Abstract
To determine whether the spheroid culture can be used to evaluate drug efficacy, we have evaluated the toxicity of free or carrier-associated doxorubicin as a single drug or in combination with other antineoplastic agents using the spheroid cultures of drug-resistant cancer cells. Paclitaxel, cisplatin, dexamethasone, mitoxantrone, sclareol or methotrexate were used in combination with doxorubicin. The effect of the treatment protocols on free, micellar and liposomal doxorubicin accumulation in spheroids and on resulting toxicity was evaluated by fluorescence and lactate dehydrogenase release, respectively. Enhanced doxorubicin accumulation and toxicity were observed after spheroid pretreatment with mitoxantrone or paclitaxel. Effects of the drug combination with doxorubicin were sequence dependent, use of doxorubicin as the first drug being the least inducer of toxicity. Finally, spheroids were recognized by a cancer cell-specific antibody. Our results suggest the usefulness of spheroids to evaluate chemotherapy combinations.
Introduction
Together with surgery and radiotherapy, chemotherapy prolongs survival of patients with solid tumors.Citation1 Howevever this benefit may not be a cure because of tumor drug resistance mechanisms.Citation2,Citation3 One of the resistance mechanisms is the limited drug access to the tumor mass.Citation3,Citation4 Resistance to drug penetration is a critical parameter not reproduced in monolayer cultures of cancer cells, which also lack heterogeneity.Citation5,Citation6 The chemotherapeutic priming as well as the increase in tumor blood flow have been used to selectively increase tumor drug penetration.Citation7,Citation8
Spheroids, three dimensional architectures of cancer cells, are found in cancer patients with sizes of 250–750 µm.Citation9 Spheroids formed with cancer cell lines mimic both architecture and share the limited drug penetration properties since drugs are largely confined to the outer cell layers.Citation5 In other ways, spheroids represent a better model for drug resistance compared with monolayer cultures.Citation10-Citation12 where the correlation between apoptosis induction in spheroids by free and micellar doxorubicin and its therapeutic efficacy in xenografts have been reported.Citation13 Finally, spheroids were as predictive as tumor histocultures of dog melanoma tumors in their response to herpes simplex thymidine kinase/ganciclovir therapy.Citation14
Considerable evidence suggests that drug combination protocols give better results in spheroids and tumor xenograftsCitation6,Citation8,Citation15 and in patients.Citation16-Citation18 Cell death induction in xenografts and ex vivo patient tumors by paclitaxel decreased interstitial blood pressure, enhanced paclitaxel penetration and therapeutic efficacy.Citation7,Citation8,Citation19,Citation20 Because the degree of apoptosis induction has recently been positively correlated with the clinical outcome, the assessment of the toxicity in the challenging spheroid model is of clear therapeutic relevance.Citation5,Citation21 As cells within the spheroid core are exposed to suboptimal drug concentrations,Citation22-Citation24 high concentrations and/or drug combinations are needed to harness antineoplastic agents efficiency. NCI-ADR-RES, doxorubicin-resistant spheroids, were used as a model in our study.Citation6
Several studies have investigated doxorubicin accumulation and toxicity in spheroids.Citation6,Citation15,Citation25,Citation26 We evaluated combinations of clinically relevant drugs belonging to different groups: mitoxantrone (anthracenedione), cisplatin (alkylating-like), dexamethasone (glucocorticosteroid), methotrexate (antifolate), sclareol (labdane diterpene), paclitaxel (taxane) and doxorubicin (anthracycline). In vitro use of these drugs in combination protocols was reported for sclareol and methotrexate,Citation27,Citation28 in vivo for paclitaxel, cisplatin and dexamethasone;Citation8,Citation29-Citation31 and with patients for doxorubicin, paclitaxel, mitoxantrone, methotrexate.Citation16-Citation18,Citation32-Citation34 Their sequential combination with doxorubicin in a spheroid model was studied in this report. In addition, we used doxorubicin in its free, micellar and liposomal form, which allowed us to compare the permeability of spheroids for different drug formulations.
The results show an improved accumulation of doxorubicin after pretreatment with paclitaxel or mitoxantrone. The cell death induction by sclareol, paclitaxel and mitoxantrone (but not by cisplatin, methotrexate and dexamethasone) potentiated doxorubicin toxicity in spheroid cultures. In addition, the correlation between size of different doxorubicin formulations (micellar or liposomal doxorubicin) and toxicity was also analyzed. Finally, the binding of a cancer-specific anti-nucleosome antibody to cancer cell monolayers and spheroids was compared.
Results
Cell death induction by pretreatment
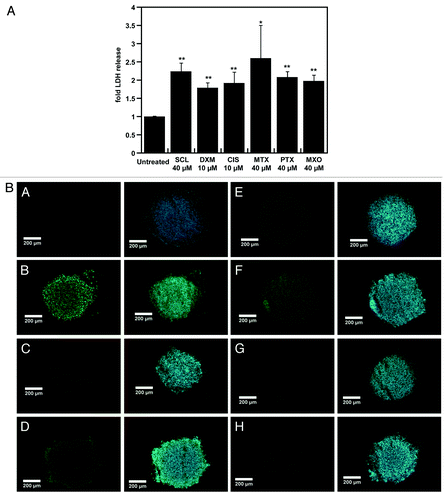
Spheroid cultures are characterized by their limited drug and nanoparticle penetrationCitation5,Citation20,Citation26,Citation35 along with altered protein expression patterns in spheroids compared with monolayers and are now being used as advanced in vitro tumor models.Citation5,Citation10-Citation12 Using spheroids we tested combination protocols that could allow enhanced doxorubicin penetration and resulting cytotoxicity. Since facilitation of antineoplastic drug or nanoassembly penetration of spheroids or tumors was shown to require the induction of apoptosis, we determined cytotoxic concentrations for six different drugs in spheroids.Citation8,Citation19,Citation20,Citation36 For this purpose, spheroids were cultured for 48 h with concentrations of drugs allowing lactate dehydrogenase (LDH) release as a marker of cell death. LDH release was increased by 1.5- to 2.5-fold after 48 h incubations with 10 µM of dexamethasone or cisplatin or with 40 µM of sclareol, methotrexate, paclitaxel or mitoxantrone (). These drug concentrations are in accordance with those used in previous reports.Citation22,Citation23 The use of such high doses of these drugs is supported on the one hand by the doxorubicin IC50 above 100 µM in NCI-ADR-RES spheroidsCitation6 and on the other hand, by the data on limited peripheral penetration of cisplatin and doxorubicin in HCT116 spheroids.Citation23 Induction of cell death by this pretreatment was confirmed by TUNEL staining (). Compared with DNase I (positive control), pretreatments with lower drug concentrations resulted in lower quantities of dead cells, confirming the need for higher cell death-inducing drug concentrations as reported.Citation8,Citation20 Almost no fragmented DNA was detected in untreated spheroids, as previously reported.Citation10,Citation25 Fragmented DNA signal intensities were apparent for the six-inducers used in mentioned concentrations based on the corresponding LDH releases observed (). Thus, the concentrations selected above were used in combination with doxorubicin.
