Abstract
Tamoxifen is a standard therapeutical treatment in patients with estrogen receptor positive breast carcinoma. However, less than 50% of estrogen receptor positive breast cancers do not respond to tamoxifen treatment whereas 40% of tumors that initially respond to treatment develop resistance over time. The underlying mechanisms for tamoxifen resistance are probably multifactorial but remain largely unknown. The primary aim of this study was to investigate the impact of PTEN tumor suppressor gene on acquiring resistance to tamoxifen by analyzing loss of heterozygosity (LOH) and immunohystochemical expression of PTEN in 49 primary breast carcinomas of patients treated with tamoxifen as the only adjuvant therapy. The effect of PTEN inactivation on breast cancer progression and disease outcome was also analyzed. Reduced or completely lost PTEN expression was observed in 55.1% of samples, while 63.3% of samples displayed LOH of PTEN gene. Inactivation of PTEN immunoexpression significantly correlated with the PTEN loss of heterozygosity, suggesting LOH as the most important genetic mechanism for the reduction or complete loss of PTEN expression in primary breast carcinoma. Most importantly, LOH of PTEN and consequential reduction of its immunoexpression showed significant correlation with the recurrence of the disease. Besides, our study revealed that LOH of PTEN tumor suppressor was significantly associated with shorter disease free survival, breast cancer specific survival and overall survival. In summary, our results imply that LOH of PTEN could be used as a good prognostic characteristic for the outcome of breast cancer patients treated with tamoxifen.
Introduction
Breast cancer is the most commonly occurring cancer in women. It comprises 22% of all cancersCitation1 and is second only to lung cancer as a cause of cancer related death in women.Citation2 Over the past few decades, there has been no significant decrease in the incidence of this disease. Current therapies delay tumor progression significantly, but recurrence is almost inevitable, resulting in high mortality rates in advanced stages.
Tamoxifen, which functions as a cell type-specific antiestrogen, has dominated endocrine treatment of breast cancer for over 30 years. It is a standard therapeutical treatment in patients with estrogen receptor (ERα) positive breast carcinoma with demonstrated efficacy in metastatic breast cancer, adjuvant therapy, preoperative treatment and chemoprevention.Citation3 However, less than 50% of ERα-positive breast cancers do not respond to tamoxifen treatment (intrinsic or de novo resistance) whereas 40% of tumors that initially respond to treatment develop resistance over time (acquired resistance), despite continued expression of ERα.Citation4 The underlying mechanisms for tamoxifen resistance are probably multifactorial but remain largely unknown. However, there is a compelling evidence which suggests that increased growth factor signaling, in particular the epidermal growth factor receptor (EGFR), human epidermal growth factor receptor type 2 (HER2) and insulin-like growth factor-1 receptor (IGF-1R) signaling pathways, contribute to this resistance.Citation5,Citation6 Moreover, the activity of kinases that functions downstream of these receptors, such as AKT, are often elevated in non-responsive tumors that exhibit either de novo or acquired resistance.Citation7 AKT, also known as protein kinase B (PKB), plays an important role in cell proliferation, survival and endocrine resistance. Several studies have now demonstrated that PKB/Akt can protect breast cancer cells from tamoxifen-induced apoptosis by modulating ERα activity.Citation8,Citation9 These data suggest a significant role of the PKB/Akt signaling pathway in hormone-refractory breast cancer.
PKB/AKT is activated by PI3K leading to substrate phosphorylation and cell survival and is counter-balanced by the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10). PTEN has dual phosphatase activity and dephosphorilates both lipid and protein substrates. The main lipid target of PTEN tumor suppressor is phosphatidylinositol-(3,4,5)-triphosphate (PIP3) acting as key negative regulator of PI3K/Akt signaling pathway thus regulating cell cycle progression and survival, cell growth, angiogenesis and genome stability.Citation10 It has recently been shown that, in addition to these very important tumor suppressing functions, PTEN could play an important role in acquiring resistance to tamoxifen.Citation11,Citation12 Specifically, Shoman et al.Citation12 showed that decreased PTEN expression significantly correlated with the resistance to tamoxifen in ERα-positive breast carcinoma patients.
