Abstract
The EGFR (epidermal growth factor receptor) a member of the family of transmembrane protein kinase receptors known as the erbB family shows a significant correlation with the presence of metastases and poorly differentiated oral cancer. Aim of the present work is to define the key-role of EGFR in oral cancer prognosis. We have analyzed the EGFR expression on 149 cases of oral squamous cell cancers (OSCC) and we have found that it was poorly expressed in normal oral epithelium, but its expression was significantly increased in OSCCs. Moreover, we have recorded that both pEGFR-Tyr 845 and pEGFR-Tyr 1068 were mainly distributed in high histological grading and in advanced stages. Western blotting has confirmed the total absence of EGFR phosphorylation in normal oral epithelium and the higher level of protein phosphorylation in representative cases of OSCCs. The EGF-R amplification was found by fluorescence in situ hybridization (FISH) in 14% of OSCC; interestingly, EGF-R amplification was mainly observed in OSCC with higher histological grading (G2 and G3) and advanced stage (pT4) sub-groups. Kaplan-Meyer survival analysis suggested that patients with positive pEGFR-Tyr 845 tumors had a worse prognosis and were bad responders to chemotherapy. These results confirm the central role of EGF-R activation status as a prognostic biomarker in OSCC.
Keywords: :
Introduction
Oral Squamous cell carcinoma (OSCC) remains a significant cause of morbidity and mortality, with approximately 540,000 in the Western Countries.Citation1 Despite recent advances in cancer treatment, survival rate for oral cancer has not changed significantly over the last four to five decades.Citation2,Citation3 The understanding of the genetic alterations and protein expression profiling in these tumors might reveal new prognostic factors that accurately predict the biological behavior of the disease and allow for a more accurate prognostic characterization of individual tumors.Citation4 In addition, these markers might lead to individually targeted therapeutic approaches specifically designed to inhibit several biochemical events in the pathogenesis of this cancer.Citation5-Citation7 The EGFR (epidermal growth factor receptor) is a member of the family of transmembrane protein kinase receptors known as the erbB or HER receptor family: EGFR (HER1 or erbB1), erbB2 (HER2), erbB3 (HER3) and erb4 (HER4). The EGFR is codified by a gene localized in locus 7p11.2 of chromosome 7.Citation8 Upon binding of specific polypeptide ligands, including EGF, transforming growth factor-α, beta-cellulin, heparin-binding EGF, epiregulin, and amphiregulin, EGFR undergoes homo- or hetero-dimerization and activation of its intrinsic tyrosine kinase activity.Citation9,Citation10 The primary risk factors of OSCC are tobacco, smoking, and elevated levels of alcohol consumption. Other potential risk factors include diet, human papillomavirus (particularly HPV16), and various oral factors, including oral hygiene.Citation11 In fact, high levels of colonization of OSCC by facultative oral streptococci were observed in the saliva of OSCC subjects.Citation12,Citation13 More recently, viable bacteria have been isolated from both superficial and deep parts of OSCC,Citation14 revealing that the tumor microenvironment is well suited for bacterial survival. The role of bacteria in the development of oral cancer has not been delineated, but the persistent presence of bacteria at tumor sites in the oral cavity raises intriguing questions about the role of bacteria in the progression of OSCC. The phosphorylation of EGFR activates multiple biological processes including apoptosis, differentiation, cellular proliferation, motility, invasion, adhesion, DNA repair and survival.Citation15 Several strategies have been developed to inactivate the EGFR pathway including monoclonal antibodies against the extracellular domain of EGFR.Citation16-Citation18 Expression of EGFR varies widely in several tumors, including head and neck (80–100%). In human tumors, high expression of EGFR correlates with a more aggressive clinical course, and has been reported to be a useful diagnostic and prognostic marker. In recent years, EGFR has been considered a promising target for monoclonal antibody therapy, and, in particular recently performed clinical trials have established the clinical importance of administer monoclonal antibodies against EGFR together with chemotherapy or radiotherapy in the treatment of advanced head and neck cancers.Citation19-22 However, these treatments are potentially toxic and, as for today, there are not established criteria to distinguish responsive patients from non-responders.Citation23 The activation status of EGFR is determined by its autophosphorylation rate that is responsible for the triggering of the downstream signaling cascade. Five autophosphorylation sites have been identified in vivo in EGFR including Tyr1068.Citation24 Tyr 1068 is a classic RTK auto-phosphorylation site and potentiates docking of growth factor receptor binding protein Grb1 to EGFR, with subsequent activation of MAPK\extracellular signal-regulated kinase and phosphoinositide 3 kinase\AKT signaling cascades, respectively.Citation25,Citation26 These events lead to the autophosphorylation of multiple tyrosine residues in the COOH-terminal tail of the molecule that serve as binding sites for cytosolic signaling proteins containing Src homology 2 (SH2) domains and phosphotyrosine binding domains. Previous results demonstrated an association between c-Src and EGFR that results in the appearance of two tyrosine phosphorylations, one of which is Tyr 845.Citation27 Accumulating evidences indicate that c-Src-mediated phosphorylation of EGFR Tyr 845 is involved in regulation of receptor function, as well as in tumor progression.Citation28,Citation29 Src is overexpressed in a high percentage of human neoplasms, including head and neck cancer, and its deregulation is identified as one of the major oncogenic signatures found in cancer.Citation30,Citation31 Early studies on cells show that cells transiently expressing EGFR baring a Tyr to Phe mutation at Tyr 845 are impaired in their ability to synthesize DNA in response to EGF, suggesting that this c-Src mediated phosphorylation site is important for receptor function. Accumulating evidences indicate that interactions between the EGFR and the non receptor tyrosine kinase c-Src may contribute to an aggressive phenotype in multiple human tumors. Aim of the work was to evaluate EGFR expression and its phosphorylation in Tyr 845 and Tyr 1068 by immunohistochemistry together with EGFR amplification by Fluorescence in situ Hybridization (FISH) in prognostic tissue microarrays (TMA) of paraffin-embedded tissue specimens from 149 patients who underwent surgical treatment for squamous cell carcinoma of oral cavity in the period between 1996–2007.
Results
Clinical characteristics of the patients
The OSCC-149 tissue microarray contained representative tumor samples from 149 patients affected by OSCC. Clinical and pathological data of the retrospectively studied population have been reported in . Interestingly, adjuvant radiotherapy has been used in 110 (74%) patients and adjuvant chemotherapy in 45 patients (30%); finally, only 23 (15%) patients in this cohort did not received adjuvant therapy. The criteria employed to administer adjuvant therapy had been exclusively clinical, as mentioned in Materials and Methods. TMA technology has allowed high throughput immunohistochemical analysis of archival material and in particular FISH technology has been used in order to evaluate the percentage of EGFR gene amplification in the studied OSCC cases, comparing gene amplification with immunohistochemical EGFR expression (EGFR, pEGFR-Tyr 845, and pEGFR-Tyr 1068).