Figure 1. The effect of cytotoxic doses of priming drugs on NCI-ADR-RES spheroids. NCI-ADR-RES spheroids were cultured with sclareol (SCL), dexamethasone (DXM), cisplatin (CIS), methotrexate (MTX), paclitaxel (PTX) or mitoxantrone (MXO) for 48 h before determination of LDH release with a Cytotox 96 cell viability kit (A) and apoptosis induction by a Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (B). Concentrations used are indicated in micromolars. LDH release was normalized to untreated cells cultured in the same conditions. Pretreated spheroids were fixed and submitted to TUNEL assay: A, untreated spheroids; B, DNase I (positive control); C, SCL; D, MTX; E, CIS; F, DXM; G, MXO; H, PTX. Scale bar represents 200 µm. Data represent the mean ± SD, n = 3. Student’s t-test; *p < 0.05, **p < 0.01 compared with untreated cells.
Enhancement of free doxorubicin accumulation by cell death induction
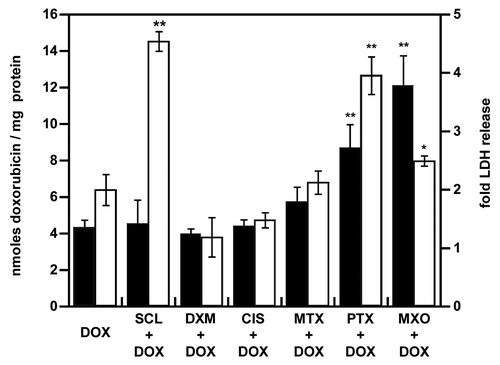
We next analyzed to what extent cell death induction by paclitaxel, mitoxantrone, cisplatin, dexamethasone, sclareol or methotrexate influenced doxorubicin accumulation (). Strong differences in doxorubicin accumulation were observed: only mitoxantrone and paclitaxel increased doxorubicin accumulation (by 2.8- and 2-fold, respectively). The doxorubicin analog mitoxantrone allowed the highest doxorubicin accumulation. Furthermore, a similar doxorubicin accumulation was detected after the pretreatment with 40, 60 or 80 µM of mitoxantrone (data not shown). The increase in fluorescence was not due to the auto-fluorescence of mitoxantrone or paclitaxel. No signal was detected without the addition of doxorubicin.
Figure 2. Enhancement of doxorubicin accumulation and toxicity after pretreatment of NCI-AR-RES spheroids. NCI-ADR-RES spheroids were preincubated with cytotoxic doses of priming drugs before the addition of doxorubicin (DOX, 100 µM): sclareol (SCL + DOX), dexamethasone (DXM + DOX), cisplatin (CIS + DOX), methotrexate (MTX + DOX), paclitaxel (PTX + DOX), mitoxantrone (MXO + DOX) or doxorubicin alone (DOX). The accumulation of doxorubicin (black bars) was quantitated by fluorescence and was expressed in nanomoles of doxorubicin per mg of protein. LDH release (white bars) was measured (). Same concentrations as in were used. Data represent the mean ± SD, n = 3. Student’s t-test; *p < 0.05, **p < 0.01 compared with DOX alone group.
Enhancement of free doxorubicin toxicity
We next evaluated the influence of the pretreatment on doxorubicin toxicity. Enhanced doxorubicin accumulation when spheroids were pretreated with mitoxantrone or paclitaxel was correlated with an increased toxicity (1.4- and 2.2-fold compared with doxorubicin alone, respectively) (). Although mitoxantrone further improved doxorubicin distribution in spheroids compared with paclitaxel, the latter showed superior toxicity when combined with doxorubicin (2.5- and 4-fold increased LDH release, respectively). Interestingly, sclareol, which did not enhance doxorubicin accumulation, potentiated its toxicity with a 4.5-fold increase in LDH release. No potentiation of doxorubicin toxicity by methotrexate, dexamethasone or cisplatin was detected.
Enhancement of doxorubicin nanoformulations accumulation and toxicity
Using the same drug combinations, we evaluated the influence of doxorubicin formulation as doxorubicin-loaded micelles or liposomes on its accumulation and resulting toxicity ()()(). Without the pretreatment, micellar doxorubicin accumulation was similar to the free drug (5.8 and 4.3 nmol per mg of protein, respectively; ), whereas for the liposomal doxorubicin (as Lipo-Dox) it was lower than for free doxorubicin (0.7 and 4.3 nmol per mg of protein, respectively; ); a similar pattern was reported by Tsukioka et al.Citation26 Sclareol, methotrexate, dexamethasone and cisplatin did not modify micellar and liposomal doxorubicin accumulation and toxicity. Although paclitaxel has been shown to modify liposomal doxorubicin pharmacokinetics with selective doxorubicin tumor accumulation,Citation8 in our study, an enhanced doxorubicin accumulation was observed only with free doxorubicin (8.7 nmol per mg of protein with the pretreatment vs. 4.3 without; ) and micellar doxorubicin (8.2 and 5.8 nmol/mg of protein, with and without the pretreatment, respectively; ), but not with the liposomal doxorubicin (0.9 and 0.8 nmol / mg of protein, with and without the pretreatment, respectively; ).