Multiple mechanisms of somatic PTEN inactivation occur depending on the type of neoplasia involved. Reported mechanisms for PTEN inactivation are mutations, homozygous deletions, promoter hypermethylation and loss of heterozygosity (LOH).Citation13 The most frequent mechanism of reduction or loss of PTEN expression in sporadic breast carcinoma is loss of heterozygosity,Citation14 particularly in late stages of disease.Citation15 The aim of this study was to investigate the impact of PTEN tumor suppressor gene on breast cancer progression and, specifically, to evaluate its role in acquiring resistance to tamoxifen, by analyzing loss of heterozygosity and immunohystochemical expression of PTEN in primary breast carcinomas of patients treated with tamoxifen as the only adjuvant therapy. To that end, we analyzed the association of PTEN expression with the recurrence of the disease, survival rate, stage, grade, tumor size and tumor expression of estrogen receptor-α (ER-α), progesterone receptor (PR) and Her-2/neu expression.
Results
Clinical and pathological findings
The patients consisted of 49 postmenopausal steroid receptor positive women who underwent modified radical mastectomy. Thirty-nine of them were treated by radiation therapy while all patients received standard adjuvant tamoxifen therapy. Patients’ characteristics are summarized in . The mean patient follow-up was 114 mo, ranging from 11–220 mo. At the completion of the study, 26 (53.1%) patients had died and 23 (46.9%) were alive. Twenty-eight patients had invasive ductal breast carcinomas while 21 had invasive lobular carcinoma. Out of the total number of 49 patients, lymph node metastasis was present in 46 (93.9%), whereas only 3 (6.1%) were node negative. Disease recurrence occurred in 22 (44.9%) of 49 patients.
Table 1. Clinical and histopathological characteristics of patients
Immunophenotype of breast cancer patients
For the purpose of testing the patients’ suitability for tamoxifen therapy, immunohistochemical analyses were used to evaluate the expression of steroid receptors that is estrogen receptor (ER) and progesterone receptor (PR). Estrogen receptor positive status was determined in all 49 patients (100%) while 39 (79.6%) patients were progesterone receptor positive. In addition, the expression of Human Epidermal growth factor Receptor 2 (HER2) was analyzed and only 6 (12.2%) patients showed positive status. The results are summarized in .
Table 2. Breast cancer immunophenotype
Disease relapse developed in 22 of 49 patients and did not display any correlation with progesterone negative status (p = 0.7199, 2-tailed Fisher’s exact test) or HER2 positive status (p = 0.3794, 2-tailed Fisher’s exact test). Imunohistochemistry was used to analyze the expression of PTEN tumor-suppressor in order to test the hypotheses that PTEN might play a role in intrinsic or acquired resistance to tamoxifen. High PTEN immunostaining was revealed for 22 samples. Immunoreactivity was present in nuclei and/or cytoplasm of malignant cells ( and , respectively) with obvious heterogenity of immunoexpression on the same slices of tumor tissue (). Since cutoff values for reduced PTEN expression by using immunohistochemical methods have not been defined so far, the mean value of PTEN histo-score (H-score) was used to designate reduced expression. As a result, 27 (55.1%) samples exhibited total or partial inactivation of PTEN expression. Normal breast tissue was used as the internal control. In the absence of normal breast tissue, immunoreactivity of fibrocytes, nerves and endothelium were used as internal control ().
Figure 1. Immunocytochemical analysis of PTEN expression in breast cancers. (A) Nuclear PTEN immunoreactivity in invasive lobular breast carcinoma (IHCx40); (B) cytoplasmatic PTEN immunoreactivity in invasive ductal breast carcinoma (IHCx200); (C) heterogeneity of nuclear PTEN immunoreactivity in invasive ductal breast carcinoma (IHCx40); (D) negative PTEN immunoreactivity in invasive ductal breast carcinoma; nerve and fibrocytes as positive controls (IHCx100).