Table 1. Clinic-pathological characteristic of study population
EGFR expression and phosphorylation is associated with worse grading and prognosis of OSCC
EGFR is expressed in normal epithelium at low levels and in particular in the basal and supra-basal proliferating layers (); as expected, EGFR is highly over-expressed in OSCCs, both in membrane and in cytoplasm, and its staining is associated with high graded and advanced staged tumors. Representative IHC findings of EGFR, pEGFR-Tyr 1068 and pEGFR-Tyr 845 can be observed respectively in and . We also observed discrete nuclear immunoreactivity of EGFR as the functional roles of nuclear EGFR were extensively studied in recent reports. Regarding immunohistochemistry for pEGFR-Tyr 845 and pEGFR-Tyr 1068 only percentage of cell staining has been evaluated, irrespective of the sub-cellular localization. The frequencies of OSCC showing phosphorylation of EGFR on Tyrosine 845 and on Tyrosine 1068 can be observed in : both phosphorylations are mainly distributed in high histological degrees and in advanced stages. In fact, 83 (56%) cases were positive for pEGFR Tyr 845 and 37 (25%) for pEGFR Tyr 1068. Moreover, among pEGFR Tyr 845 positive OSCCs, 80% were Grade 2-3 tumors (67 cases) and 42% stage 4 (35 cases) while, among pEGFR Tyr 1068 positive cases, 71% (26 cases) were G2-3 tumors and 39% (14 cases) were stage 4 cancers. These data suggested that higher EGFR phosphorylation is correlated with more advanced OSCC grading and staging. We have also evaluated EGFR expression and phosphorylation by western blotting of some representative cases of OSCCs and relative normal mucosal tissues. We have found an increased expression of EGFR in all the examined OSCCs that was however expressed at significant levels also in normal mucosa. These findings were paralleled by the total absence of EGFR phosphorylation in normal oral epithelium while high levels of protein phosphorylation of both evaluated tyr sites were observed in all representative cases of OSCCs (). Interestingly, these OSCC cases had trisomic and amplified EGFR (see below).
Figure 1. EGFR expression in normal peri-tumor oral epithelium as evaluated by immunohistochemistry and in representative cases of OSCC’swith amplified EGFR. (A) EGFR shows a high expression limited to the basal proliferative layer, whereas spinous epithelial layer demonstrated faint-intermediate expression at membrane malpighian bridges ( LSAB-HRP, original magnification x63, inset original magnification x100). (B) Photos A and B show two different cases with strong EGFR expression. Amplified cases showed very strong circumferential staining of the membrane that appeared remarkable thickened; in addition, cytoplasms were stained (A1 and B1) and in some fields (A1) the nuclei appeared positive (LSAB-HRP, nuclear counterstaining with haematoxylin; Ventana pre-diluted Ab not specific for activating phosphorylations).
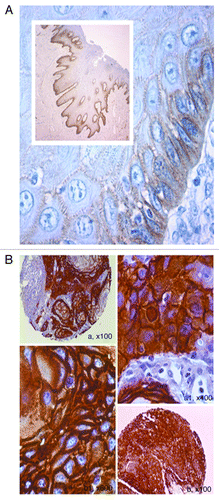
Figure 2. Phosphorylated EGFR-p-tyr1068 and p-tyr845 in oral cancer as evaluated by TMA-based immunohistochemistry. (A) A TMA core of positive p-EGFR-tyr 1068 is showed in (A), at larger magnification in (B), demonstrating strong cytoplasmic expression of the activated receptor (LSAB-HRP, nuclear counterstaining with haematoxylin). (B) Phosphorylation of EGFR on Tyrosine 845 in oral cancer as evaluated by TMA-based IHC. A, A1, A2 show high cytoplasmic expression of p-845 EGFR in a representative case of OSCC with vascular invasion. B, B1, B2 show p-845 EGFR in a case of OSCC with poor differentiation (A, A1, A2, B, B1, B2: IHC- LSAB-HRP, nuclear counterstaining with haematoxylin; phosphorylated tyr845 EGFR Ab).
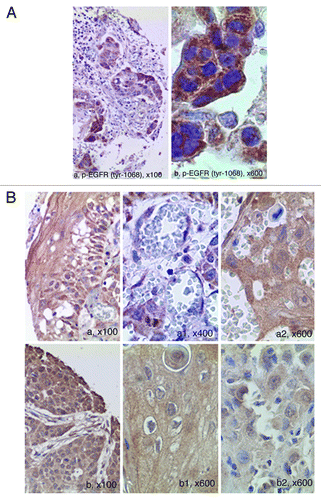
Table 2. Clinic-pathological characteristics of pEGFR Tyr 845/1068 positive and negative OSCCs
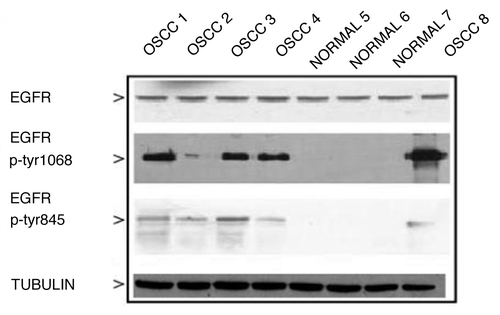
Figure 3. Western blotting of representative normal epithelia, EGFR-trisomic and EGFR-amplified OSCC’s using phosphorylated Tyr 845 EGFR antibody. Both trisomic and amplified OSCC’s show EGFR phosphorylation on both Tyr sites, whereas normal epithelia are negative. For further details see “Materials and Methods” section.