Figure 3. Enhancement of micellar doxorubicin accumulation and toxicity after pretreatment of NCI-AR-RES spheroids. NCI-ADR-RES spheroids were preincubated with cytotoxic doses of priming drugs before addition of micellar doxorubicin (100 µM): sclareol (SCL + MDOX), dexamethasone (DXM + MDOX), cisplatin (CIS + MDOX), methotrexate (MTX + MDOX), paclitaxel (PTX + MDOX), mitoxantrone (MXO + MDOX) or media as control (MDOX). The accumulation of doxorubicin (black bars) was quantitated (see ). LDH release (white bars) was measured (see ). Data represent the mean ± SD, n = 3. Student’s t-test; *p < 0.05, **p < 0.01 compared with MDOX alone group.
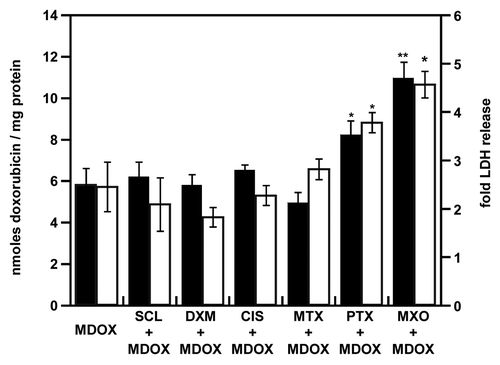
Figure 4. Cytotoxicity of empty and doxorubicin-loaded micelles. NCI-ADR-RES spheroids were preincubated with cytotoxic doses of priming drugs before addition of PEG 2000 micelles (MIC) either empty (white bars) or DOX-loaded (black bars). LDH release was measured with the Cytotox 96 cell viability kit and normalized to untreated cells. Data represent the mean ± SD, n = 3. Student’s t test; *p < 0.05, **p < 0.01 between empty and DOX-loaded micelles.
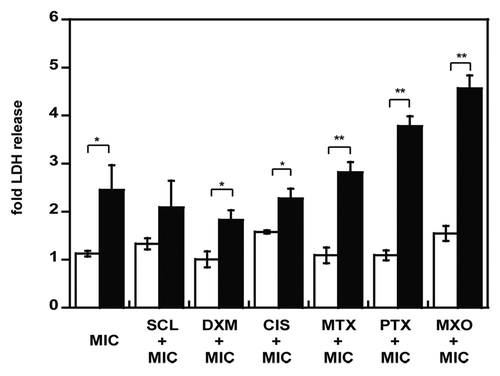
Figure 5. Enhancement of liposomal doxorubicin accumulation and toxicity after pretreatment of NCI-ADR-RES spheroids. NCI-ADR-RES spheroids were preincubated with cytotoxic doses of priming drugs before addition of Lipo-Dox (LDOX, 100 µM): sclareol (SCL + LDOX), dexamethasone (DXM + LDOX), cisplatin (CIS + LDOX), methotrexate (MTX + LDOX), paclitaxel (PTX + LDOX), mitoxantrone (MXO + LDOX) or LDOX alone (LDOX). The accumulation of doxorubicin (black bars) was quantitated (). LDH release (white bars) was measured (). Data represent the mean ± SD, n = 3. Student’s t-test; **p < 0.01 compared with unprimed group.
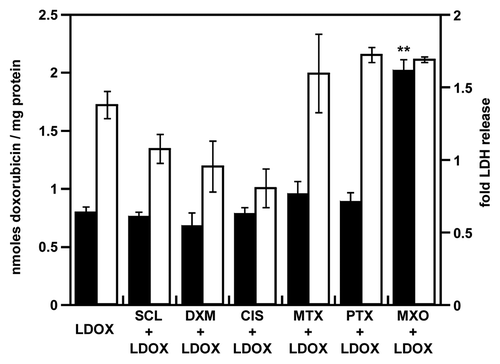
Conversely, mitoxantrone promoted the accumulation of free doxorubicin (12.1 nmol per mg of protein with the pretreatment vs. 4.3 without; ), micellar doxorubicin (10.9 nmol per mg with the pretreatment vs. 5.9 without it) and liposomal doxorubicin (2 nmol per mg with vs. 0.8 without mitoxantrone pretreatment). Although LDH release obtained with MDOX and free doxorubicin was similar, the toxicity of MDOX was increased 1.5-fold with paclitaxel and 1.8-fold with mitoxantrone pretreatments.
Scheduling effect of drug administration on resulting toxicity
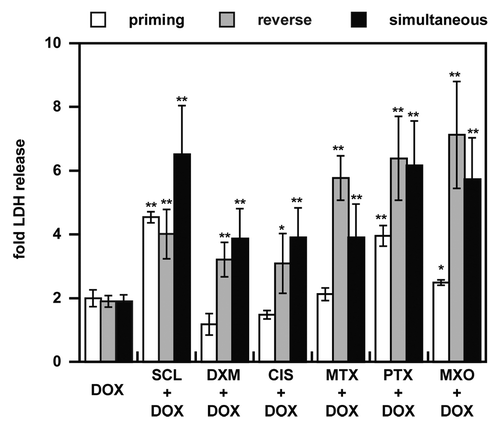
We next used the spheroid model to compare three sequences of drug combinations for toxicity induction: doxorubicin as the second drug (priming by cell death induction), doxorubicin as the first drug (reverse) and co-administration (). Except for sclareol, the priming schedule was the poorest inducer of LDH release. Use of doxorubicin as the first drug resulted in the highest resulting LDH release. A lower toxicity with priming compared with reverse and co-administration schedules should be correlated with its lower adverse effects in clinical trials.Citation16,Citation37,Citation38
Figure 6. Schedule-dependency of drug combinations on NCI-ADR-RES spheroids. Three regimens of drug combinations were evaluated, priming (inducer drug for 48 h before DOX 24 h, white bars), reverse priming (DOX for 48 h as a first line agent before second drug for 24 h, gray bars) and co-administration of both drugs (black bars). LDH release was measured 72 h after addition of the second drug or their co-administration and was normalized to untreated cells. Data represent the mean ± SD, n = 3. Student’s t-test; *p < 0.05, **p < 0.01 compared with DOX alone.