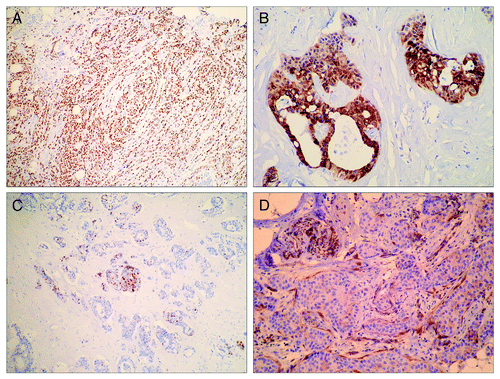
PTEN immunoexpression was analyzed in relation to clinicopathological parameters including tumor size, histological type of tumor, tumor grade, lymph node status and tumor expression of ER, PR and HER2. No correlation was found between any of these parameters and inactivation of PTEN expression (summarized in ). However, inactivation of PTEN immunoexpression significantly correlated with the recurrence of the disease ().
Table 3. Association between LOH of PTEN / reduction of PTEN immunoexpression and histopathological parameters
Table 4. Association between inactivation of PTEN and recurrence of disease
Loss of heterozygosity (LOH) of PTEN tumor-suppressor
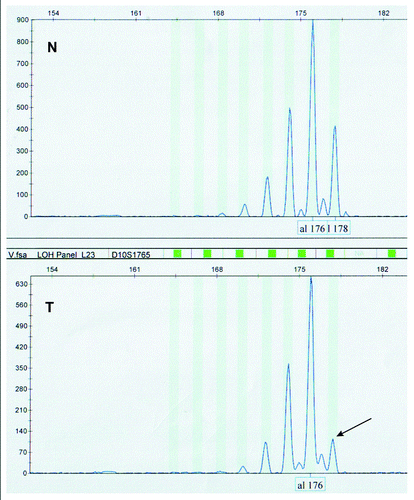
LOH of PTEN tumor suppressor gene was evaluated by fragment analysis in order to examine genetic bases of the inactivation of PTEN immunoexpression (). Fragment analysis was performed utilizing five highly polymorphic microsatellite markers, D10S579, D10S1765, D10S541, AFM086wg9 and D10S215, mapping at the chromosomal region of PTEN gene. All microsatellite markers were informative. Forty-six of forty-nine analyzed samples were informative for at least one microsatellite marker while only three samples were not informative at all, that is did not show heterozygosity for any locus. Allelic loss for at least one marker was observed in 31 (63.3%) of the 49 cases. Eighteen samples showed allelic loss at multiple markers. D10S1765 was the most frequently lost marker, with the rate of 58%.
Figure 2. Representative example of LOH analyses of PTEN tumor suppressor gene with D10S1765 microsatellite marker, showing loss of allele 178 (arrow) in tumor sample (T) compared with its normal counterpart (N).
Inactivation of PTEN gene by LOH was analyzed in relation to clinicopathological parameters: tumor subtype, tumor grade, tumor size, lymph node status and tumor expression of ER, PR and HER2. No correlation was found between any of these parameters and inactivation of PTEN by LOH (), as was the case with PTEN immunoexpression. Following the same pattern, inactivation of PTEN by LOH and disease recurrence showed statistically significant correlation (). Finally, LOH of PTEN significantly correlated with the inactivation of PTEN immunoexpression (). Accordingly, it could be concluded that inactivation of PTEN immunoexpression is, in the vast majority of cases, the consequence of LOH of PTEN gene.