Amplification of EGFR correlates with EGFR overexpression and advanced grading and staging of OSCCs
To more comprehensively study the relevance of the EGFR amplification in OSCC (), the protein levels of EGFR were investigated by immunohistochemistry on the same tissue slides used for FISH analysis. Overall, no evidence of gene amplification was found on chromosome 7 in any samples of normal oral epithelia. Tumor tissues with higher EGFR immunointensity had higher FISH signal ratios. In the same way, cases with amplified EGFR showed very strong circumferential staining of the membrane that appeared remarkable thickened; in addition, cytoplasms were diffusely stained and in some fields the nuclei appeared positive. Polysomic cases showed medium-strong immunohistochemical staining with frequent membrane staining interruptions. Therefore, EGFR immunoreactivity might serve as a surrogate marker to predict EGFR amplification in OSCC. Frequencies of gene amplification according to pathological correlations has been reported in . EGFR gene amplification was found in 21 (14%) OSCCs in our series; OSCCs with amplified EGFR were mainly distributed in higher histological degree (G2-G3, 20 cases, 97%) and advanced stage (ST4, 15 cases, 67%) OSCCs. We have also assessed the correlations among EGFR immunohistochemical expression, gene amplification as evaluated by FISH, and pathological findings. Interestingly, women have higher percentage of EGFR expression in euploid not amplified tumors, but lower percentage in polysomic and/or amplified cancers.. In not EGFR amplified OSCCs EGFR over-expression is more evident in advanced and undifferentiated tumors, while in polysomic and amplified cancers this correlation is lost.
Figure 4. FISH analysis for the detection of EGFR amplification. (A) An OSCC showing euploid chromosome 7 and not amplified EGFR; (B) a case of OSCC showing triploid chromosome 7 and three copies of EGFR; (C) a case of OSCC demonstrating aneuploidy at chromosome 7 and multiple copies of EGFR (polysomic not amplified EGFR); (D) a case of OSCC showing euploid chromosome 7 and multiple copies of EGFR visualized as nuclear clusters (amplified EGFR gene) [FISH: LSI EGFR Dual-Color Probe-Hyb Set, LSI EGFR Spectrum Orange/Cep-7 Spectrum Green; DAPI II (4,6-diamino-2-phenyindole-2-hydrochloride) was used for chromatin counterstaining; a ratio of LSI EGFR Spectrum Orange/Cep-7 Spectrum Green >2 has been considered as amplified; for further details see Materials and Methods].
![Figure 4. FISH analysis for the detection of EGFR amplification. (A) An OSCC showing euploid chromosome 7 and not amplified EGFR; (B) a case of OSCC showing triploid chromosome 7 and three copies of EGFR; (C) a case of OSCC demonstrating aneuploidy at chromosome 7 and multiple copies of EGFR (polysomic not amplified EGFR); (D) a case of OSCC showing euploid chromosome 7 and multiple copies of EGFR visualized as nuclear clusters (amplified EGFR gene) [FISH: LSI EGFR Dual-Color Probe-Hyb Set, LSI EGFR Spectrum Orange/Cep-7 Spectrum Green; DAPI II (4,6-diamino-2-phenyindole-2-hydrochloride) was used for chromatin counterstaining; a ratio of LSI EGFR Spectrum Orange/Cep-7 Spectrum Green >2 has been considered as amplified; for further details see Materials and Methods].](/cms/asset/4581b544-a85e-4a69-b103-0436ad1a45d4/kcbt_a_10920991_f0004.gif)
Table 3. Frequencies of FISH amplification according to pathological correlations
EGFR activating phosphorylations and survival analysis
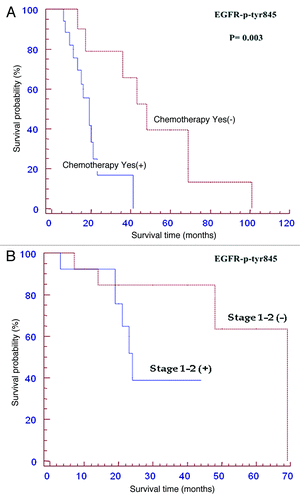
Clinical outcome of the patients showing EGFR activating phosphorylations compared to the respective phosphorylation negative cases has been studied by Kaplan-Meier curves; these topics are reported in and . The survival curves showing percentage survival according to EGFR-p-Tyr 845 in males and females (), and EGFR-p-Tyr 1068 in males and females () were not statistically significant. In fact, the median OS of positive EGFR-p-Tyr 845 males was 59 months (CI 0.37-1.81; p = 0.836) vs 69 months of negative patients (). However, inside females affected by OSCC with EGFR-p-Tyr 845 negative staining a trend for a better survival was observed (median OS: 69 months) if compared to the patients positive for EGFR-p-Tyr 845 (median OS: 24 months, CI: 0.80-13.62; p = 0.098) (). The median OS of positive EGFR-p-Tyr 1068 male patients was 33 months (CI: 0.37-1.79, p = 0.812) vs 59 months of negative patients (). The median OS of positive EGFR-p-Tyr 1068 female patients was 46 months (CI: 0.12-5.07, p = 0.78) vs 48 months of negative patients (). A significant result of the present study is the demonstration that the survival of patients with early tumor stages (stage 1 and 2) and of those subjected to adjuvant chemotherapy can be predicted by the phosphorylation status of EGFR-Tyr 845. In fact, the median OS of the patients subjected to adjuvant chemotherapy and positive for EGFR-Tyr 845 was 19 months (CI: 1.66-13.15, p = 0.003) vs 48 months of negative patients (). The median OS of the early staged tumors (T1-T2) positive for EGFR-Tyr 845 was 59 months (CI: 1.01-10.16; p = 0.048) vs 69 months of negative patients (). No correlation was found between the phosphorylation status of EGFR and the lymph nodal status of disease. The study of survival for pEGFR-Tyr 1068 has given rise to negative statistical results as regard to the clinical outcome (data not shown). All the remaining correlations with other clinical and pathological factors were all not significant (data not shown).
Figure 5. Kaplan Meier curvesof patients positive for p-Tyr 845 and 1068EGFR stratified for gender. Survival curves showing percentage survival according to sex and EGFR-p-Tyr 845 (A, B), and EGFR-p-Tyr 1068 (C, D). Interestingly, inside the group of females affected by OSCC the EGFR-p-Tyr 845 negative subgroup showed a trend for a better survival if compared to the EGFR-p-Tyr 845 positive subgroup (for details, see the text).
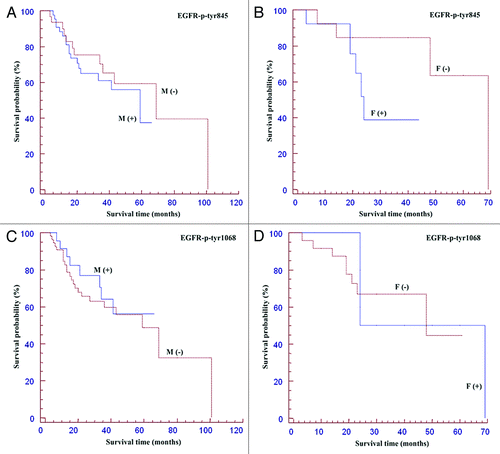
Figure 6. Kaplan Meier curves of patients positive forp-Tyr-845 EGFR expression subjected to adjuvant chemotherapy (A) or with early stage tumours (B). Among patients receiving adjuvant chemotherapy p-Tyr 845 negative cases had a better survival (p<0.05). Among T1-T2 tumors p-Tyr 845 negative cases had a better survival (p<0.05). See the text for details.
