 3+, respectively.
| Abbreviations: | ||
| PDAC | = | pancreatic ductal adenocarcinoma |
| VLTS | = | very long-term survivors |
| STS | = | short-term survivors |
| IHC | = | immunohistochemistry |
| IPMN | = | intraductal papillary mucinous neoplasm |
| TMA | = | tissue microarray |
| FFPE | = | formalin fixed paraffin embedded |
Additional material
Download Zip (81 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Ralph H. Hruban for his assistance with histopathology of the Johns Hopkins cases. We are grateful to the Proteomics Shared Resource Lab at Fred Hutchinson Cancer Research Center for the mass spectrometric analysis. This study was support in part with federal funds from the National Institutes of Health under grants R01CA107209, K07CA116296, K25CA137222, R01DK081368, R21CA161575, R21CA149772, P50CA062924, P01CA134292, and R01CA113669; and funding from Mary Ann Walters Pancreatic Cancer Foundation.
Supplementary Material
Supplementary material may be downloaded here:
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010; 7:163 - 72; http://dx.doi.org/10.1038/nrclinonc.2009.236; PMID: 20101258
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al, Groupe Tumeurs Digestives of Unicancer, PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817 - 25; http://dx.doi.org/10.1056/NEJMoa1011923; PMID: 21561347
- Adham M, Jaeck D, Le Borgne J, Oussoultzouglou E, Chenard-Neu MP, Mosnier JF, et al. Long-term survival (5-20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: a series of 30 patients collected from 3 institutions. Pancreas 2008; 37:352 - 7; http://dx.doi.org/10.1097/MPA.0b013e31818166d2; PMID: 18665012
- Bradley EL 3rd. Long-term survival after pancreatoduodenectomy for ductal adenocarcinoma: the emperor has no clothes?. Pancreas 2008; 37:349 - 51; http://dx.doi.org/10.1097/MPA.0b013e31818e9100; PMID: 18953246
- Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg 2004; 198:722 - 31; http://dx.doi.org/10.1016/j.jamcollsurg.2004.01.008; PMID: 15110805
- Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996; 223:273 - 9; http://dx.doi.org/10.1097/00000658-199603000-00007; PMID: 8604907
- Han SS, Jang JY, Kim SW, Kim WH, Lee KU, Park YH. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas 2006; 32:271 - 5; http://dx.doi.org/10.1097/01.mpa.0000202953.87740.93; PMID: 16628082
- Mosca F, Giulianotti PC, Balestracci T, Di Candio G, Pietrabissa A, Sbrana F, et al. Long-term survival in pancreatic cancer: pylorus-preserving versus Whipple pancreatoduodenectomy. Surgery 1997; 122:553 - 66; http://dx.doi.org/10.1016/S0039-6060(97)90128-8; PMID: 9308613
- Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RD, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg 2009; 250:282 - 7; http://dx.doi.org/10.1097/SLA.0b013e3181ae9f93; PMID: 19638918
- Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery 2006; 140:764 - 72; http://dx.doi.org/10.1016/j.surg.2006.04.006; PMID: 17084719
- Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible?. Ann Surg 2008; 247:456 - 62; http://dx.doi.org/10.1097/SLA.0b013e3181613142; PMID: 18376190
- Shimada K, Sakamoto Y, Nara S, Esaki M, Kosuge T, Hiraoka N. Analysis of 5-year survivors after a macroscopic curative pancreatectomy for invasive ductal adenocarcinoma. World J Surg 2010; 34:1908 - 15; http://dx.doi.org/10.1007/s00268-010-0570-9; PMID: 20376443
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011; 364:2128 - 37; http://dx.doi.org/10.1056/NEJMsa1010705; PMID: 21631325
- Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP 2008; 9:99 - 132; PMID: 18326920
- Kim R, Tan A, Lai KK, Jiang J, Wang Y, Rybicki LA, et al. Prognostic roles of human equilibrative transporter 1 (hENT-1) and ribonucleoside reductase subunit M1 (RRM1) in resected pancreatic cancer. Cancer 2011; 117:3126 - 34; http://dx.doi.org/10.1002/cncr.25883; PMID: 21264835
- Carpelan-Holmström M, Nordling S, Pukkala E, Sankila R, Lüttges J, Klöppel G, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut 2005; 54:385 - 7; http://dx.doi.org/10.1136/gut.2004.047191; PMID: 15710987
- Jørgensen MT, Fenger C, Klöppel G, Lüttges J. Long-term survivors among Danish patients after resection for ductal adenocarcinoma of the pancreas. Scand J Gastroenterol 2008; 43:581 - 3; http://dx.doi.org/10.1080/00365520701834943; PMID: 18415751
- D’Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg 2004; 239:400 - 8; PMID: 15075659
- Maire F, Hammel P, Terris B, Paye F, Scoazec JY, Cellier C, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut 2002; 51:717 - 22; http://dx.doi.org/10.1136/gut.51.5.717; PMID: 12377813
- Raimondo M, Tachibana I, Urrutia R, Burgart LJ, DiMagno EP. Invasive cancer and survival of intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol 2002; 97:2553 - 8; http://dx.doi.org/10.1111/j.1572-0241.2002.06022.x; PMID: 12385438