Recognition of spheroids by a cancer cell-specific antibody
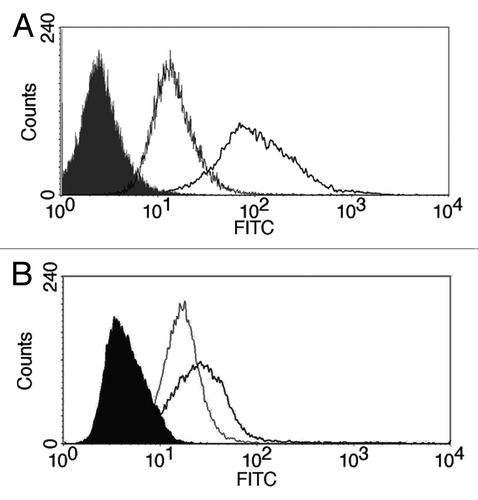
Finally, as we observed clear differences between drug formulations including free, micellar or liposomal doxorubicin, we wanted to determine whether spheroids could be used as a model for targeted delivery of drug formulations. The recognition of spheroids by a cancer-specific monoclonal antibody (2C5) that reacts with cancer cell surface-associated nucleosomes was studied ().Citation35 Earlier, the 2C5 conjugation allowed the selective enhancement of doxorubicin-loaded liposomes and paclitaxel-loaded micelles accumulation in tumors together with increased therapeutic activity.Citation35,Citation36 We found that anticancer nucleosome-specific antibodies binds selectively to NCI-ADR-RES spheroids compared with the control isotype-matching antibody (). Moreover, in spheroids, the signal intensity of 2C5-treated cells was 6.9 times higher than for the control antibody (), but was only 1.6 times higher than control in monolayer cultures () suggesting higher selectivity of this antibody for spheroids.
Discussion
We report for the first time the use of a spheroid cancer model to evaluate the activity of drug combinations with different doxorubicin forms (free, micellar or liposomal) as well as administration schedules. We tested drug combinations for their ability to promote doxorubicin delivery in cancer cell spheroids. Although dexamethasone was shown to increase the accumulation of carboplatin and gemcitabine in tumor xenografts of lung, colon or breast cancers,Citation30 we observed no effect in our case with NCI-ADR-RES cells, similar to the results reported with ovarian carcinoma xenografts.Citation29 Chemotherapy combinations may be tumor type specific, since earlier the ovarian cancer cell line failed to demonstrate any doxorubicin potentiation, and no influence of cisplatin on doxorubicin accumulation was observed.Citation29 A different incubation time with cisplatin may be required to observe an enhancement since this agent’s sensitizing property was shown to be time-dependent both in vitro and in vivo.Citation29,Citation31 We observed no enhancement in doxorubicin delivery by sclareol or methotrexate, contradictory to previous reports.Citation18,Citation29 On the other hand, as expected based on the previous reports, paclitaxel permitted an increase in doxorubicin distribution in spheroid cells.Citation8,Citation19,Citation20 The 40 µM dose of paclitaxel used to improve doxorubicin accumulation is 400 times higher than the 100 nM used by Wong et al. to increase siRNA lipoplexes penetration in FaDu spheroids.Citation19 This 400-fold increase in effective paclitaxel concentration between FaDu and NCI-ADR-RES spheroids should be correlated with the 520-fold lower IC50 value of NCI-ADR-RES monolayers compared with FaDu cells monolayers lines (7.3 nM and 3.8 µM, respectively).Citation37,Citation38 Moreover, the use of a high paclitaxel dose of 200 mg/m2 in combination with doxorubicin induced high rates of partial and complete responses in breast cancer patients (29 and 58%, respectively).Citation37
Paclitaxel and mitoxantrone allowed an increased distribution of doxorubicin in spheroids, and paclitaxel, mitoxantrone and sclareol enhanced doxorubicin toxicity. While the increase of doxorubicin accumulation with mitoxantrone priming was superior to that obtained with paclitaxel previously shown to enhance drug delivery and therapeutic efficacy in mice,Citation8 the resulting toxicity was higher with paclitaxel before doxorubicin.
Regarding the mechanism of the phenomena observed, we would like to speculate as follows. The enhancement of doxorubicin accumulation by mitoxantrone might be due to the inhibition of Hypoxia Inducible Factor 1α (HIF-1α) by this drug.Citation39 Indeed, HIF-1α silencing decreased the resistance to cisplatin and doxorubicin in the non-small cell lung cancer in vitro.Citation40 Finally, the additive reduction in cell viability observed after the incubation with doxorubicin and mitoxantrone could be due to a synergistic action of these drugs on DNA intercalation and unwinding.Citation39 Recently, mitoxantrone was reported to inhibit the activity of cancer-associated kinases, focal adhesion kinase (FAK) among them.Citation40 Interestingly, FAK silencing in colon carcinoma spheroids sensitized them to 5-fluorouracil suggesting an indirect doxorubicin potentiation by mitoxantrone.Citation42
Potentiation of doxorubicin toxicity by paclitaxel pretreatment corresponds well to the previous reports.Citation8,Citation43 While sclareol did not promote doxorubicin accumulation, it increased its toxicity. A synergistic action of sclareol and doxorubicin has been demonstrated with monolayers,Citation27 and our results support the existence of this synergy using three-dimensional cultures.
When drug combination experiments were repeated with micellar or liposomal doxorubicin, mitoxantrone allowed an almost 2-fold increase in micellar (13 nm sized) and liposomal (100 nm sized) doxorubicin accumulation. This increased penetration of 100 nm nanoassemblies is in agreement with increased tumor accumulation of 200 nm latex beads obtained after tumor pretreatment with paclitaxel.Citation8 On the contrary, paclitaxel only promoted increased distribution of free and micellar doxorubicin. The previous studies that have reported increased delivery of drug- or siRNA-loaded liposomes in spheroids or tumors after paclitaxel pretreatment used oropharyngeal FaDu cells.Citation19,Citation43 In our study, we used doxorubicin-resistant NCI-ADR-RES cells and this may explain the difference of the penetration of the liposomal doxorubicin.