Discussion
OSCC represents one of the major health issues, with over 540,000 new cases reported in Western Countries annually.Citation31-Citation35 Though improvements in screening and early diagnosis have dramatically reduced the incidence of this neoplasm in recent years, the 5-year-disease-free survival is still poor, despite the great scientific and financial efforts. For many years, the main prognostic factors of OSCC have been the conventional grading, staging and site of tumor. The molecular mechanisms involved in the oral carcinogenesis are not yet fully understood and the complete genetic profile of the cancer cells is still to be characterized. Recent reports show a significant correlation among the amplification status of EGFR, and both the presence of metastases and poorly differentiated tumors. Therefore, EGFR over-expression in OSCC has been suggested as a valuable prognostic marker for shortened survival and metastatic spread.Citation36,Citation37 On the other hand, current literature on prognostic role of EGFR has been limited by small series of cases characterized by low time of follow-up and lacking of complete and detailed information about diagnosis and therapy. The present retrospective study investigates about the correlation among EGFR expression, EGFR phosphorylation, amplification or polysomic status and the clinical and pathological characteristics in a large series of oral cancers of different grade and stage, by combining different types of molecular diagnostic methods, as immunohistochemistry, FISH and Western Blotting on TMA. TMA are a high-throughput technology that allows the simultaneous in situ analysis of a large number of tumors at the protein, DNA and RNA levels using immunohistochemistry or in situ hybridization techniques.Citation38-Citation40 In the recent years molecular technologies such as Fluorescent in situ Hybridization (FISH) has become an essential tool in the diagnosis and management of a variety of solid tumors and hematologic malignancies in the clinical setting, as well as an aid in the identification of particular genetic disorders. FISH yielding information about chromosomal changes can also be considered an innovative method of choice to select patients for individualized targeted cancer therapies. In our study, combining different molecular techniques and morphological/histological information, the key role of EGFR and its phosphorylated forms has been brought out. Amplification of 7p11.2 is frequently found in many cancer types, including lung and breast cancer, and glioblastomas. Our study showed that the 7p11.2 amplification and the centromere polysomy were the most common DNA copy number change also in OSCC and provided cogent evidence that EGFR was the key gene contributing to OSCC development. We identified amplification and polysomic status in OSCC tissues, by validated FISH analysis, a sensitive and specific method of assessing gene copy number, on a large series of samples (n = 149). By comparing genetic contents and expression/phosphorylation levels, we concluded that the key ‘‘cancer-driving’’ gene, EGFR plays an important role in oral cancer development. The present study confirms that high EGFR expression is present in OSCCs and that an uncontrolled tumor growth may be mediated by abnormal EGFR expression. EGFR expression extent and intensity scores suggest that EGFR expressing carcinomas display pathological features of more aggression which may be attributable to the activation of different signaling pathways that control diverse biological processes. As reported by other investigators, EGFR expression involved all epithelial layers in OSCC specimens while in normal oral epithelia it was localized to the basal cell layer. Similar results were reported by other investigators. Since the squamous epithelium maintains a continuous physiological regeneration in normal conditions, it is reasonable that the basal cells interpret signals of EGF by binding to EGFR, while its expression beyond basal localization in cancerous tissue suggests that a correlation between EGFR and tumor progression may exist. The expression was mainly localized to the peripheries of highly undifferentiated tumor nests. This finding confirms the presence of this receptor on more undifferentiated cells and explains that the staining reaction varies with cellular differentiation. Moreover, it may explain that peripheral tumor cells receive a signal from EGF resulting in the additional proliferation of cancer tissues. Other aim of our work has been to assess whether heterogeneity of EGFR immunoreactivity in OSCC is related to non-standardized criteria for staining evaluation. Different methods of immunohistochemical evaluation led to different results, strengthening the need for standardization, especially against a background of rapidly evolving EGFR targeted cancer treatment strategies. In numerous immonohistochemical studies, EGFR staining has been observed in the cytoplasm and in nucleus as well as in the membrane, but the subcellular localization of EGFR has so far received little attention.Citation41,Citation42 In a series of benign and malignant skin tumors, loss of membrane staining and increased cytoplasmic accumulation of EGFR were observed in malignant cutaneous epithelial tumors when compared to normal tissue. Furthermore, this alteration in EGFR distribution was predominant in less well-differentiated tumors, indicating increased cytoplasmic localization with increasing malignancy.Citation43 Some authors showed that strong cytoplasmic EGFR staining was significantly associated with extra-thyroidal growth of the primary papillary carcinoma and related to decreased recurrence-free survival in surgically treated tumours.Citation44 Cytoplasmic staining has also been associated with high renal tumour stage and high renal tumor grade. It has been suggested that the cytoplasmic overexpression of EGFR plays a significant role in the progression of pancreatic ductal adenocarcinoma, especially in the invasion and acquisition of aggressive clinical behavior. Taken together, the available information concerning EGFR expression and subcellular localization are consistent with an aberrant function of the EGFR located in the cytoplasm. Our results are consistent with the existent model of translocation of membranous EGFR to the cytoplasm and afterwards to the nucleus acting as a transcriptional factor and fostering cell proliferation. The exact mechanism by which cell-surface EGFR translocates into the cytoplasm and the cell nucleus remains largely unknown. EGFR co-localizes and interacts with importins α1/β1, carriers that are critical for macromolecules nuclear import. EGFR variant mutated at the nuclear localization signal (NLS) is defective in associating with importins and in entering the nuclei indicating that EGFR's NLS is critical for EGFR/importins interaction and EGFR nuclear import. Moreover, our data suggest that a critical factor determining the prognosis of cancers is the activation status of EGFR. In fact, we have found that pTyr 845 is correlated with a worse prognosis in early stage OSCCs and in tumors subjected to adjuvant chemotherapy. Tyr845 is a known target of c-SrcCitation45,Citation46 and c-Src has been correlated with the progression and poor prognosis of OSCC. In fact, it has been recently reported that CD133 (a stem cell marker) via Src activation mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer.Citation47 Moreover, high expression of Src protein (labeling indices >50%) was correlated with larger tumor size (p = .017), positive lymph node metastasis (p = .030), more advanced clinical stages (p = .007), and recurrence (p < .001) of OSCC.Citation48 Our data suggest that the detection of phosphorylation in Tyr 845 of EGFR could be a surrogate marker of activation of Src in OSCC and could have a role in predicting poor prognosis in the early stages discriminating patients who require a more aggressive therapeutic strategy despite the initial extension of the disease. This is still more important if we consider that choose of the best treatment strategy is presently based exclusively on the grading and staging of OSCC. Finally, we have also found that pTyr 845 EGFR can be a predictive marker of resistance to chemotherapy in OSCC. This finding discloses a new scenario in which the use of Src inhibitors such as dasatinib should be explored in the treatment of pTyr845 EGFR positive OSCCs. In fact, it was recently demonstrated in preclinical models that the simultaneous inhibition of Scr and Met signaling can be an attractive and effective new strategy in the treatment of OSCC.Citation49 HPV infection plays an important role in pathogenesis of OSCC as recently reviewed by our group.Citation50 Moreover, there is according evidence that HPV positive cases are more responsive to chemo- and radiation treatments whereas HPV negative are associated with worse prognosis and need further research to detect future targeted treatments. However, the cohort of cases analyzed in this study is mainly constituted by HPV negative cases. In the present study we have analyzed only cancers of the oral cavity carefully excluding the oropharyngel cancers that, in turn, show a very high HPV incidence. In fact, consecutive slides of TMA used for EGFR and p-EGFR were studied by p-16 immunohistochemistry, and in situ hybridization in order to detect LR-HPV and HR-HPV and they resulted HR-HPV negative.Citation51 Further studies based on consensus PCR to detect HR-HPV confirmed that the cases were HPV negative. This finding is in agreement with some Italian retrospective studies showing a very low frequency of HPV in squamous cell carcinoma limited to the oral cavity.Citation52 Since the cohort of OSCC cases studied by TMA was mainly HPV negative the percentage of cases showing EGFR amplification was lower than that reported in other studies.
