Abstract
Orally administered small molecule receptor tyrosine kinase inhibitors (RTKIs) are increasingly common treatments for cancer, both alone and in combination with chemotherapy. However, their side effect profiles and the underlying mechanisms of such are not yet fully elucidated. Management of their most common dose limiting side effect, diarrhea, has been hampered by a lack of suitable animal models. We aimed to develop a clinically relevant rat model of RTKI-induced diarrhea that could be utilized for investigating supportive care interventions and pharmacokinetics. Albino Wistar rats were treated daily for 4 weeks with various concentrations of lapatinib to determine the optimal dose for development of diarrhea. This was then followed by an experiment with addition of paclitaxel once weekly for 4 weeks to observe effects of combination drug treatment on diarrhea. Data regarding animal tolerance to the treatment, organ weights, circulating lapatinib concentration and histopathology were collected weekly. Lapatinib caused diarrhea in rats that was dose-dependent. Diarrhea occurred without causing significant intestinal histopathology. Follow up experiments are currently underway to determine the exact pathogenesis and mechanisms of lapatinib-induced diarrhea and potential protective strategies.
Introduction
Orally administered small molecule receptor tyrosine kinase inhibitors (RTKIs) are increasingly common treatments for cancer. However, their side effect profiles are not yet fully elucidated. Toxic effects can include cardiac, skin, hepatic and gastrointestinal.Citation1 While skin toxicity has been extensively studied and is associated with response,Citation2 gastrointestinal toxicity has received relatively little attention. Importantly, diarrhea is one of the most common adverse events recorded following treatment with small molecule RTKI’s.Citation3 The recent TEACH trial found that 60% of lapatinib-treated patients experienced diarrhea and this was the most frequent cause of dose reduction.Citation4 Diarrhea may be detrimental for achieving full dosage of orally administered agents,Citation5 although the influence diarrhea might have on drug absorption and efficacy has yet to be investigated.
Lapatinib (GW572016/Tykerb® GlaxoSmithKline) is an orally administered small molecule RTKI targeting ErbB-1 (EGFR) and ErbB-2 (HER2).Citation6 Lapatinib’s anti-cancer effect in HER2 amplified breast cancer is mediated through inhibition of downstream signaling to extracellular signal-related kinase (ERK)-1/2 and phosphatidylinositol 3′kinase (PI3K)/Akt pathways. ERK and PI3K have numerous roles within the cell primarily concerning growth, proliferation and survival.Citation7 In 2007, the U.S. Food and Drug Administration granted approval for lapatinib in combination with capecitabine for the treatment of advanced and metastatic breast cancer in patients that have previously received an anthracycline, a taxane and trastuzumab and whose tumors overexpress HER2.Citation8 More recently, thanks to positive results from the large multinational trial EGF30008,Citation9 lapatinib has been granted accelerated approval for treatment of postmenopausal women with hormone receptor positive metastatic breast cancer that overexpress HER2 and for whom hormonal therapy is indicated. There are also numerous trials currently underway investigating lapatinib in first line metastatic disease (COMPLETE trial), neoadjuvant (NEO-ALTO trial) and adjuvant therapy (ALTO and TEACH trials).
Diarrhea is the most frequently reported side effect of lapatinib monotherapy.Citation10 A pooled analysis of diarrhea events has also shown that diarrhea is worsened when lapatinib is combined with capecitabine.Citation11 More recently, increased incidence and severity of diarrhea has also been observed when lapatinib is combined with taxane chemotherapy, leading to a need for dose reduction in this setting.Citation12 Current favored theories for the underlying pathology of diarrhea induced by therapies which target EGFR include a relative increase in chloride secretion or direct mucosal damage.Citation13 EGFR has been shown to play an important role in regulation of chloride secretion in the normal and inflamed colon.Citation14 Studies using EGFR knockout mice and other small molecule EGFR inhibitors have described mucosal atrophy supporting a role for direct mucosal damage.Citation15,Citation16 Inhibition of HER2 alone with trastuzumab has not been associated with as frequent gastrointestinal toxicities clinically,Citation12 which may be due to mode of delivery (intravenous vs oral) or indicate that EGFR blockage, rather than HER2 blockade, is primarily responsible for intestinal dysfunction. However, further in vivo experiments are required to develop these hypotheses and to gain a better understanding of lapatinib-specific intestinal changes and effects on drug absorption.