Similar toxicities observed here for doxorubicin and MDOX in spheroids are in agreement with previous studies.Citation13,Citation26 Whereas paclitaxel and mitoxantrone promoted MDOX accumulation, toxicities were not different from that for the free drug. This does parallel the lower therapeutic efficacy of MDOX compared with doxorubicin.Citation13 Since dose-dependent tumor growth retardation with doxorubicin micelles was reported, a higher concentration of MDOX may be required for increased cytotoxicity.Citation47 Although mitoxantrone increased liposomal doxorubicin accumulation, no toxicity was noticed, in accordance with Tsukioka et al.Citation26
When we analyzed the resulting toxicities obtained after the use of doxorubicin as either first or second drug or simultaneous administration of doxorubicin and either paclitaxel, mitoxantrone, methotrexate, cisplatin, dexamethasone, sclareol, it was the use of doxorubicin as the first drug that resulted in the highest LDH release. The lower toxicity of dexamethasone followed by doxorubicin is in agreement with the higher in vitro survival of cardiomyocytes when incubated with doxorubicin 24 h after dexamethasone.Citation44 In contrast, high toxicity obtained after simultaneous or reverse administrations is consistent with the good clinical results of liposomal doxorubicin (Caelyx), dexamethasone and thalidomide in myeloma patients.Citation38 Synergy between doxorubicin and sclareol was demonstrated in vitro after their co-administration.Citation27 We obtained a similar LDH release with the three sequences, suggesting that the interaction between these two antineoplastic agents is not schedule-dependent. The higher LDH release observed after co-administration of doxorubicin and methotrexate contradicts reports on the absence of any benefit of methotrexate addition in doxorubicin treatment of sarcoma.Citation45 No superior LDH release was obtained from methotrexate pretreated spheroids compared with those receiving doxorubicin. This result is consistent with the low clinical effectiveness of methotrexate and 5-fluorouracil pretreatment before doxorubicin administration.Citation46
Although the most toxic drug combination schedule identified in our experiments, namely doxorubicin before methotrexate, has not been used in clinical trials, a doxorubicin before paclitaxel schedule induces more complete clinical responses than paclitaxel before doxorubicin.Citation16 Similar toxicities measured with doxorubicin and paclitaxel in priming, reverse, and simultaneous schedules are consistent with the breast cancer clinical studies.Citation16,Citation37 Indeed, the three schedules allowed clinical responses with less toxicity in the priming schedule.Citation16,Citation37 In addition, higher cardiac toxicity was reported when the two drugs were injected within a 15 min interval compared with a 12 or 16 h interval.Citation16,Citation37 While clinical responses were detected for both doxorubicin preceding and following cisplatin, the 5-y survival of ovarian cancer patients treated first with doxorubicin then cisplatin was superior to the opposite schedule (44 and 11%, respectively).Citation47 We observed higher LDH release from spheroids treated first with doxorubicin then cisplatin than with cisplatin then doxorubicin (3- and 1.4-fold LDH release, respectively). LDH release was higher after co-administration of the two drugs compared with doxorubicin alone, although no clinical improvement with this combination was observed in hepatoblastoma patients.Citation48 Concerning the interaction between doxorubicin and mitoxantrone, their coadministration or incubation with doxorubicin prior to mitoxantrone resulted in at least 2-fold higher LDH release than the incubation with mitoxantrone prior to doxorubicin suggesting schedule-dependency for these two drugs. While similar LDH release was obtained with the reverse and simultaneous schedule, the treatment of breast cancer patients with mitoxantrone and doxorubicin did not show any clinical improvement over doxorubicin alone.Citation34
Our results suggest that induction of cell death is not sufficient to enhance the toxicity of doxorubicin since while similar LDH release and TUNEL signals were obtained with methotrexate, mitoxantrone, paclitaxel, sclareol, dexamethasone and methotrexate (), only sclareol, paclitaxel and mitoxantrone potentiated doxorubicin toxicity evaluated following the LDH release (–). Enhancing doxorubicin toxicity is a major concern since the acquisition of doxorubicin resistance is linked to a poor prognosis.Citation2 We report evaluation of chemotherapy regimens involving doxorubicin in a spheroid model. Combination of doxorubicin with sclareol, paclitaxel or mitoxantrone increased doxorubicin toxicity. The use of doxorubicin as the second drug resulted in less toxicity than the addition of mitoxantrone, paclitaxel, cisplatin, sclareol, dexamethasone or methotrexate after doxorubicin or their co-administration, the priming regimen was also demonstrated to have low off-target toxicity in clinical trials.Citation16,Citation37,Citation38
In addition, this study outlines higher anticancer nucleosome-specific monoclonal antibody (2C5) binding to spheroids compared with monolayers. This enhanced selectivity toward spheroids may be due to the higher fraction of membrane associated nucleosomes in spheroids compared with monolayer cultures. Indeed, induction of cell death enhanced 2C5 binding to surviving ST 49 cells after apoptosis induction and evidence of dead cells in untreated spheroids was reported.Citation25,Citation49
In conclusion, this study strengthens the likelihood that spheroids can be used to adequately assess drug combinations as the schedule-dependencies observed for dexamethasone, cisplatin and paclitaxel were in agreement with the data from clinical studiesCitation16,Citation37,Citation38,Citation47 and in accordance with in vitro studies for sclareol and dexamethasone.Citation27,Citation44 Finally, the different efficacies obtained with drug combinations including free, micellar or liposomal doxorubicin and the cancer cell-specific antibody binding to spheroids open opportunities for the testing of tumor-targeted drug formulations in spheroid models.
Materials and Methods
Materials
NCI-ADR-RES cells were obtained from the National Cancer Institute. DMEM (Dulbecco’s modified Eagle’s medium), heat-inactivated fetal bovine serum (FBS), streptomycin and penicillin were purchased from Cellgro (Herndon). Doxorubicin hydrochloride (D1515-10MG), paclitaxel (T7402-5MG), dexamethasone (D1756-25MG), mitoxantrone dihydrochloride (M6545-10MG), HEPES [(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], DNase I, Triton X-100, triethylamine, methotrexate (M9929-25MG) and sclareol (357995-1G) were from Sigma Aldrich. DSPE-PEG 2000 (1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000) (ammonium salt, 880128C) was from Avanti Polar Lipids. Cisplatin was from Bedford Laboratories. Donkey anti-mouse FITC (fluorescein isothiocyanate) antibody was from (715-096-150, Jackson Immuno Research). Hoechst 33342 was purchased from Invitrogen (H3570). Monoclonal cancer-specific antinuclear autoantibody 2C5 was produced by Harlan Bioproducts using a hybridoma cell line from our laboratory.Citation35 Mouse myeloma ascites IgG2a was purchased from MP Biomedicals. Amicon Ultracel-100K filters were obtained through Millipore (UFC810024). Sodium dodecyl sulfate (SDS), plates, flasks, agarose with a high gelling temperature (ICN800666, Superfrost* plus gold slides (12-550-15), and fluoromount were from Fisher Scientific. Optimal cutting temperature medium (RT 62550-01) was from Electron Microscopy Sciences. Liposomal doxorubicin (Lipo-Dox) was purchased from Sun Pharma. Accumax was from Innovative Cell Technologies, Inc. The CytoTox 96 Non-Radioactive Cytotoxicity Assay kit was from Promega (G1788).