In conclusion, the search for molecular prognostic markers for cancer is still a major clinical and therapeutic issue. The determination of the expression of Ghrelin, type I interferons and IGF-I may be important prognostic markers and promising approaches for novel treatment strategies, even if the literature characterizes better the role of these markers in neuroendocrine tumors rather than squamous.Citation53-Citation56 However, detailed studies will be required for better understanding of the complex mechanism of carcinogenesis relating to OSCCs and to improve chemoradiotherapy through the discovery of new therapeutic markers. Our data lead support to the need of detecting the expression of EGFR and its phosphorylated isoforms in clinical practice and diagnostic management of OSCCs.Citation57 The study of EGFR Tyr phosphorylation could be also a useful surrogate marker of activation of downstream signal transduction pathways in the cell and could be helpful in guiding the therapeutic decisions in OSCC patients. In fact, target-based therapies are widely accepted as the future of cancer treatmentCitation58,Citation59 and detection of EGFR status in oral cancer patients may identify patients who will benefit from the use of new anti-cancer agents.Citation60,Citation61
Materials and Methods
Study cases
Paraffin blocks from OSCC resections were retrieved from the archives of National Cancer Institute of Naples. A single block for each case was selected for use in the construction of the tissue microarray (TMA). Tissues from 149 cases were included, representing different clinical and pathological categories. Clinical information including demographic, therapeutic and clinical outcome variables were retrieved from patient medical records and were considered for correlation analysis (). This tissue microarray has been named OSCC-149. The study was approved by the Research Ethics Boards of Fondazione ‘G.Pascale’. All the patients included in the OSCC-149 have been treated according to standard diagnostic and therapeutic criteria. Briefly, for oral cancers trans-oral resections plus sentinel lymphadenectomy have been performed for T1-2/N0 while resection of primary plus neck lymphadenectomy (levels I-IV; for any T with N+, resection of T plus neck lymphadenectomy) have been made for T3-4/N0. For maxillary and ethmoidal tumors of any T, total maxillectomy or extended maxillectomy was performed while for the same tumors of any T with N+ total maxillectomy or extended maxillectomy plus neck lymphadenectomy (levels I-IV) was made. Adopted criteria for adjuvant therapy after primitive cancer removal and neck dissection were the following: 1) positive margins or close (<5 mm); 2) T3-T4 primary tumor; 3) vascular invasion; 4) perineural invasion; 5) N>1 (more than one positive lymph-node including micrometastasis and extracapsular spreads).
Tissue microarray based immunohistochemistry
For tissue microarray construction, areas of interest rich in non-necrotic tumoral cells were identified on corresponding haematoxylin and eosin-stained sections and marked on the source paraffin block. The source block was cored and a 0.6 mm core transferred to the recipient master block using Galileo TMA CK 3500 Tissue Microarrayer (ISE TMA Software, Integrated System Engineering). Moreover, two cores from different areas (a superficial one and one representative of the deep invasion) and, whenever possible, one core of normal mucosa of the same tissue block were arrayed for each case. All the donor cores were formatted into one recipient block. H&E staining of a 4-µm TMA section was used to verify all samples. Immunohistochemical analysis on 4-µm TMA serial sections was performed by using Ventana Benchmark® XT autostainer and/or manual standard linked streptavidin-biotin horseradish peroxidase technique (LSAB-HRP), according to the best protocol for each antibody used in our laboratory: pre-diluted primary anti-EGFR (clone3C6); primary anti-p-EGFR Tyr 1068 (code2234 phosphoTyr 1068-EGF receptor, Cell Signaling) diluted 1:400 in PBS and primary anti-p-EGFR Tyr 845 (code2231 phosphoTyr 845-EGF receptor Cell Signaling) diluted 1: 400 with PBS and incubated overnight. Negative control slides without primary antibodies were included for each staining. The results of the immunohistochemical staining were evaluated separately by two observers (RF, GB). In each tissue section 10 representative high power fields (HPFs) were analyzed at optical microscope (OLYMPUS BX41, at x40) and were selected for EGFR positive tumor cells with an average of 1,000 tumor cells per case and 200 tumor cells per field. The topographical staining pattern was also evaluated and recorded as membranous (M), cytoplasmic (C), or mixed, and nuclear (N). For each case, the cumulative percentage of positive cells among all sections examined was determined. Since till now there is not standardized criteria for EGFR staining evaluation, we have chosen to grade and score the extent of EGFR immunostaining as follows: 0 points for negative staining of the considered cells, (1) <10%, (2) 10-50%, (3) 51-80% and (4) ≥ 80% positive staining of the considered cells. The intensity of staining was scored as 0, no staining; +, weak; ++, moderate; +++, strong. For pEGFR-Tyr 845 and pEGFR-Tyr 1068 immunohistochemical evaluation, we selected a dichotomized indicator variable. In order to determine the best logical cut-off point for the presence and absence of expression, we used a specific model formally known as the Martingale residuals. In this way we have established a cut-off point at 5%: the cells were considered positive when ≥5% of them showed a cytoplasm staining, and negative when no staining was observed or <5% of cells stained for the marker. Inter-rate reliability between the two investigators blindly and independently examining the immunostained sections was assessed by the Cohen’s K test, yielding K values higher than 0.70 in almost all instances.