Table 5. Association between LOH and immunoexpression of PTEN gene
Association between inactivation of PTEN by LOH and survival
Kaplan-Meier survival curves were generated to evaluate the effect of PTEN inactivation by LOH on disease free survival (DFS), overall survival (OS) and breast cancer specific survival (BCSS). The most significant observation of this study was that LOH of PTEN tumor suppressor was significantly associated with shorter disease free survival (p = 0.038) and shorter breast cancer specific survival (p = 0.048) regardless of tumor size, type, tumor gradus or lymph node metastasis (). In addition, comparison of survival curves of patients with altered and wild type PTEN showed that LOH of PTEN gene was also significantly associated with poorer overall survival (p = 0.049) ().
Figure 3. Kaplan-Meier survival curves. Patients with reduced or lost immunoexpression of PTEN due to loss of heterozygosity showed significantly shorter (A) disease free survival, (B) breast cancer specific survival and (C) overall survival.
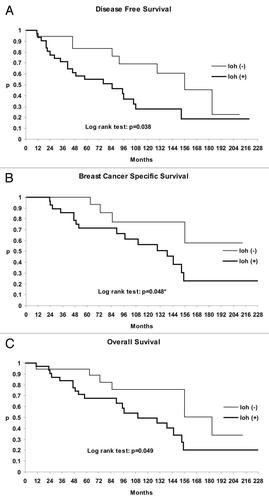
Cox regression analysis showed inactivation of PTEN by LOH to be an independent important determinant of breast cancer prognosis. Namely, patients with the LOH of PTEN tumor suppressor gene had poor prognosis for disease free survival (p = 0.0348; hazard ratio, HR = 2.32; 95% confidential interval, CI = 1.02–5.29), breast cancer specific survival (p = 0.0384; HR = 2.89, 95% CI = 0.962–8.69) and overall survival (p = 0.0472; HR = 2.32, 95% CI = 0.967–5.59).
Discussion
The main reason why treatment with tamoxifen fails in the majority of patients with ER-positive breast cancer is the development of drug resistance, either intrinsic or acquired. Multiple factors have been implicated in the generation of tamoxifen resistance, including the anomalous activation of the PI3K/Akt signaling pathway.Citation9,Citation16 This increase in active Akt can result, among other things, from the absence or decreased levels of the PIP3 phosphatase PTEN.Citation17 The aim of this study was to examine the potential role of PTEN tumor suppressor in acquiring resistance to tamoxifen in primary breast carcinoma patients. We found a high frequency (55.1%) of decreased or complete loss of PTEN expression by immunostaining in a series of 49 primary breast carcinomas, considerably higher than in previously reported studies.Citation12,Citation18 We further investigated LOH as the major mechanism responsible for the decreased PTEN expression in sporadic breast carcinomaCitation14 using fragment analyses. Fragment analyses revealed 63.3% of samples having lost at least one of five examined markers defining PTEN, which is within the frequency range of samples with reduced PTEN immunoexpression. Importantly, inactivation of PTEN immunoexpression significantly correlated with the PTEN loss of heterozygosity, confirming LOH as the most important genetic mechanism for the inactivation of PTEN in primary breast carcinoma.