To address the current gap in knowledge regarding mechanisms of small molecule RTKI-induced diarrhea, we have developed a rat model of lapatinib-induced diarrhea. Through a series of experiments, we have shown for the first time that EGFR/HER2 inhibition by lapatinib leads to diarrhea without significant damage to intestinal mucosa in rats, and that diarrhea is potentiated by paclitaxel without reducing drug exposure.
Results
Lapatinib-induced diarrhea
The first experiment aimed to establish a dose of lapatinib which induced diarrhea in 50% of rats, which is an incidence that is similar to what is observed in clinical trials. One hundred and twenty rats were randomly assigned to receive; vehicle, 100, 240 or 500 mg/kg lapatinib daily by oral gavage for 28 d (n = 30/arm). Groups of rats from each arm were killed at the end of each week (n = 6). One group of rats were observed for a further 16 weeks to assess any late onset diarrhea or chronic tissue changes. Observations were conducted twice daily. All animals completed treatment without signs of distress or adverse reaction to lapatinib. Two rats were removed from the study (1 from 500 mg/kg and 1 from control group) due to biting off the gavage needle and swallowing it which lead to weight loss. Growth rates of lapatinib treated rats were not significantly different to control animals (Fig. S1). At day 28, the control group had on average gained 22.2 ± 4.03, the 100 mg/kg group had gained 27.8 ± 4.78, the 240 mg/kg group had gained 18.3 ± 4.56, and the 500 mg/kg group had gained 16.3 ± 2.82 percent body weight compared with day 1 of treatment (p > 0.05).
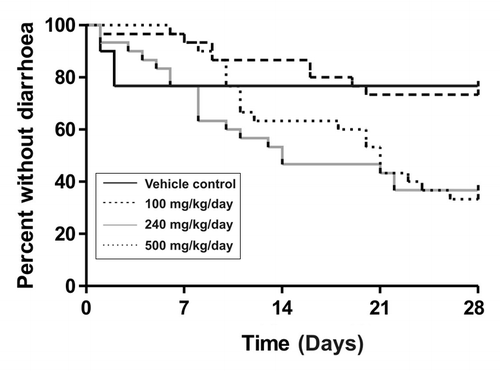
Diarrhea was dose-dependent and mild (Grade 1) to moderate (Grade 2) in response to lapatinib treatment. Mild diarrhea was defined as soft stools (G1), moderate diarrhea as loose stools with perianal staining of fur (G2), and severe diarrhea as watery stools +/− mucous with fur staining incorporating hind legs (Grade 3). A total of 23, 27, 63 and 67% of rats had diarrhea during treatment with vehicle, 100, 240 and 500 mg/kg lapatinib, respectively (). Lapatnib-induced diarrhea was intermittent and short lived (lasting 1 to 3 d only). Diarrhea incidence peaked in week 3 in the 240 mg/kg rats and in week 4 in the 500 mg/kg rats. Diarrhea resolved within 2 d of treatment cessation. All diarrhea seen in vehicle control animals occurred during the first week, lasted for 1 d, and did not reappear. This very early diarrhea was considered to be associated with restraint stress rather than the vehicle as later diarrhea was not noted. The 240 mg dose was chosen for all further experiments.
Figure 1. Diarrhea onset and group proportions in lapatinib dose-finding experiment. Survival curves show days to first diarrhea in rats treated with different doses of lapatinib (n = 30).