- Allen PJ. Pancreatic adenocarcinoma: putting a hump in survival. J Am Coll Surg 2007; 205:Suppl S76 - 80; http://dx.doi.org/10.1016/j.jamcollsurg.2007.06.331; PMID: 17916524
- Pan S, Chen R, Stevens T, Bronner MP, May D, Tamura Y, et al. Proteomics portrait of archival lesions of chronic pancreatitis. PLoS One 2011; 6:e27574; http://dx.doi.org/10.1371/journal.pone.0027574; PMID: 22132114
- Pan S, Chen R, Reimel BA, Crispin DA, Mirzaei H, Cooke K, et al. Quantitative proteomics investigation of pancreatic intraepithelial neoplasia. Electrophoresis 2009; 30:1132 - 44; http://dx.doi.org/10.1002/elps.200800752; PMID: 19373808
- Dennis G Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003; 4:3; http://dx.doi.org/10.1186/gb-2003-4-5-p3; PMID: 12734009
- Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44 - 57; http://dx.doi.org/10.1038/nprot.2008.211; PMID: 19131956
- Chen R, Pan S, Brentnall TA, Aebersold R. Proteomic profiling of pancreatic cancer for biomarker discovery. Mol Cell Proteomics 2005; 4:523 - 33; http://dx.doi.org/10.1074/mcp.R500004-MCP200; PMID: 15684406
- Chen R, Brentnall TA, Pan S, Cooke K, Moyes KW, Lane Z, et al. Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol Cell Proteomics 2007; 6:1331 - 42; http://dx.doi.org/10.1074/mcp.M700072-MCP200; PMID: 17496331
- Daroqui CM, Ilarregui JM, Rubinstein N, Salatino M, Toscano MA, Vazquez P, et al. Regulation of galectin-1 expression by transforming growth factor beta1 in metastatic mammary adenocarcinoma cells: implications for tumor-immune escape. Cancer Immunol Immunother 2007; 56:491 - 9; http://dx.doi.org/10.1007/s00262-006-0208-9; PMID: 16900348
- Spano D, Russo R, Di Maso V, Rosso N, Terracciano LM, Roncalli M, et al. Galectin-1 and its involvement in hepatocellular carcinoma aggressiveness. Mol Med 2010; 16:102 - 15; http://dx.doi.org/10.2119/molmed.2009.00119; PMID: 20200618
- Zhong LP, Wei KJ, Yang X, Pan HY, Ye DX, Wang LZ, et al. Overexpression of Galectin-1 is negatively correlated with pathologic differentiation grade in oral squamous cell carcinoma. J Cancer Res Clin Oncol 2010; 136:1527 - 35; http://dx.doi.org/10.1007/s00432-010-0810-2; PMID: 20157731
- Chen R, Yi EC, Donohoe S, Pan S, Eng J, Cooke K, et al. Pancreatic cancer proteome: the proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology 2005; 129:1187 - 97; http://dx.doi.org/10.1053/j.gastro.2005.08.001; PMID: 16230073
- Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology 2006; 16:137R - 57R; http://dx.doi.org/10.1093/glycob/cwl025; PMID: 16840800
- Bian CF, Zhang Y, Sun H, Li DF, Wang DC. Structural basis for distinct binding properties of the human galectins to Thomsen-Friedenreich antigen. PLoS One 2011; 6:e25007; http://dx.doi.org/10.1371/journal.pone.0025007; PMID: 21949831
- Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 2004; 5:241 - 51; http://dx.doi.org/10.1016/S1535-6108(04)00024-8; PMID: 15050916
- Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res 2010; 70:6216 - 24; http://dx.doi.org/10.1158/0008-5472.CAN-09-4150; PMID: 20647324
- Thijssen VL, Poirier F, Baum LG, Griffioen AW. Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood 2007; 110:2819 - 27; http://dx.doi.org/10.1182/blood-2007-03-077792; PMID: 17591944
- Gál P, Vasilenko T, Kostelníková M, Jakubco J, Kovác I, Sabol F, et al. Open Wound Healing In Vivo: Monitoring Binding and Presence of Adhesion/Growth-Regulatory Galectins in Rat Skin during the Course of Complete Re-Epithelialization. Acta Histochem Cytochem 2011; 44:191 - 9; http://dx.doi.org/10.1267/ahc.11014; PMID: 22096259
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004; 432:332 - 7; http://dx.doi.org/10.1038/nature03096; PMID: 15549095
- Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem 2007; 101:887 - 907; http://dx.doi.org/10.1002/jcb.21209; PMID: 17266048
- De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 2008; 123:2229 - 38; http://dx.doi.org/10.1002/ijc.23925; PMID: 18777559
- De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol 2003; 200:429 - 47; http://dx.doi.org/10.1002/path.1398; PMID: 12845611
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol 1997; 32:141 - 74; http://dx.doi.org/10.3109/10409239709108551; PMID: 9145286
- Cui X, Song B, Hou L, Wei Z, Tang J. High expression of osteoglycin decreases the metastatic capability of mouse hepatocarcinoma Hca-F cells to lymph nodes. Acta Biochim Biophys Sin (Shanghai) 2008; 40:349 - 55; http://dx.doi.org/10.1111/j.1745-7270.2008.00392.x; PMID: 18401533
- Rucci N, Rufo A, Alamanou M, Capulli M, Del Fattore A, Ahrman E, et al. The glycosaminoglycan-binding domain of PRELP acts as a cell type-specific NF-kappaB inhibitor that impairs osteoclastogenesis. J Cell Biol 2009; 187:669 - 83; http://dx.doi.org/10.1083/jcb.200906014; PMID: 19951916
- Holcomb B, Yip-Schneider M, Schmidt CM. The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy. Pancreas 2008; 36:225 - 35; http://dx.doi.org/10.1097/MPA.0b013e31815b3207; PMID: 18362834