Observed frequency of PTEN LOH is significantly higher than previously reportedCitation14,Citation15,Citation19 and the explanation of this discrepancy may lie in the evolution of tumor under selective pressure provided by tamoxifen treatment. Namely, correlation analysis displayed a significant association between inactivation of PTEN immunoexpression by LOH and recurrence of the disease which suggests poor response to tamoxifen treatment. Accepting the fact that genomic instability is the hallmark of cancer that drives cancerogenesis under unfavorable conditionsCitation20,Citation21 such as the presence of tamoxifen, genotypes with favorable effects on cell growth are likely to be promoted. In other words, we think that the loss of heterozygosity of PTEN tumor suppressor gene is an important genetic event leading to a reduction or complete loss of PTEN protein expression, the consequence of which is anomalous activation of the PI3K/Akt survival pathway followed by acquiring resistance to tamoxifen and recurrence of the disease. Our supposition is limited by the fact that we have not examined the PI3K/Akt signaling pathway but in the light of information from cell culture studies,Citation17,Citation22 it may be proposed that reduced PTEN expression can result in increased activity of PI3K/Akt survival pathway, which interferes with cellular actions of tamoxifen thus resulting in tumor recurrence. Our results obtained from the follow-up studies are supportive of above thesis. Kaplan-Meier survival curves revealed that LOH of PTEN tumor suppressor was significantly associated with shorter disease free survival, shorter breast cancer specific survival and shorter overall survival. These findings were independent of tumor subtype, tumor grade, tumor size, lymph node status and tumor expression of ER, PR and HER2. In agreement with our findings are those of Shoman et al.Citation12 who obtained significant association between reduced PTEN expression and shorter relapse-free survival and shorter disease-specific survival in breast cancer patients on tamoxifen treatment. In addition, they showed a significant negative association between PTEN expression and the presence of lymph node metastasis suggesting that loss of PTEN may also be involved in promotion of the invasive properties of the tumor. Similar results were disclosed by Depowski et al.Citation18 who showed negative association between PTEN expression and the presence of lymph node metastasis and Garcia et al.Citation19 who found statistically significant difference between PTEN LOH and lymph node metastases, as well as between PTEN LOH and tumor gradus. Contrary to these findings, our results did not reveal correlation between any of the examined clinicopathological parameters and reduction of PTEN expression or PTEN LOH. Based on these results we cannot argue whether loss of PTEN expression is an early or a late event in breast carcinogenesis. Some reports suggest that it is a late event associated with tumor progression and not tumor initiation, at least in the breast cancer model.
In conclusion, our findings suggest that PTEN loss of heterozygosity is the major mechanism responsible for the loss or reduction of PTEN expression which may interfere with the effects of tamoxifen leading to tamoxifen resistance and disease recurrence with poor outcome. Moreover, as revealed by Kaplan-Meier test and Cox regression analysis, PTEN LOH showed to be a good prognostic characteristic for the disease outcome of breast cancer patients treated with tamoxifen.
Material and methods
Patients
This is a retrospective study comprised of 49 ER and/or PR positive primary breast cancer and 49 corresponding normal tissue samples obtained from postmenopausal women. All patients underwent modified radical mastectomy between 1988 and 1995 at the Institute of Oncology and Radiology of Serbia. As per protocol at that time, all of them received adjuvant endocrine therapy tamoxifen for 5 y (without adjuvant chemotherapy) and 39 of 49 patients were postoperatively irradiated. All relevant clinical parameters [age, tumor size, lymphonodal status, disease free survival (DFS), overall survival (OS), breast cancer specific survival (BCSS)] were retrieved from patients medical records.
Collected tumor specimens and corresponding normal tissue were formalin-fixed, paraffin-embedded and hematoxylin-eosin (HE) stained. Histological type and grade of each carcinoma sample were determined after hematoxylin-eosin staining. The carcinomas were graded (I–III) according to Scarff-Bloom-Richardson scoring system.Citation23 All histopathological parameters were reviewed by two independent pathologists blinded to clinical outcome, results obtained by other pathologist and results of LOH analysis. Axillary lymph node status was determined as score 0 for absence of metastases and score 1 for 1–3 metastatic lymph nodes.
Signed informed consent was obtained from each of the patient. The written consent was acquired according to the ethical standards laid down in the 1964 Declaration of Helsinki, the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS), Geneva 1993 and the Guidelines for Good Clinical Practice (CPMP/ICH/135/95), September 1997.
Immunohistochemistry
We performed a manual immunohystochemical technique with primary monoclonal mouse anti-human PTEN clone (1:100, Clone 6H2.1, Dako) with EnVision+ system (HRP Labeled Polymer, K4000, Dako) and chromogen Dako Dab liquid (K3468). Labeled streptavidin-biotin-LSAB+ method together with immunoperoxidase was used according to recommended procedure for commercial primary monoclonal mouse antibody: anti-human ERα clone (1:50; Clone 1D5; Dako) and anti-human PR clone (1:50; Clone PgR 636; Dako), as well as for policlonal rabbit antibody anti-human c-erbB2/HER2 oncoprotein (1:300; Dako) with Dako LSABTM+/HRP kit (K0679). Slices were contrasted with Mayer hematoxylin.