Tissue microarray based FISH
The interphase FISH was performed on representative sections of Prognostic OSCC TMA. The sections were cut onto positively charged slides at 5µm thickness. Deparaffinization of sections was carried out with two 10 m immersion in bio-clear, followed by three 3 m immersion in ethanol 100%, 70%, 50%. The slides were rinsed in distilled water by immersing the slides in citrate buffer (pH 6) for 15 m at 90°C. The slides were then rinsed in distilled water for 5 m for two times. The slides pre-treatment and protease incubation were performed according the manufactures illustrated in datasheet of Vyses (paraffin pre-treatment reagent kit II). The used probes were the commercial LSI EGFR Dual-Color Probe-Hyb Set (Vysis/Abbott Molecular) LSI EGFR Spectrum Orange/Cep-7 Spectrum Green in order to simultaneously visualize EGFR gene and chromosome 7 copy number according to manufacturer’s instructions. DAPI II (4,6-diamino-2-phenyindole-2-hydrochloride) was used for chromatin counterstaining. The fluorescence signals (orange for LSI EGFR, green for Cep-7 and blue for nuclear chromatin) were evaluated under epifluorescence microscope (Olympus). Image acquisition was done by CCD microscopy camera (Olympus). Signals were evaluated by two independent evaluators (RF, GB) scoring at least 100 interphase nuclei in four different high power fields (HPF). The FISH results were scored as follow: specimens with the ratio LSI EGFR/CEP-7≥2.0 were considered as amplified; polysomic were considered cases showing three or more CEP-7 signals per cell in more than 30% of the evaluated cells.Citation31,Citation32
Tissue microarray based Western Blotting
Cancer cells and normal epithelial tissues have been microdissected from the donor blocks punching two cores of 1 mm each from the same fields selected for IHC and FISH with the help of H&E stained slides. Expression levels of EGFR, pEGFR-Tyr 1068 and pEGFR-Tyr 845 proteins were determined by immunoblotting,Citation33 using anti pEGFR-Tyr 1068 and pEGFR-Tyr 845 antibodies. Representative cases of normal epithelia together with trisomic and amplified EGFR OSCC have been selected for this analysis. All the donor cores were dewaxed in xylene.
Statistical analysis
The data were analyzed by the Stanton Glantz statistical software 3 (MS-DOS) and Graph Pad Prism software version 4.00 for Windows (Graph Pad software). Differences between the groups were determined using the one-way analysis of variance (ANOVA) and the Student-Newman-Keuls test. Only p values < 0.05 were considered significant. Disease free interval (DFI) and overall survival (OS) were calculated from the date of pathological diagnosis to the date of recurrence or death or the last event. Estimates of the survival probability were obtained using Kaplan-Meier method, by Chi-square test and reporting values of the Hazard Ratio, at 95% CI and the median survival in months.
Caudell JJ, Sawrie SM, Spencer SA, Desmond RA, Carroll WR, Peters GE, et al. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys 2008; 71:676 - 81; PMID: 18355979
| Abbreviations: | ||
| EGFR | = | epidermal growth factor receptor |
| OSCC | = | oral squamous cell cancers |
| FISH | = | fluorescence in situ hybridization |
| SH2 | = | Src homology 2 |
| TMA | = | tissue microarrays |
| IHC | = | immunohistochemistry |
| OS | = | overall survival |
| NLS | = | nuclear localization signal |
| HPFs | = | high power fields |
| LSAB-HRP | = | streptavidin-biotin horseradish peroxidase technique |
| DFI | = | Disease free interval |
| DAPI II | = | 4,6-diamino-2-phenyindole-2-hydrochloride |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
First of all, we thank all patients or their relatives for their volun- tary participation in this study. Then, a particular thanks to the financial support by Fondazione Banca del Monte Foggia-Italy and Associazione Italiana Ricerca sul Cancro (AIRC). Michele Caraglia received a grant from MIUR PRIN 2009 and FIRB Programma quadro 2011.
References
- Son YH, Kapp DS. Oral cavity and oropharyngeal cancer in a younger population. Review of literature and experience at Yale. Cancer 1985; 55:441 - 4; http://dx.doi.org/10.1002/1097-0142(19850115)55:2<441::AID-CNCR2820550225>3.0.CO;2-5; PMID: 3965099
- Chen YJ, Lin SC, Kao T, Chang CS, Hong PS, Shieh TM, et al. Genome-wide profiling of oral squamous cell carcinoma. J Pathol 2004; 204:326 - 32; http://dx.doi.org/10.1002/path.1640; PMID: 15372456
- Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. J Natl Cancer Inst 1993; 85:862 - 74; http://dx.doi.org/10.1093/jnci/85.11.862; PMID: 8492315
- Sciubba JJ. Oral cancer. The importance of early diagnosis and treatment. Am J Clin Dermatol 2001; 2:239 - 51; http://dx.doi.org/10.2165/00128071-200102040-00005; PMID: 11705251
- Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001; 37:Suppl 4 S3 - 8; http://dx.doi.org/10.1016/S0959-8049(01)00230-1; PMID: 11597398
- Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck 2002; 24:165 - 80; http://dx.doi.org/10.1002/hed.10004; PMID: 11891947
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57:43 - 66; http://dx.doi.org/10.3322/canjclin.57.1.43; PMID: 17237035
- Eley GD, Reiter JL, Pandita A, Park S, Jenkins RB, Maihle NJ, et al. A chromosomal region 7p11.2 transcript map: its development and application to the study of EGFR amplicons in glioblastoma. Neuro-oncol 2002; 4:86 - 94; PMID: 11916499
- Baba Y, Nosho K, Shima K, Hayashi M, Meyerhardt JA, Chan AT, et al. Phosphorylated AKT expression is associated with PIK3CA mutation, low stage, and favorable outcome in 717 colorectal cancers. Cancer 2011; 117:1399 - 408; http://dx.doi.org/10.1002/cncr.25630; PMID: 21425139