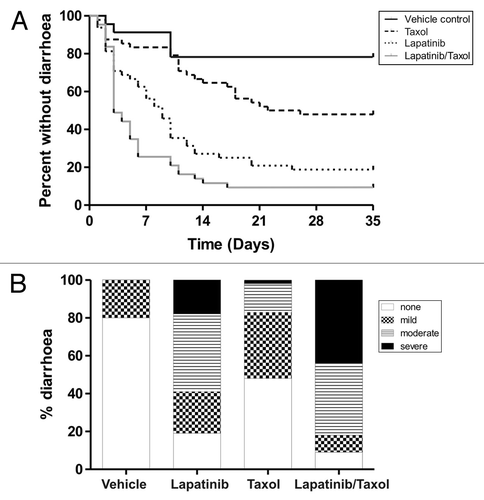
The second experiment investigated the combination of paclitaxel and lapatinib in the development of diarrhea. 168 rats were randomly assigned to; control, lapatinib, paclitaxel or combined lapatinib/paclitaxel groups (n = 48/treatment arm and n = 24 control arm). Lapatinib was given daily by gavage for 4 weeks at 240 mg/kg. At the start of each week paclitaxel was administered (at the same time as lapatinib) intraperitoneally at 12 mg/kg. This dose was chosen based on a small pilot study which found 12 mg/kg paclitaxel was safe to administer over 4 weeks (Fig. S2). Rats (control group n = 4, treatment groups n = 8) were killed at the end of each week for 5 weeks. The 5th week kill point was to observe the recovery period as rats did not receive any treatment during that week. A final group from each experimental arm were killed at the end of week 20. At the first dose, addition of 12 mg/kg paclitaxel to 240 mg/kg lapatinib caused severe weight loss which peaked on day 5 (‑7.4 ± 6.2% loss of bodyweight). Indeed, two rats had to be removed for excess weight loss (> 15% of total starting body weight) as required by the ethical constraints of the study. Severe intestinal pathology was noted in these animals (Fig. S3). All other groups had similar weight gain during the treatment period (). The subsequent doses of paclitaxel (given weeks 2–4) were therefore administered at 9 mg/kg, which prevented weight gain for 5 d but did not induce significant weight loss. Rats treated with combined lapatinib and paclitaxel reached control weights by day 40. General appearance of rats in the lapatinib + paclitaxel treatment arm included mild piloerection, chromodacryorrhea, and malaise, as assessed by conventional clinical record scoring specific for rat which has been used extensively by our group. Diarrhea was observed in 92% of rats treated with paclitaxel and lapatinib on at least one occasion, and 88% advanced to at least moderate severity. The combination group had diarrhea with an earlier onset (p = 0.034) that was more severe in nature (p = 0.0002) compared with lapatinib alone. Paclitaxel on its own caused diarrhea in 52% of rats, of which 19% progressed to moderate severity. 81% of rats treated with lapatinib alone had diarrhea on at least one occasion, of which 67% was at least moderate severity (). Severity of diarrhea varied greatly from mild to severe, and lasted 1 - 4 d during 1 or more episodes. A small proportion of vehicle control animals (20%) again had early onset, short lived mild diarrhea during the experiment. All diarrhea was resolved by day 33. No chronic onset diarrhea was observed for either experiment.
Figure 2. Daily bodyweight in combination treatment experiment. Graph shows the daily weight gain or loss of rats over 5 weeks in each group. Data presented is mean ± SEM (n = 24 in the control arm and n = 48 in the treatment arms).
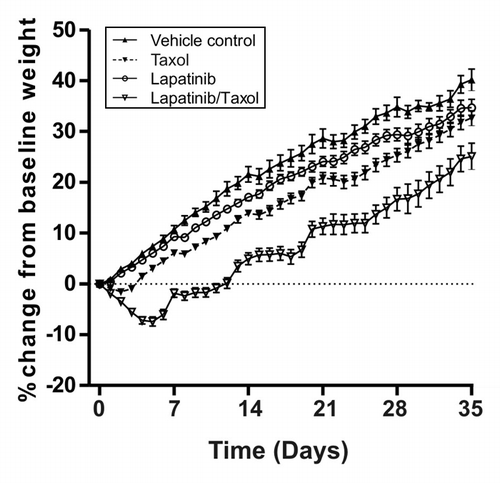
Figure 3. Diarrhea onset and severity in combination treatment experiment. (A) Survival curves show days to first diarrhea in rats across the different groups Rats treated with combined lapatinib and paclitaxel (Taxol) had significantly earlier diarrhea (p = 0.034, Log Rank Test) compared with lapatinib only treated rats. (B) Graph shows proportions of diarrhea severity across the different groups (n = 24 in control arm, n = 48 in treatment arms). Rats treated with combined lapatinib and paclitaxel (Taxol) had a significantly higher proportion of severe diarrhea (p = 0.0002, Chi Squared Test) compared with lapatinib only treated rats.
Pathology
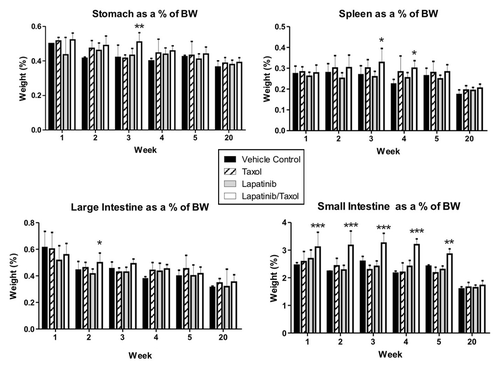
At necropsy, organ weights were not significantly different across groups for the dose-finding monotherapy experiment. In the combination treatment experiment, paclitaxel added to lapatinib caused a significant increase in small intestine weight relative to body weight at weeks 1 to 5 (p < 0.001) compared with controls, lapatinib only and paclitaxel only (). Absolute small intestinal weight was also higher in the lapatinib + paclitaxel treated animals (data not shown).