We assessed the immunoreactivity of PTEN using the semiquantitative method based on the score of percentage of stained cells-cytoplasm/nuclei (0, no immunoreactivity; 1, 1–10%; 2, 11–50%; 3, 51–100%) and intensity of staining (0, no immunoreactivity; 1, reduced staining intensity relative to the corresponding normal cells; 2, same as normal cells staining; 3, mildly increased staining; 4, moderately increased staining; 5, intensely increased staining). Immunoreactivity of normal surrounding breast tissue (duct epithelium, myoepithelial cells, endothelium, fibrocytes and nerves was used for the internal positive control).Citation24 Since cutoff levels for reduced PTEN expression by using immunohistochemical methods have not been defined so far, we used the mean PTEN score as a cutoff point to designate reduced expression.Citation12 Accordingly, PTEN status was defined as follows: low expression if score was ≤ 5; high expression if score was > 5.
The evaluation of steroid receptors (ER, PR) was based on the scoring system which included percentage of stained malignant nuclei (0–5) and their intensity of staining (0–3); positive (high expression) cases were with score ≥ 4 while negative (low expression) cases were with score < 4.Citation25 HER2 status was determined using DAKO scoring system and HER2 positive status was defined if IHC score was 2+/3+.Citation26
DNA Extraction and LOH Analysis
DNA was isolated from formalin-fixed / paraffin-embedded tissue of 49 pared, tumor and corresponding normal, samples. Tissue sections were cut into 5 μm thick slices and DNA extracted according to the protocol described by Shi et al.Citation27 Tumor DNA was isolated only from slices that contained 70% or more of tumor cells.
Loss of heterozygosity of PTEN tumor suppressor was determined by fragment analysis using a set of five highly polymorphic microsatellite markers that map at the 10q23 chromosome region: D10S579, D10S215, D10S1765, AFMa086wg9 and D10S541. All markers were CA-dinucleotide repeats and are presented in a centromere-to-telomere orientation. Forward primers for all markers were 5′-labeled with Fam fluorescent dye, except for AFMa086wg9 which was PET labeled (Applied Biosystems). Markers were chosen from published sources following the criteria of heterozygosity (heterozygosity value greater than 0.7) in miscellaneous human populations and the position of marker (markers had to span the whole PTEN loci). Locus-specific PCR reagents and conditions were as described previously.Citation28,Citation29
Locus specific, florescent dye-labeled PCR products were prepared according to the manufacturer’s instructions. Amplicons were mixed with HiDi formammide and GeneScan-500 LIZ Size Standard, denaturated at 95°C for 5 min, cooled on ice for 10 min and, finally, separated by capillary array electrophoresis (filed with polymer type 7, POP-7, Applied Biosystems) on an ABI Prizm 3130 automated Genetic Analyzer (Applied Biosystems). Row data were collected and subsequently analyzed using GeneMapper Software. DNA from normal breast tissue of the same patient was used as a reference. A marker was defined as informative when two allelic peaks were identified in florescent histogram of reference DNA (it was heterozygous). When only one allelic peak was noted in control DNA, a marker was considered non-informative (it was homozygous) and therefore not useful for LOH scoring. The same way of reasoning was applied for separate samples—a sample was considered informative when it was heterozygous for at least one locus and non-informative when it was homozygous for all five loci. LOH candidates were determined after calculating allelic imbalance (AI) between normal and tumor tissues. For all informative cases, allelic imbalance was determined by comparing the calculated peak height ratios of microsatellite alleles between normal and tumor tissue of the same patient according to the following formula: (peak high of normal allele 2)/(peak high of normal allele 1) divided by (peak high of tumor allele 2)/(peak high of tumor allele 1). A sample was flagged as LOH candidate for particular marker if the value of allelic imbalance (AI) was above 1.35 or below 0.67 (AI > 1.35 or AI < 0.67).