Preparation of doxorubicin micelles
Micelles containing doxorubicin were prepared according to Shuai et al. and Tang et al.Citation47 Doxorubicin HCl in methanol was first reacted with triethylamine (1:2 molar ratio, 1 h RT). Then, doxorubicin base was mixed with PEG-2000-DSPE in chloroform (molar ratio doxorubicin / PEG 1:2). The polymer/drug film was formed by removal of organic solvent using rotary evaporation. The film was hydrated with 10 mM HEPES 150 mM NaCl pH 7.4 for 30 min at 37°C with orbital shaking. Finally, the preparation was filtered through 0.45 µm and Ultracel-100K filters to remove un-encapsulated drug. Size and size distribution of the micelles obtained were determined by dynamic light scattering using a Beckman Coulter N4 PLUS size analyzer (Brea, CA) using weight analysis. The encapsulation efficiency of doxorubicin estimated by fluorescence was 65–80%.
Formation of spheroids
NCI-ADR-RES cells were grown at 37°C at 5% CO2 in DMEM supplemented with 50 U/mL penicillin, 50 µg/mL streptomycin and 10% FBS. Spheroids of 400–500 µm diameter were formed from 10,000 cells in 96-well plates according to Yang et al. with modifications.Citation10 DMEM with 1.5% agarose was used instead of poly(2-hydroxyethyl) methacrylate to prevent cell adhesion, and plates were centrifuged for 15 min at 1,500 rcf instead of 5 min at 800 rpm. Spheroid formation was monitored using a Nikon Eclipse E400 microscope (Nikon, Inc.) at 10 X magnification and with a Spot Insight 3.2.0 camera with Spot Advanced software (Spot Imaging).
Sectioning
For sections, spheroids were first fixed overnight with neutral buffered formalin containing 0.5% methylene blue. They were then embedded in freezing medium and cut with a Minotome Plus Cryosta (Triangle Biomedial Systems) microtome to obtain 16 µm sections. Finally, the sections were counterstained with 5 μM Hoechst 33342 and imaged by epifluorescence microscopy with the Nikon Eclipse E400 microscope.
Doxorubicin accumulation
Doxorubicin accumulation in spheroids was evaluated by fluorescence according to the work of Mellor and Callaghan with modifications.Citation6 Cells were incubated with drug 24 h and dispersed by incubation for 2 h with 2% SDS at 37°C and vortexing. Briefly, spheroids were incubated with doxorubicin in medium. After dispersal, doxorubicin accumulation was measured with a Multi-mode microplate reader (Synergy HT, Biotek; λex = 485/20 nm λem = 590/35 nm). Doxorubicin concentration was calculated based on standards in 2% SDS. The amount of doxorubicin was expressed as nmol/mg of protein after protein determination using a micro BCA assay (Pierce). For pretreatment experiments, spheroids were incubated with cisplatin, sclareol, dexamethasone, methotrexate, mitoxantrone or paclitaxel at 40 µM either for 48 h prior to incubation with doxorubicin for an additional 24 h. In experiments performed with liposomal doxorubicin (LDOX) or micellar doxorubicin (MDOX), formulations were used at an equivalent concentration of free doxorubicin.
Antinuclear antibody binding
Spheroids were dissociated by incubation with Accumax for 30 min at 37°C before the resuspension in PBS. Cells from monolayers were grown to about 80% confluency, detached and suspended in PBS. Cells from spheroids or monolayers were incubated for 1 h at 4°C with the 2C5 monoclonal anti-nucleosome antibody (final concentration 30 ng/mL) or the corresponding isotype-matched control antibody in PBS with 1% bovine serum albumin. After washing, cells were stained with the FITC-conjugated secondary antibody at 1/100 for 1 h at 4°C. Finally, cells from monolayers or spheroids were analyzed by flow cytometry with a FACSCalibur flow cytometer (Beckton Dickinson) recording 20,000 gated events. The ratio of mean fluorescence intensity values of 2C5 to isotype-matched IgG control incubated cells was used to evaluate binding selectivity.
DNA fragmentation assay
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was done with a Fluorescein FragEL DNA Fragmentation Detection Kit (QIA33-1EA, Calbiochem) as indicated by supplier. A positive control (DNase I treatment) was included. Samples were analyzed with the Nikon Eclipse 400 epifluorescence microscope.
Cell viability
Cell viability after treatments was measured with a Cytotox 96 Non-Radioactive Cytotoxicity kit (Promega) as reported by Howes et al.Citation52 Spheroids were cultured in a medium containing 5% of serum as recommended by the supplier. Lactate dehydrogenase (LDH) release was normalized to total LDH content following spheroid lysis with a medium containing 0.9% Triton X-100.
Disclosure of Potential Conflicts of Interest
No conflict of interest are to be declared.
Acknowledgments
This work was supported by the NIH grant U54CA151881 to V.P.T.
References
- World Cancer Report, Boyle P and Levin B, Editors. 2008, International Agency for Research on Cancer: Lyon.