Figure 4. Organ weights in combination treatment experiment. Graphs show organ weights as a percentage of total body weight across the different groups. Data presented is mean ± SD (n = 4 in control arm and n = 8 in treatment arms). Relative small intestinal was significantly higher between weeks 1 and 5 in rats treated with combined lapatinib and paclitaxel (Taxol) compared with all other groups (p < 0.001, Two-way ANOVA with Bonferroni post-test). Relative large intestinal weight was significantly increased in the combined lapatinib and paclitaxel (Taxol) rats at week 2 compared with lapatinib only treated rats (p < 0.05, Two-way ANOVA with Bonferroni post-test). Relative stomach weight was significantly increased at week 3 in the combined lapatinib and paclitaxel (Taxol) group compared with lapatinib only treated and paclitaxel (Taxol) only treated groups (p < 0.01, Two-way ANOVA with Bonferroni post-test). Relative spleen weight was significantly increased at week 3 in the combined lapatinib and paclitaxel (Taxol) group compared with lapatinib only treated rats (p < 0.05, Two-way ANOVA with Bonferroni post-test) and at week 4 compared with vehicle controls (p < 0.05, Two-way ANOVA with Bonferroni post-test).
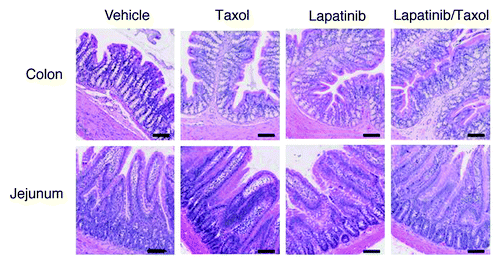
No significant pathology was reported in the intestines of rats treated with lapatinib monotherapy at any dose. Addition of paclitaxel to lapatinib in the combination experiment resulted in an occasional apoptotic enterocyte in colonic crypts. The jejunum was unremarkable (). No changes due to paclitaxel alone were observed.
Circulating lapatinib concentrations
Trough lapatinib levels were analyzed by LC/MS/MS weekly in the lapatinib dose finding experiment and the combination treatment experiment (). The average Cmin for lapatinib in the dose finding experiment was 64.0 ± 17.7 ng/mL, 370.6 ± 88.4 ng/mL, and 1757 ± 390.6 ng/mL for the 100 mg/kg, 240 mg/kg and 500 mg/kg group, respectively.
Figure 6. Serum lapatinib concentration. (A) Serum lapatinib concentrations over time in the lapatinib dose finding experiment (n = 6). (B) Overall group mean serum lapatinib concentrations in the lapatinib dose finding experiment (n = 6, data combined over 4 weeks). (C) Serum lapatinib concentrations over time in the combination treatment experiment (n = 8). (D) Overall group mean serum lapatinib concentrations in the combination treatment experiment (n = 8, data combined over 4 weeks). Rats in the combination group had significantly higher mean lapatinib concentrations compared with lapatinib only treated rats (p = 0.027, Repeated measures Two-way ANOVA). Data presented is mean ± SEM.
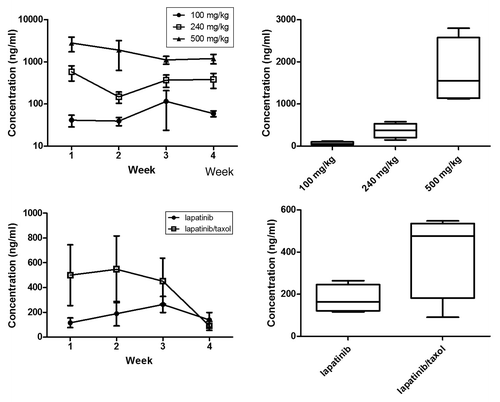
In the combination experiment, the average Cmin was 177.1 ± 32.8 ng/mL for 240 mg/kg lapatinib only treated rats and 397.7 ± 104.1 ng/mL for the 9 mg/kg paclitaxel and 240 mg/kg lapatinib treated rats. Concurrent treatment with paclitaxel significantly increased systemic lapatinib concentrations (p = 0.027).