Appearance of a new or additional peak or peaks, representing new allele form in tumor DNA when compared with the corresponding normal tissue was regarded as microsatellite instability (MSI). When MSI was detected in two or more markers of the same sample it was considered high, while MSI was regarded as low when it was present in one microsatellite marker.
Statistical Analysis
Statistical analyses were conducted with SPSS 11.0 for Windows (SPSS, Inc.). Pearson χ2 test, Fisher’s exact (2-tailed P values) and Linear-by-Linear Association χ2-tests were used to determine the significance of the associations between different variables. The comparison of LOH of PTEN, PTEN expression, tumor size, lymph node metastasis, tumor grade, disease recurrence, estrogen receptor staining (ER) status, progesterone receptor (PR) status and HER2 receptor status with each other and with the survival (disease free survival, overall survival and breast cancer specific survival) were performed by univariate and multivariate analysis using the Cox proportional hazards model and the Kaplan-Meier test. The level of significance was set at 0.05.
Acknowledgments
This research was supported by the Ministry of Education and Science of Republic of Serbia, grant number. III41031 and grant number ON173049. The authors are grateful to Dr Boban Stanojevic of the Laboratory for Radiobiology and Molecular Genetics, Institute of Nuclear Sciences “Vinča,” Belgrade, Serbia for the experimental contribution at the very beginning of the realization of this project.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
References
- Parkin DM. International variation. Oncogene 2004; 23:6329 - 40; http://dx.doi.org/10.1038/sj.onc.1207726; PMID: 15322508
- Stewart SL, King JB, Thompson TD, Friedman C, Wingo PA. Cancer mortality surveillance--United States, 1990-2000. MMWR Surveill Summ 2004; 53:1 - 108; PMID: 15179359
- Jordan VC. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol 2006; 147:Suppl 1 S269 - 76; http://dx.doi.org/10.1038/sj.bjp.0706399; PMID: 16402113
- Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer 2004; 11:643 - 58; http://dx.doi.org/10.1677/erc.1.00776; PMID: 15613444
- Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res 2008; 68:826 - 33; http://dx.doi.org/10.1158/0008-5472.CAN-07-2707; PMID: 18245484
- Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer 2005; 12:Suppl 1 S99 - 111; http://dx.doi.org/10.1677/erc.1.01005; PMID: 16113104
- Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 2005; 207:139 - 46; http://dx.doi.org/10.1002/path.1829; PMID: 16088978
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 2001; 276:9817 - 24; http://dx.doi.org/10.1074/jbc.M010840200; PMID: 11139588
- Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther 2002; 1:707 - 17; PMID: 12479367
- Li L, Ross AH. Why is PTEN an important tumor suppressor?. J Cell Biochem 2007; 102:1368 - 74; http://dx.doi.org/10.1002/jcb.21593; PMID: 17972252
- Pfeiler G, Horn F, Lattrich C, Klappenberger S, Ortmann O, Treeck O. Apoptotic effects of signal transduction inhibitors on human tumor cells with different PTEN expression. Oncol Rep 2007; 18:1305 - 9; PMID: 17914589
- Shoman N, Klassen S, McFadden A, Bickis MG, Torlakovic E, Chibbar R. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod Pathol 2005; 18:250 - 9; http://dx.doi.org/10.1038/modpathol.3800296; PMID: 15475931
- Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J 2004; 382:1 - 11; http://dx.doi.org/10.1042/BJ20040825; PMID: 15193142
- Singh B, Ittmann MM, Krolewski JJ. Sporadic breast cancers exhibit loss of heterozygosity on chromosome segment 10q23 close to the Cowden disease locus. Genes Chromosomes Cancer 1998; 21:166 - 71; http://dx.doi.org/10.1002/(SICI)1098-2264(199802)21:2<166::AID-GCC13>3.0.CO;2-P; PMID: 9491329
- Bose S, Wang SI, Terry MB, Hibshoosh H, Parsons R. Allelic loss of chromosome 10q23 is associated with tumor progression in breast carcinomas. Oncogene 1998; 17:123 - 7; http://dx.doi.org/10.1038/sj.onc.1201940; PMID: 9671321
- deGraffenried LA, Friedrichs WE, Russell DH, Donzis EJ, Middleton AK, Silva JM, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res 2004; 10:8059 - 67; http://dx.doi.org/10.1158/1078-0432.CCR-04-0035; PMID: 15585641
- DeGraffenried LA, Fulcher L, Friedrichs WE, Grünwald V, Ray RB, Hidalgo M. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol 2004; 15:1510 - 6; http://dx.doi.org/10.1093/annonc/mdh388; PMID: 15367412
- Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol 2001; 14:672 - 6; http://dx.doi.org/10.1038/modpathol.3880371; PMID: 11454999
- Garcia JM, Silva JM, Dominguez G, Gonzalez R, Navarro A, Carretero L, et al. Allelic loss of the PTEN region (10q23) in breast carcinomas of poor pathophenotype. Breast Cancer Res Treat 1999; 57:237 - 43; http://dx.doi.org/10.1023/A:1006273516976; PMID: 10617300
- Cahill DP, Kinzler KW, Vogelstein B, Lengauer C. Genetic instability and darwinian selection in tumours. Trends Cell Biol 1999; 9:M57 - 60; http://dx.doi.org/10.1016/S0962-8924(99)01661-X; PMID: 10611684
- Breivik J, Gaudernack G. Genomic instability, DNA methylation, and natural selection in colorectal carcinogenesis. Semin Cancer Biol 1999; 9:245 - 54; http://dx.doi.org/10.1006/scbi.1999.0123; PMID: 10448112
- Frogne T, Jepsen JS, Larsen SS, Fog CK, Brockdorff BL, Lykkesfeldt AE. Antiestrogen-resistant human breast cancer cells require activated protein kinase B/Akt for growth. Endocr Relat Cancer 2005; 12:599 - 614; http://dx.doi.org/10.1677/erc.1.00946; PMID: 16172194
- Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 1957; 11:359 - 77; http://dx.doi.org/10.1038/bjc.1957.43; PMID: 13499785
- Perren A, Weng LP, Boag AH, Ziebold U, Thakore K, Dahia PL, et al. Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol 1999; 155:1253 - 60; http://dx.doi.org/10.1016/S0002-9440(10)65227-3; PMID: 10514407
- Leake R, Barnes D, Pinder S, Ellis I, Anderson L, Anderson T, et al. Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. J Clin Pathol 2000; 53:634 - 5; http://dx.doi.org/10.1136/jcp.53.8.634; PMID: 11002770
- HercepTestTM, For determination of HER2 protein overexpression. Catalog Products and Services, DAKO 2007; 86-87.
- Shi SR, Cote RJ, Wu L, Liu C, Datar R, Shi Y, et al. DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: heating under the influence of pH. J Histochem Cytochem 2002; 50:1005 - 11; http://dx.doi.org/10.1177/002215540205000802; PMID: 12133903
- Feilotter HE, Nagai MA, Boag AH, Eng C, Mulligan LM. Analysis of PTEN and the 10q23 region in primary prostate carcinomas. Oncogene 1998; 16:1743 - 8; http://dx.doi.org/10.1038/sj.onc.1200205; PMID: 9582022
- Hahn M, Wieland I, Koufaki ON, Görgens H, Sobottka SB, Schackert G, et al. Genetic alterations of the tumor suppressor gene PTEN/MMAC1 in human brain metastases. Clin Cancer Res 1999; 5:2431 - 7; PMID: 10499615