- Poppleton HM, Wiepz GJ, Bertics PJ, Patel TB. Modulation of the protein tyrosine kinase activity and autophosphorylation of the epidermal growth factor receptor by its juxtamembrane region. Arch Biochem Biophys 1999; 363:227 - 36; http://dx.doi.org/10.1006/abbi.1998.1095; PMID: 10068444
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008; 100:1672 - 94; http://dx.doi.org/10.1093/jnci/djn389; PMID: 19033571
- Tateda M, Shiga K, Yoshida H, Saijo S, Yokoyama J, Nishikawa H, et al. Management of the patients with hypopharyngeal cancer: eight-year experience of Miyagi Cancer Center in Japan. Tohoku J Exp Med 2005; 205:65 - 77; http://dx.doi.org/10.1620/tjem.205.65; PMID: 15635275
- Shiga K, Tateda M, Saijo S, Hori T, Sato I, Tateno H, et al. Presence of Streptococcus infection in extra-oropharyngeal head and neck squamous cell carcinoma and its implication in carcinogenesis. Oncol Rep 2001; 8:245 - 8; PMID: 11182034
- Hooper SJ, Crean SJ, Lewis MA, Spratt DA, Wade WG, Wilson MJ. Viable bacteria present within oral squamous cell carcinoma tissue. J Clin Microbiol 2006; 44:1719 - 25; http://dx.doi.org/10.1128/JCM.44.5.1719-1725.2006; PMID: 16672398
- Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer 2006; 94:184 - 8; http://dx.doi.org/10.1038/sj.bjc.6602941; PMID: 16434982
- Hiraishi Y, Wada T, Nakatani K, Tojyo I, Matsumoto T, Kiga N, et al. EGFR inhibitor enhances cisplatin sensitivity of oral squamous cell carcinoma cell lines. Pathol Oncol Res 2008; 14:39 - 43; http://dx.doi.org/10.1007/s12253-008-9020-5; PMID: 18347929
- Park SJ, Kim MJ, Kim YK, Kim SM, Park JY, Myoung H. Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer Lett 2010; 292:54 - 63; http://dx.doi.org/10.1016/j.canlet.2009.11.004; PMID: 19959278
- Li T, Ling YH, Perez-Soler R. Tumor dependence on the EGFR signaling pathway expressed by the p-EGFR:p-AKT ratio predicts erlotinib sensitivity in human non-small cell lung cancer (NSCLC) cells expressing wild-type EGFR gene. J Thorac Oncol 2008; 3:643 - 7; http://dx.doi.org/10.1097/JTO.0b013e3181753b24; PMID: 18520805
- Kalish LH, Kwong RA, Cole IE, Gallagher RM, Sutherland RL, Musgrove EA. Deregulated cyclin D1 expression is associated with decreased efficacy of the selective epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in head and neck squamous cell carcinoma cell lines. Clin Cancer Res 2004; 10:7764 - 74; http://dx.doi.org/10.1158/1078-0432.CCR-04-0012; PMID: 15570011
- Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol 2001; 19:3234 - 43; PMID: 11432891
- Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010; 11:21 - 8; http://dx.doi.org/10.1016/S1470-2045(09)70311-0
- Lord HK, Junor E, Ironside J. Cetuximab is effective, but more toxic than reported in the Bonner trial. Clin Oncol (R Coll Radiol) 2008; 20:96; http://dx.doi.org/10.1016/j.clon.2007.09.003; PMID: 17928207
- Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature 1984; 311:483 - 5; http://dx.doi.org/10.1038/311483a0; PMID: 6090945
- Yip WK, Leong VC, Abdullah MA, Yusoff S, Seow HF. Overexpression of phospho-Akt correlates with phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal carcinoma. Oncol Rep 2008; 19:319 - 28; PMID: 18202777
- Wallerand H, Cai Y, Wainberg ZA, Garraway I, Lascombe I, Nicolle G, et al. Phospho-Akt pathway activation and inhibition depends on N-cadherin or phospho-EGFR expression in invasive human bladder cancer cell lines. Urol Oncol 2010; 28:180 - 8; http://dx.doi.org/10.1016/j.urolonc.2008.09.041; PMID: 19070520
- Kannangai R, Sahin F, Torbenson MS. EGFR is phosphorylated at Ty845 in hepatocellular carcinoma. Mod Pathol 2006; 19:1456 - 61; http://dx.doi.org/10.1038/modpathol.3800665; PMID: 16936701
- Tsakiridis T, Cutz JC, Singh G, Hirte H, Okawara G, Corbett T, et al. Association of phosphorylated epidermal growth factor receptor with survival in patients with locally advanced non-small cell lung cancer treated with radiotherapy. J Thorac Oncol 2008; 3:716 - 22; http://dx.doi.org/10.1097/JTO.0b013e31817c6094; PMID: 18594316
- McMillen E, Ye F, Li G, Wu Y, Yin G, Liu W. Epidermal growth factor receptor (EGFR) mutation and p-EGFR expression in resected non-small cell lung cancer. Exp Lung Res 2010; 36:531 - 7; http://dx.doi.org/10.3109/01902148.2010.482176; PMID: 20939760
- Rusnak DW, Affleck K, Cockerill SG, Stubberfield C, Harris R, Page M, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res 2001; 61:7196 - 203; PMID: 11585755
- Kazkayasi M, Hücümenoğlu S, Siriner GI, Hücümenoğlu M. Over-expression of p53 and c-erbB-2 oncoproteins in laryngeal carcinoma. Eur Arch Otorhinolaryngol 2001; 258:329 - 35; http://dx.doi.org/10.1007/s004050100346; PMID: 11699821
- Franchi A, Fondi C, Paglierani M, Pepi M, Gallo O, Santucci M. Epidermal growth factor receptor expression and gene copy number in sinonasal intestinal type adenocarcinoma. Oral Oncol 2009; 45:835 - 8; http://dx.doi.org/10.1016/j.oraloncology.2008.12.005; PMID: 19213595
- Macarenco RS, Uphoff TS, Gilmer HF, Jenkins RB, Thibodeau SN, Lewis JE, et al. Salivary gland-type lung carcinomas: an EGFR immunohistochemical, molecular genetic, and mutational analysis study. Mod Pathol 2008; 21:1168 - 75; http://dx.doi.org/10.1038/modpathol.2008.113; PMID: 18587327
- Di Domenico M, Castoria G, Bilancio A, Migliaccio A, Auricchio F. Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res 1996; 56:4516 - 21; PMID: 8813150
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 2006; 24:2666 - 72; http://dx.doi.org/10.1200/JCO.2005.04.8306; PMID: 16763281
- Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol 2006; 24:4170 - 6; http://dx.doi.org/10.1200/JCO.2006.07.2587; PMID: 16943533