Discussion
The purpose of this experiment was to develop a rat model of orally administered small molecule receptor tyrosine kinase inhibitor-induced diarrhea that could be interrogated for mechanisms of gastrointestinal mucosal injury and then be used to trial potential interventions and provide information about the influence of diarrhea on active serum concentrations. This study was conducted over two phases; the first phase a dose-finding experiment to determine the optimal concentration of lapatinib administration to induce 50% diarrhea, and the second phase a combination treatment experiment to determine the effect of concurrent chemotherapy on lapatinib-induced diarrhea. We were successful in designing a model that reflects the clinical picture by inducing moderate diarrhea in 60% of rats with daily lapatinib treatment. Analysis of diarrhea events in clinical trials of lapatinib have found that around 51% of patients experience diarrhea which is predominantly mild to moderate in nature.Citation11 However, it is worth noting that the dose required to cause diarrhea in this rat model was higher than that used clinically in patients.
A phase I dose escalation trial by Burris et al. (2005) found the geometric mean Cmin in patients treated with 1200 mg daily lapatinib to be 0.3 μg per mL.Citation19,Citation20 Our dose-finding experiment found that giving 240 mg/kg of daily lapatinib resulted in an average Cmin of approximately 0.37 μg per mL. Since this dose caused more than 50% moderate diarrhea at a reasonable Cmin, it was used in the further combination treatment experiment. Pharmacokinetic studies of lapatinib have found that lapatinib exposure varies considerably in patients,Citation21 which was also noted in our rat study. The causes for this are currently unknown, but may be influenced by the presence of food in the stomach which has been shown to increase bioavailability of lapatinib.Citation21 As such, lapatinib is directed to be taken at least one hour before or after food. In our study, rats were not fasted before administration of lapatinib which may have contributed to the variation seen. Paclitaxel has also been shown to cause increased exposure to lapatinib.Citation22 Our current study found a significant increase in lapatinib Cmin in rats co-treated with paclitaxel, despite increased diarrhea severity and incidence in this group. Further investigation is warranted to assess the changes in drug exposure following concurrent treatment regimens, since in theory, more rapid intestinal transit due to induction of diarrhea might reduce absorption of oral drugs and hence interfere with their anticancer effect, although our current study findings did not support this concern. Indeed, sub-analysis of rats treated with lapatinib or combination lapatinib plus paclitaxel that experienced diarrhea showed similar circulating lapatinib concentrations to rats that did not experience diarrhea in the same group (data not shown).
Our study found no significant tissue pathology in lapatinib-treated rats. Therefore our study does not support the theory of TKI-induced direct mucosal damage leading to diarrhea.Citation13 Treatment with conventional chemotherapy drugs causes atrophy of intestinal mucosa leading to a secretory/osmotic-type diarrhea due to an inability to control solute absorption and secretion through decreased surface area.Citation23 Lapatinib-treated intestinal tissue appeared histologically similar to vehicle-treated control tissue, and did not exhibit atrophy such as seen in mice treated with another EGFR inhibitor, gefitinib.Citation15 The lack of histological injury may explain the quick resolution of diarrhea seen with treatment removal in our study. It may also provide a plausible explanation as to why simple anti-diarrheal medications are generally effective in treating lapatinb-induced diarrhea. Further investigations are required to examine alternative mechanisms of diarrhea induced by lapatinib, and the effect of anti-diarrheal agents on lapatinib pharmacokinetics.
A limitation of the current study was our tissue collection time point. All histological samples were collected at the end of each week, effectively 7 d after injection of paclitaxel. Assessing tissue changes at this late time point potentially underestimates early tissue damage caused by the chemotherapy agent. It is likely that any early damage, such as epithelial atrophy, would be generally resolved by day 7 due to the speed of epithelial renewal of the intestinal epithelium. It is expected that paclitaxel may have caused a brief and mild epithelial injury in our rat model due to the frequent gastrointestinal toxicity which has been observed in patients treated with paclitaxel-based chemotherapy regimens.Citation24,Citation25 In addition, a single injection of intraperitoneal paclitaxel has been shown to induce epithelial apoptosis, loss of mucosal structure and bacterial translocation in rats,Citation26 albeit at a higher dose than that used in this study. However, further research is required to unequivocally confirm histological injury in our model. The actual dose used in the current study was determined by a small pilot experiment of clinically relevant doses,Citation27 and review of the literature based on paclitaxel-induced neuropathy.Citation28,Citation29 We aimed to find a dose that induced mild gastrointestinal effects without causing substantial weight loss as we were aware of the potential for more severe toxicity in the combination groups and wanted to avoid excessive loss of animals.