- Aas T, Børresen AL, Geisler S, Smith-Sørensen B, Johnsen H, Varhaug JE, et al. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med 1996; 2:811 - 4; http://dx.doi.org/10.1038/nm0796-811; PMID: 8673929
- Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res 2002; 8:878 - 84; PMID: 11895922
- Lankelma J, Dekker H, Luque FR, Luykx S, Hoekman K, van der Valk P, et al. Doxorubicin gradients in human breast cancer. Clin Cancer Res 1999; 5:1703 - 7; PMID: 10430072
- Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance?. Crit Rev Oncol Hematol 2000; 36:193 - 207; http://dx.doi.org/10.1016/S1040-8428(00)00086-X; PMID: 11033306
- Mellor HR, Callaghan R. Accumulation and distribution of doxorubicin in tumour spheroids: the influence of acidity and expression of P-glycoprotein. Cancer Chemother Pharmacol 2011; 68:1179 - 90; http://dx.doi.org/10.1007/s00280-011-1598-8; PMID: 21400240
- Marcucci F, Corti A. How to improve exposure of tumor cells to drugs: promoter drugs increase tumor uptake and penetration of effector drugs. Adv Drug Deliv Rev 2012; 64:53 - 68; http://dx.doi.org/10.1016/j.addr.2011.09.007; PMID: 21983328
- Wang J, Lu Z, Gao Y, Wientjes MG, Au JL. Improving delivery and efficacy of nanomedicines in solid tumors: role of tumor priming. Nanomedicine (Lond) 2011; 6:1605 - 20; http://dx.doi.org/10.2217/nnm.11.141; PMID: 22077464
- Casey RC, Burleson KM, Skubitz KM, Pambuccian SE, Oegema TR Jr., Ruff LE, et al. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol 2001; 159:2071 - 80; http://dx.doi.org/10.1016/S0002-9440(10)63058-1; PMID: 11733357
- Yang TM, Barbone D, Fennell DA, Broaddus VC. Bcl-2 family proteins contribute to apoptotic resistance in lung cancer multicellular spheroids. Am J Respir Cell Mol Biol 2009; 41:14 - 23; http://dx.doi.org/10.1165/rcmb.2008-0320OC; PMID: 19097992
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 2010; 148:3 - 15; http://dx.doi.org/10.1016/j.jbiotec.2010.01.012; PMID: 20097238
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007; 130:601 - 10; http://dx.doi.org/10.1016/j.cell.2007.08.006; PMID: 17719539
- Kim TH, Mount CW, Gombotz WR, Pun SH. The delivery of doxorubicin to 3-D multicellular spheroids and tumors in a murine xenograft model using tumor-penetrating triblock polymeric micelles. Biomaterials 2010; 31:7386 - 97; http://dx.doi.org/10.1016/j.biomaterials.2010.06.004; PMID: 20598741
- Gil-Cardeza ML, Villaverde MS, Fiszman GL, Altamirano NA, Cwirenbaum RA, Glikin GC, et al. Suicide gene therapy on spontaneous canine melanoma: correlations between in vivo tumors and their derived multicell spheroids in vitro. Gene Ther 2010; 17:26 - 36; http://dx.doi.org/10.1038/gt.2009.107; PMID: 19741734
- Meng E, Taylor B, Ray A, Shevde LA, Rocconi RP. Targeted inhibition of telomerase activity combined with chemotherapy demonstrates synergy in eliminating ovarian cancer spheroid-forming cells. Gynecol Oncol 2012; 124:598 - 605; http://dx.doi.org/10.1016/j.ygyno.2011.11.018; PMID: 22115853
- Frassineti GL, Zoli W, Silvestro L, Serra P, Milandri C, Tienghi A, et al. Paclitaxel plus doxorubicin in breast cancer: an Italian experience. Semin Oncol 1997; 24:Suppl 17 S17 - 9, S17-25; PMID: 9374087
- Vollmer T, Panitch H, Bar-Or A, Dunn J, Freedman MS, Gazda SK, et al. Glatiramer acetate after induction therapy with mitoxantrone in relapsing multiple sclerosis. Mult Scler 2008; 14:663 - 70; http://dx.doi.org/10.1177/1352458507085759; PMID: 18424479
- Imazawa M, Kojima T, Boku N, Onozawa Y, Hironaka S, Fukutomi A, et al. Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer 2009; 12:153 - 7; http://dx.doi.org/10.1007/s10120-009-0517-8; PMID: 19890695
- Wong HL, Shen Z, Lu Z, Wientjes MG, Au JL. Paclitaxel tumor-priming enhances siRNA delivery and transfection in 3-dimensional tumor cultures. Mol Pharm 2011; 8:833 - 40; http://dx.doi.org/10.1021/mp1004383; PMID: 21417439
- Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther 1999; 290:871 - 80; PMID: 10411604
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore VdelG, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 2011; 334:1129 - 33; http://dx.doi.org/10.1126/science.1206727; PMID: 22033517
- Davies CdeL, Lundstrøm LM, Frengen J, Eikenes L, Bruland S ØS, Kaalhus O, et al. Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res 2004; 64:547 - 53; http://dx.doi.org/10.1158/0008-5472.CAN-03-0576; PMID: 14744768
- Fayad W, Brnjic S, Berglind D, Blixt S, Shoshan MC, Berndtsson M, et al. Restriction of cisplatin induction of acute apoptosis to a subpopulation of cells in a three-dimensional carcinoma culture model. Int J Cancer 2009; 125:2450 - 5; http://dx.doi.org/10.1002/ijc.24627; PMID: 19670329
- Trédan O, Garbens AB, Lalani AS, Tannock IF. The hypoxia-activated ProDrug AQ4N penetrates deeply in tumor tissues and complements the limited distribution of mitoxantrone. Cancer Res 2009; 69:940 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-08-0676; PMID: 19176397
- Dunkern TR, Mueller-Klieser W. Quantification of apoptosis induction by doxorubicin in three types of human mammary carcinoma spheroids. Anticancer Res 1999; 19:4B 3141 - 6; PMID: 10652603
- Tsukioka Y, Matsumura Y, Hamaguchi T, Koike H, Moriyasu F, Kakizoe T. Pharmaceutical and biomedical differences between micellar doxorubicin (NK911) and liposomal doxorubicin (Doxil). Jpn J Cancer Res 2002; 93:1145 - 53; http://dx.doi.org/10.1111/j.1349-7006.2002.tb01217.x; PMID: 12417045