- Lin SC, Liu CJ, Ko SY, Chang HC, Liu TY, Chang KW. Copy number amplification of 3q26-27 oncogenes in microdissected oral squamous cell carcinoma and oral brushed samples from areca chewers. J Pathol 2005; 206:417 - 22; http://dx.doi.org/10.1002/path.1790; PMID: 15906274
- Hiraishi Y, Wada T, Nakatani K, Negoro K, Fujita S. Immunohistochemical expression of EGFR and p-EGFR in oral squamous cell carcinomas. Pathol Oncol Res 2006; 12:87 - 91; http://dx.doi.org/10.1007/BF02893450; PMID: 16799709
- Schwartz JL, Muscat JE, Baker V, Larios E, Stephenson GD, Guo W, et al. Oral cytology assessment by flow cytometry of DNA adducts, aneuploidy, proliferation and apoptosis shows differences between smokers and non-smokers. Oral Oncol 2003; 39:842 - 54; http://dx.doi.org/10.1016/S1368-8375(03)00107-6; PMID: 13679208
- Franco R, Caraglia M, Facchini G, Abbruzzese A, Botti G. The role of tissue microarray in the era of target-based agents. Expert Rev Anticancer Ther 2011; 11:859 - 69; http://dx.doi.org/10.1586/era.11.65; PMID: 21707283
- Psyrri A, Yu Z, Weinberger PM, Sasaki C, Haffty B, Camp R, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res 2005; 11:5856 - 62; http://dx.doi.org/10.1158/1078-0432.CCR-05-0420; PMID: 16115926
- Bei R, Pompa G, Vitolo D, Moriconi E, Ciocci L, Quaranta M, et al. Co-localization of multiple ErbB receptors in stratified epithelium of oral squamous cell carcinoma. J Pathol 2001; 195:343 - 8; http://dx.doi.org/10.1002/path.965; PMID: 11673832
- Rubin Grandis J, Melhem MF, Barnes EL, Tweardy DJ. Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 1996; 78:1284 - 92; http://dx.doi.org/10.1002/(SICI)1097-0142(19960915)78:6<1284::AID-CNCR17>3.0.CO;2-X; PMID: 8826952
- Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med 1993; 328:184 - 94; http://dx.doi.org/10.1056/NEJM199301213280306; PMID: 8417385
- Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinog 2005; 44:262 - 73; http://dx.doi.org/10.1002/mc.20138; PMID: 16167350
- Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 1999; 274:8335 - 43; http://dx.doi.org/10.1074/jbc.274.12.8335; PMID: 10075741
- Chen YS, Wu MJ, Huang CY, Lin SC, Chuang TH, Yu CC, et al. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS ONE 2011; 6:e28053; http://dx.doi.org/10.1371/journal.pone.0028053; PMID: 22140506
- Cheng SJ, Kok SH, Lee JJ, Yen-Ping Kuo M, Cheng SL, Huang YL, et al. Significant association of SRC protein expression with the progression, recurrence, and prognosis of oral squamous cell carcinoma in Taiwan. Head Neck 2011; http://dx.doi.org/10.1002/hed.21923; PMID: 22052839
- Sen B, Peng S, Saigal B, Williams MD, Johnson FM. Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin Cancer Res 2011; 17:514 - 24; http://dx.doi.org/10.1158/1078-0432.CCR-10-1617; PMID: 21106725
- Pannone G, Santoro A, Papagerakis S, Lo Muzio L, De Rosa G, Bufo P. The role of human papillomavirus in the pathogenesis of head & neck squamous cell carcinoma: an overview. Infect Agent Cancer 2011; 6:4; http://dx.doi.org/10.1186/1750-9378-6-4; PMID: 21447181
- Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization. Infect Agent Cancer 2012; 7:4; http://dx.doi.org/10.1186/1750-9378-7-4; PMID: 22376902
- Scapoli L, Palmieri A, Rubini C, Martinelli M, Spinelli G, Ionna F, et al. Low prevalence of human papillomavirus in squamous-cell carcinoma limited to oral cavity proper. Mod Pathol 2009; 22:366 - 72; http://dx.doi.org/10.1038/modpathol.2008.180; PMID: 18978731
- Delhanty PJ, van Koetsveld PM, Gauna C, van de Zande B, Vitale G, Hofland LJ, et al. Ghrelin and its unacylated isoform stimulate the growth of adrenocortical tumor cells via an anti-apoptotic pathway. Am J Physiol Endocrinol Metab 2007; 293:E302 - 9; http://dx.doi.org/10.1152/ajpendo.00377.2006; PMID: 17405826
- Vitale G, van Eijck CH, van Koetsveld Ing PM, Erdmann JI, Speel EJ, van der Wansem Ing K, et al. Type I interferons in the treatment of pancreatic cancer: mechanisms of action and role of related receptors. Ann Surg 2007; 246:259 - 68; http://dx.doi.org/10.1097/01.sla.0000261460.07110.f2; PMID: 17667505
- Vitale G, van Koetsveld PM, de Herder WW, van der Wansem K, Janssen JA, Colao A, et al. Effects of type I interferons on IGF-mediated autocrine/paracrine growth of human neuroendocrine tumor cells. Am J Physiol Endocrinol Metab 2009; 296:E559 - 66; http://dx.doi.org/10.1152/ajpendo.90770.2008; PMID: 19141687
- Caraglia M, Marra M, Tagliaferri P, Lamberts SW, Zappavigna S, Misso G, et al. Emerging strategies to strengthen the anti-tumour activity of type I interferons: overcoming survival pathways. Curr Cancer Drug Targets 2009; 9:690 - 704; http://dx.doi.org/10.2174/156800909789056980; PMID: 19508175
- Dimery IW, Hong WK. Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst 1993; 85:95 - 111; http://dx.doi.org/10.1093/jnci/85.2.95; PMID: 8418313
- Green MR. Targeting targeted therapy. N Engl J Med 2004; 350:2191 - 3; http://dx.doi.org/10.1056/NEJMe048101; PMID: 15118072
- Gamel JW, Jones AS. Squamous carcinoma of the head and neck: cured fraction and median survival time as functions of age, sex, histologic type, and node status. Br J Cancer 1993; 67:1071 - 5; http://dx.doi.org/10.1038/bjc.1993.196; PMID: 8494700
- Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004; 22:77 - 85; http://dx.doi.org/10.1200/JCO.2004.06.075; PMID: 14701768
- Magné N, Fischel JL, Dubreuil A, Formento P, Poupon MF, Laurent-Puig P, et al. Influence of epidermal growth factor receptor (EGFR), p53 and intrinsic MAP kinase pathway status of tumour cells on the antiproliferative effect of ZD1839 (“Iressa”). Br J Cancer 2002; 86:1518 - 23; http://dx.doi.org/10.1038/sj.bjc.6600299; PMID: 11986789