The only indirect suggestion of gut damage in the experiment was increased small intestinal weight in rats treated with combined lapatinib and paclitaxel. Increased small intestinal weight has been consistently observed with regenerative crypt hyperplasia following chemotherapy insult to the intestine in previous experiments.Citation30,Citation31 Since increased small intestinal weight were not seen in the paclitaxel only group or lapatinib only groups, it can be hypothesized that the combination regimen caused some early damage and that this was a factor of lapatinib-paclitaxel interactions leading to more severe gut injury and diarrhea. In further support, the two animals that were removed early from the study due to severe weight loss underwent histopathological analysis. The intestines showed severe multifocal cryptolysis and villous atrophy in the jejunum, and extensive crytolysis in the colon as assessed by expert tissue pathologist (JF). The cull time point was three days after paclitaxel and coincides with the expected peak gut injury as seen with other chemotherapy agents in rat models.Citation32,Citation33 Future studies are needed to explore the kinetics of tissue changes following treatment with paclitaxel, with or without lapatinib, and how the combination alters tissue responses.
In conclusion, the findings from this two-part study support the Albino Wistar rat as an appropriate choice to model lapatinib-induced diarrhea. This model will enable us to better interrogate the mechanisms underlying onset and severity of lapatinib and combination therapy-induced diarrhea. It will also be applied in future experiments to examine potential diarrhea intervention agents, and adapted to other small molecule RTKIs.
Methods and Materials
Chemicals
Lapatinib (GW 572016F) was provided by GlaxoSmithKline and arrived as the ditosylate salt. For all in vivo experiments lapatinib was diluted in 0.5% (w/w) hydroxypropylmethylcellulose with 0.1% polyoxyethylenesorbitan monooleate Tween®80 (Sigma-Aldrich) in sterile distilled water to a final concentration of 100 mg/ml. Paclitaxel (Ebewe Pharm) was diluted to a final concentration of 1.2 mg/ml in sterile saline.
Animal model
This study was approved by the Animal Ethics Committees of the Institute of Medical and Veterinary Science and The University of Adelaide, and complied with the National Health and Medical Research Council (Australia) Code of Practice for Animal Care in Research and Training (2004). All experiments were conducted on male (Charles River Laboratory) Albino Wistar rats (initial age approximately 8 weeks), conventionally housed under a 12 h light/dark cycle with ad libitum access to autoclaved standard rodent pellets and water delivered by dripper bottle. Six to eight rats were included in each group. Lapatinib (at doses between 100 and 500 mg/kg) was administered daily for four weeks via oral gavage using a soft plastic feeding tube (Instech Solomon). Paclitaxel (at doses between 9 and 12 mg/kg) was administered weekly for 4 weeks via intraperitoneal (i.p.) injection. Control animals received daily gavage with lapatinib vehicle (0.5% (w/w) hydroxypropylmethylcellulose with 0.1% polyoxyethylenesorbitan monooleate Tween®80), and weekly saline i.p. injection. The dose volume for oral gavage and i.p. injection was roughly 5 ml/kg. Each day of experiment the rats were checked for signs of treatment, including diarrhea, coat appearance, weight loss and change in temperament. Groups of rats were killed at the end of each week of treatment, as well as a group which were observed for a further 4 mo without any further treatment and killed at the end of week 20. At necropsy organs were removed, flushed with saline, weighed and fixed in 10% formalin for later analysis. The jejunum and colon were the tissues primarily assessed for changes.
Serum analysis
Determination of serum lapatinib concentration (range 10–10000 ng/ml) by LC/MS/MS was developed and validated at the Pharmaceutical Science Sector Laboratory, School of Pharmacy and Medical Sciences, University of South Australia. Twenty four hours after the previous lapatinib dose, and 7 d after the previous paclitaxel dose, blood was collected from the tail artery of the same rats once weekly for 4 weeks (n = 6–8), stored briefly in clotting tubes, before spinning for collection of serum. Lapatinib and the internal standard, 13C, 2H7-lapatinib (Alsachim), was extracted from serum using a protein precipitation clean up, before separation by HPLC on a Phenomenex Luna C18 reverse phase column. Elutes were monitored by an API 3000 MS/MS detector operated in positive MRN mode. Samples with lapatinib concentrations above the linear range were diluted 1:10 with blank rat serum.
Histopathology
Haematoxylin and eosin staining for jejunum and colon histopathology was conducted as previously described for all animals (n = 6–8/group).Citation17,Citation18 Histology reporting was performed by a veterinary pathologist in a blinded fashion (J Finnie).
Statistical analysis
All data are presented as mean ± SD or SEM as indicated. Diarrhea onset and proportions were assessed with Log-rank and Chi-square tests, respectively. Lapatinib serum concentrations were assessed by repeated measures two-way ANOVA. All other data was assessed with standard two-way ANOVA. Statistical analysis was performed using Prism 5 and SPSS 15. p < 0.05 was considered significant.