- Dimas K, Papadaki M, Tsimplouli C, Hatziantoniou S, Alevizopoulos K, Pantazis P, et al. Labd-14-ene-8,13-diol (sclareol) induces cell cycle arrest and apoptosis in human breast cancer cells and enhances the activity of anticancer drugs. Biomed Pharmacother 2006; 60:127 - 33; http://dx.doi.org/10.1016/j.biopha.2006.01.003; PMID: 16527443
- Akutsu M, Furukawa Y, Tsunoda S, Izumi T, Ohmine K, Kano Y. Schedule-dependent synergism and antagonism between methotrexate and cytarabine against human leukemia cell lines in vitro. Leukemia 2002; 16:1808 - 17; http://dx.doi.org/10.1038/sj.leu.2402573; PMID: 12200697
- Son K, Huang L. Exposure of human ovarian carcinoma to cisplatin transiently sensitizes the tumor cells for liposome-mediated gene transfer. Proc Natl Acad Sci U S A 1994; 91:12669 - 72; http://dx.doi.org/10.1073/pnas.91.26.12669; PMID: 7809098
- Wang H, Li M, Rinehart JJ, Zhang R. Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: in vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res 2004; 10:1633 - 44; http://dx.doi.org/10.1158/1078-0432.CCR-0829-3; PMID: 15014014
- Kohno N, Ohnuma T, Kaneko M, Holland JF. Interactions of doxorubicin and cis-platin in squamous carcinoma cells in culture. Br J Cancer 1988; 58:330 - 4; http://dx.doi.org/10.1038/bjc.1988.213; PMID: 3179185
- Leonard RC, Cornbleet MA, Kaye SB, Soukop M, White G, Hutcheon AW, et al. Mitoxantrone versus doxorubicin in combination chemotherapy for advanced carcinoma of the breast. J Clin Oncol 1987; 5:1056 - 63; PMID: 3298559
- Gabizon A, Shmeeda H, Grenader T. Pharmacological basis of pegylated liposomal doxorubicin: impact on cancer therapy. Eur J Pharm Sci 2012; 45:388 - 98; http://dx.doi.org/10.1016/j.ejps.2011.09.006; PMID: 21933707
- Bontenbal M, Planting AS, Rodenburg CJ, Dees A, Verweij J, Bartels CC, et al. Weekly low-dose mitoxantrone plus doxorubicin as second-line chemotherapy for advanced breast cancer. Breast Cancer Res Treat 1992; 21:133 - 8; http://dx.doi.org/10.1007/BF01836959; PMID: 1627816
- ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res 2009; 15:1973 - 80; http://dx.doi.org/10.1158/1078-0432.CCR-08-2392; PMID: 19276264
- Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A 2003; 100:6039 - 44; http://dx.doi.org/10.1073/pnas.0931428100; PMID: 12716967
- Gianni L, Munzone E, Capri G, Fulfaro F, Tarenzi E, Villani F, et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 1995; 13:2688 - 99; PMID: 7595726
- Offidani M, Corvatta L, Marconi M, Visani G, Alesiani F, Brunori M, et al. Low-dose thalidomide with pegylated liposomal doxorubicin and high-dose dexamethasone for relapsed/refractory multiple myeloma: a prospective, multicenter, phase II study. Haematologica 2006; 91:133 - 6; PMID: 16434383
- Toh YM, Li TK. Mitoxantrone inhibits HIF-1α expression in a topoisomerase II-independent pathway. Clin Cancer Res 2011; 17:5026 - 37; http://dx.doi.org/10.1158/1078-0432.CCR-11-0235; PMID: 21653687
- Song X, Liu X, Chi W, Liu Y, Wei L, Wang X, et al. Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol 2006; 58:776 - 84; http://dx.doi.org/10.1007/s00280-006-0224-7; PMID: 16532342
- Golubovskaya V, Ho B, Zheng M, Magis A, Ostrov D, Cance W. Mitoxantrone Targets The ATP-Binding Site Of FAK, Binds The FAK Kinase Domain And Decreases FAK, Pyk-2, C-Src, And IGF-1R, Pyk-2 In Vitro Kinase Activities. Anticancer Agents Med Chem 2012; PMID: 22292772
- Chen YY, Wang ZX, Chang PA, Li JJ, Pan F, Yang L, et al. Knockdown of focal adhesion kinase reverses colon carcinoma multicellular resistance. Cancer Sci 2009; 100:1708 - 13; http://dx.doi.org/10.1111/j.1349-7006.2009.01217.x; PMID: 19500106
- Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther 2007; 322:80 - 8; http://dx.doi.org/10.1124/jpet.107.121632; PMID: 17420296
- Dehghani L, Karbalaei KH, Nematollahi M, Nasr Esfahani MH, Baharvand H. Dexamethasone reduces doxorubicin-induced oxidative stress and caspase 3 activity in MESC-derived pure cardiomyocytes. Cell Journal (Yakhteh), 2011; 13(Suppl. 3 (7th Congress on Stem Cell Biology and Technology)): 28-29.
- Chlebowski RT, Bull F, Irwin L, Pugh R, Weiner JM, Bateman JR. Doxorubicin and methotrexate on a weekly schedule in patients with sarcomas. Oncology 1987; 44:210 - 3; http://dx.doi.org/10.1159/000226479; PMID: 3475644
- Muro H, Acuña LR, Castagnari A, Blajman C, Schmilovich A, Hidalgo A, et al. Sequential methotrexate, 5-fluorouracil (high-dose), and doxorubicin for advanced gastric cancer. Cancer Treat Rep 1986; 70:1333 - 4; PMID: 3768875
- Hrushesky WJ, Bjarnason GA. Circadian cancer therapy. J Clin Oncol 1993; 11:1403 - 17; PMID: 8315438
- Perilongo G, Maibach R, Shafford E, Brugieres L, Brock P, Morland B, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 2009; 361:1662 - 70; http://dx.doi.org/10.1056/NEJMoa0810613; PMID: 19846851
- Iakoubov LZ, Torchilin VP. Nucleosome-releasing treatment makes surviving tumor cells better targets for nucleosome-specific anticancer antibodies. Cancer Detect Prev 1998; 22:470 - 5; http://dx.doi.org/10.1046/j.1525-1500.1998.00055.x; PMID: 9727629
- Tang N, Du G, Wang N, Liu C, Hang H, Liang W. Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J Natl Cancer Inst 2007; 99:1004 - 15; http://dx.doi.org/10.1093/jnci/djm027; PMID: 17596572
- Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release 2004; 98:415 - 26; http://dx.doi.org/10.1016/j.jconrel.2004.06.003; PMID: 15312997
- Howes AL, Chiang GG, Lang ES, Ho CB, Powis G, Vuori K, et al. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther 2007; 6:2505 - 14; http://dx.doi.org/10.1158/1535-7163.MCT-06-0698; PMID: 17766839