Additional material
Download Zip (488.8 KB)Disclosure of Potential Conflicts of Interest
There are no actual or potential conflicts of interest to report for any author.
Acknowledgments
We acknowledge the technical support and expertise of Jocelyn Darby and Erin Plews (Royal Adelaide Hospital). Glaxo Smith Kline provided funding to conduct this research through an unrestricted educational grant. Dr. Bowen was supported during this study with an NHMRC funded postdoctoral fellowship.
Supplemental Materials
Supplemental materials may be found here:
http://www.landesbioscience.com/journals/cbt/article/21783/
References
- Metzger Filho O, Saini KS, Azim HAJ Jr., Awada A, on behalf of AROME. Prevention and management of major side effects of targeted agents in breast cancer. [Epub ahead of print] Crit Rev Oncol Hematol 2011; PMID: 20817545
- Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 2007; 13:3913 - 21; http://dx.doi.org/10.1158/1078-0432.CCR-06-2610; PMID: 17606725
- Keefe DM, Gibson RJ. Mucosal injury from targeted anti-cancer therapy. Support Care Cancer 2007; 15:483 - 90; http://dx.doi.org/10.1007/s00520-006-0181-z; PMID: 17103195
- Goss P, Smith I, O'Shaugnessy J, Ejlertsen B, Kaufmann M, Boyle F, et al. Results of a Randomized, Double-Blind, Multicenter, Placebo-Controlled Study of Adjuvant Lapatinib in Women with Early-Stage ErbB2-Overexpressing Breast Cancer. SABCS 2011 [abstract]
- Thomas SK, Fossella FV, Liu D, Schaerer R, Tsao AS, Kies MS, et al. Asian ethnicity as a predictor of response in patients with non-small-cell lung cancer treated with gefitinib on an expanded access program. Clin Lung Cancer 2006; 7:326 - 31; http://dx.doi.org/10.3816/CLC.2006.n.014; PMID: 16640804
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 2001; 1:85 - 94; PMID: 12467226
- Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell Signal 2006; 18:2089 - 97; http://dx.doi.org/10.1016/j.cellsig.2006.05.015; PMID: 16815674
- Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko CW, Sridhara R, et al. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist 2008; 13:1114 - 9; http://dx.doi.org/10.1634/theoncologist.2008-0816; PMID: 18849320
- Johnston S, Pippen J Jr., Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009; 27:5538 - 46; http://dx.doi.org/10.1200/JCO.2009.23.3734; PMID: 19786658
- Burris HA 3rd. Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist 2004; 9:Suppl 3 10 - 5; http://dx.doi.org/10.1634/theoncologist.9-suppl_3-10; PMID: 15163842
- Crown JP, Burris HA 3rd, Boyle F, Jones S, Koehler M, Newstat BO, et al. Pooled analysis of diarrhea events in patients with cancer treated with lapatinib. Breast Cancer Res Treat 2008; 112:317 - 25; http://dx.doi.org/10.1007/s10549-007-9860-9; PMID: 18204897
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, Aura C, De Azambuja E, et al. First Results of the NeoALTTO Trial (BIG 01-06 / EGF 106903): A Phase III, Randomized, Open Label, Neoadjuvant Study of Lapatinib, Trastuzumab, and Their Combination Plus Paclitaxel in Women with HER2-Positive Primary Breast Cancer. SABCS 2010 [abstract]
- Loriot Y, Perlemuter G, Malka D, Penault-Llorca F, Boige V, Deutsch E, et al. Drug insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy. Nat Clin Pract Oncol 2008; 5:268 - 78; http://dx.doi.org/10.1038/ncponc1087; PMID: 18349858
- McCole DF, Barrett KE. Decoding epithelial signals: critical role for the epidermal growth factor receptor in controlling intestinal transport function. Acta Physiol (Oxf) 2009; 195:149 - 59; http://dx.doi.org/10.1111/j.1748-1716.2008.01929.x; PMID: 18983445
- Hare KJ, Hartmann B, Kissow H, Holst JJ, Poulsen SS. The intestinotrophic peptide, glp-2, counteracts intestinal atrophy in mice induced by the epidermal growth factor receptor inhibitor, gefitinib. Clin Cancer Res 2007; 13:5170 - 5; http://dx.doi.org/10.1158/1078-0432.CCR-07-0574; PMID: 17785573
- Roberts RB, Arteaga CL, Threadgill DW. Modeling the cancer patient with genetically engineered mice: prediction of toxicity from molecule-targeted therapies. Cancer Cell 2004; 5:115 - 20; http://dx.doi.org/10.1016/S1535-6108(04)00032-7; PMID: 14998487
- Bowen JM, Stringer AM, Gibson RJ, Yeoh AS, Hannam S, Keefe DM. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther 2007; 6:1449 - 54; http://dx.doi.org/10.4161/cbt.6.9.4622; PMID: 17881902
- Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM. Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 2003; 18:1095 - 100; http://dx.doi.org/10.1046/j.1440-1746.2003.03136.x; PMID: 12911669
- Burris HA 3rd, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O’Neil B, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 2005; 23:5305 - 13; http://dx.doi.org/10.1200/JCO.2005.16.584; PMID: 15955900
- Burris HA 3rd, Taylor CW, Jones SF, Koch KM, Versola MJ, Arya N, et al. A phase I and pharmacokinetic study of oral lapatinib administered once or twice daily in patients with solid malignancies. Clin Cancer Res 2009; 15:6702 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-09-0369; PMID: 19825948
- Koch KM, Reddy NJ, Cohen RB, Lewis NL, Whitehead B, Mackay K, et al. Effects of food on the relative bioavailability of lapatinib in cancer patients. J Clin Oncol 2009; 27:1191 - 6; http://dx.doi.org/10.1200/JCO.2008.18.3285; PMID: 19188677
- Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther 2008; 30:1426 - 47; http://dx.doi.org/10.1016/j.clinthera.2008.08.008; PMID: 18803986
- Gibson RJ, Keefe DM. Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer 2006; 14:890 - 900; http://dx.doi.org/10.1007/s00520-006-0040-y; PMID: 16604351
- Husain A, Aptaker L, Spriggs DR, Barakat RR. Gastrointestinal toxicity and Clostridium difficile diarrhea in patients treated with paclitaxel-containing chemotherapy regimens. Gynecol Oncol 1998; 71:104 - 7; http://dx.doi.org/10.1006/gyno.1998.5158; PMID: 9784328
- Guchelaar HJ, ten Napel CH, de Vries EG, Mulder NH. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: a review. Clin Oncol (R Coll Radiol) 1994; 6:40 - 8; http://dx.doi.org/10.1016/S0936-6555(05)80367-X; PMID: 7909688
- Zhang C, Xu YG, Duan XN, Liu YH, Zhao JX, Xu L, et al. Role of granulocyte colony-stimulating factor in paclitaxel-induced intestinal barrier breakdown and bacterial translocation in rats. Chin Med J (Engl) 2011; 124:1870 - 5; PMID: 21866617
- Jamieson SM, Liu JJ, Connor B, Dragunow M, McKeage MJ. Nucleolar enlargement, nuclear eccentricity and altered cell body immunostaining characteristics of large-sized sensory neurons following treatment of rats with paclitaxel. Neurotoxicology 2007; 28:1092 - 8; http://dx.doi.org/10.1016/j.neuro.2007.04.009; PMID: 17686523
- Tatsushima Y, Egashira N, Kawashiri T, Mihara Y, Yano T, Mishima K, et al. Involvement of substance P in peripheral neuropathy induced by paclitaxel but not oxaliplatin. J Pharmacol Exp Ther 2011; 337:226 - 35; http://dx.doi.org/10.1124/jpet.110.175976; PMID: 21233199
- Chentanez V, Thanomsridejchai N, Duangmardphon N, Agthong S, Kaewsema A, Huanmanop T, et al. Ganglioside GM1 (porcine) ameliorates paclitaxel-induced neuropathy in rats. J Med Assoc Thai 2009; 92:50 - 7; PMID: 19260244
- Gibson RJ, Bowen JM, Keefe DM. Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumor-bearing DA rats. Int J Cancer 2005; 116:464 - 70; http://dx.doi.org/10.1002/ijc.21082; PMID: 15800945
- Gibson RJ, Stringer AM, Bowen JM, Logan RM, Yeoh AS, Burns J, et al. Velafermin improves gastrointestinal mucositis following irinotecan treatment in tumor-bearing DA rats. Cancer Biol Ther 2007; 6:541 - 7; http://dx.doi.org/10.4161/cbt.6.4.3848; PMID: 17457046
- Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DM. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 2008; 62:33 - 41; http://dx.doi.org/10.1007/s00280-007-0570-0; PMID: 17703303
- Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered?. Cancer Chemother Pharmacol 2009; 63:239 - 51; http://dx.doi.org/10.1007/s00280-008-0732-8; PMID: 18351341