Abstract
CD44 is a cell surface antigen expressed on acute myeloid leukemia cells and is used as a marker to isolate leukemia stem cells. CD44 ligation with the antibody A3D8 has been found to induce apoptosis in human acute promyelocytic leukemia (APL) cells via activation of caspase-8. The mechanism of A3D8-induced caspase-8 activation was studied in APL NB4 cells. A3D8 induces lipid raft clustering which causes Fas aggregation as determined with a confocal microscope. A3D8-induced apoptosis is abrogated by the lipid raft disrupting agent methyl-β-cyclodextrin and the caspase-8 inhibitor Z-IETD-fmk. Western blot analysis reveals that A3D8 binds to the standard form of CD44 (CD44s). HL-60 cells without detectable CD44s protein are not responsive to A3D8-induced apoptosis. SKNO-1 cells containing higher level of CD44s protein are more sensitive to A3D8-induced apoptosis than NB4 cells. These results indicate that A3D8 induces apoptosis in leukemia cells through caspase-8 activation by binding to CD44s protein and inducing lipid raft clustering.
Keywords: :
Introduction
CD44, a transmembrane glycoprotein and a receptor of hyaluronic acid (HA), has been found to have several functions involving regulation of extracellular matrix, cell survival and differentiation.Citation1 Multiple variant isoforms of CD44 (CD44v) with different molecular masses are generated by extensive splicing of CD44 mRNA.Citation2 There are around nine variants coded as CD44v2-v10. The isoform with no variable exons in mRNA is called CD44 standard form (CD44s). The CD44v and CD44s mRNAs have been found to express in acute myeloid leukemia (AML) blast and stem cells.Citation3,Citation4 The elevated expression of CD44v, such as CD44v6, has been found to be associated with poor prognosis of AML patients.Citation5,Citation6 Recently it has been found that CD44s enhances, but CD44v blocks, Fas ligand (FasL)-induced apoptosis.Citation7,Citation8 These observations suggest that CD44s and CD44v could render either a proapoptotic or an antiapoptotic effect in a therapy-induced apoptosis process.
CD44 ligation with an antibody in leukemia cells has been found to induce apoptosis and/or differentiation.Citation9-Citation13 Among those antibodies reported it is found that the antibody A3D8 is a potent apoptosis inducer in leukemia cells.Citation9,Citation14 However, the antibody J173 is found not to induce apoptosis alone, rather blocking apoptosis of leukemia cells treated with several chemotherapeutic agents.Citation11,Citation15 These observations suggest that CD44 ligation with different antibodies could lead to either a proapoptotic or an antiapoptotic effect. The reason of different responses of leukemia cells to A3D8 and J173 might be due to their different binding abilities to different forms of CD44. A3D8 was found to induce apoptosis in acute promyelocytic leukemia (APL) NB4 cells, but not in HL-60 cells and that A3D8-induced apoptosis in NB4 cells is correlated with the activation of caspase-8.Citation14 Caspase-8 is a main effector caspase of the Fas activation-mediated apoptotic pathway.Citation16 Since CD44s enhances FasL-induced apoptosis,Citation8 we thought that A3D8 ligation-induced apoptosis could be mediated through Fas activation due to binding to CD44s. Lipid rafts, which are membrane microdomains enriched with cholesterol, serve as platforms for concentration of apoptotic signaling molecules at the plasma membrane.Citation17 Lipid raft clustering has been found to be essential for apoptosis induction by several agents, which involves the recruitment of Fas, Fas-associated death domain (FADD) and caspase-8.Citation18,Citation19 Recently it has been found that CD44 is a down-stream target of fusion protein AML1-ETO formed due to t(8;21) translocation in AML cells.Citation20 AML cells with this fusion protein should be more effective than other types of leukemia cells to A3D8-induced apoptosis. In this study the lipid raft clustering in A3D8-treated NB4 cells was determined. The levels of CD44s and A3D8-induced apoptosis in AML1-ETO expressing SKNO-1 cells were tested and compared with that in NB4 cells.
Results
Apoptosis induction by A3D8 treatment in NB4 cells is mediated principally through caspase-8 activation
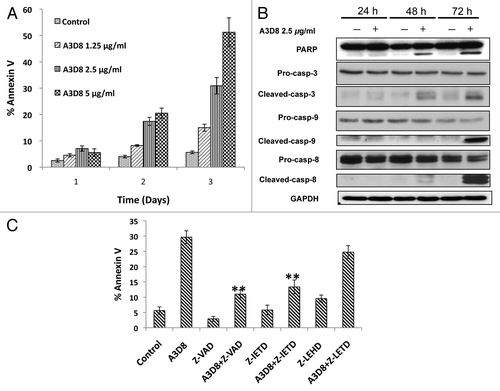
NB4 cells were treated with the A3D8 antibody (1.25 to 5 μg/ml) for 1 to 3 days and apoptotic cells were determined by FACS after staining with annexin V. Treatment with A3D8 at concentrations of 1.25 μg/ml, 2.5 μg/ml and 5 μg/ml for 3 days induced 15%, 30% and 52% of NB4 cells undergoing apoptosis, respectively (). A3D8 at a concentration of 2.5 μg/ml induced 6.0%, 16.0% and 34.2% of NB4 cells undergoing apoptosis after treatment for 1, 2 and 3 days, respectively. The levels of cleaved-PARP, -caspase-3, -caspase-8 and -caspase-9 were determined with Western blot analysis after treatment with A3D8 at a concentration of 2.5 μg/ml for 1 to 3 days. Cleaved fragments of PARP, caspase-3, caspase-8 and caspase-9 were detected after treatment with A3D8 for 3 days. Cleaved fragments of PARP, caspase-3 and caspase-8, but not caspase-9, were detected after treatment with A3D8 for 2 days (). To determine if activated caspase-8 or activated caspase-9 plays a key role in A3D8-induced apoptosis, NB4 cells were pretreated with the pancaspase inhibitor Z-VAD-fmk, the caspase-8 inhibitor Z-IETD-fmk and the caspase-9 inhibitor Z-LEHD-fmk. The pancaspase inhibitor Z-VAD-fmk and the caspase-8 inhibitor Z-IETD-fmk, but not the caspase-9 inhibitor Z-LEHD-fmk, significantly decreased the level of apoptotic cells treated with A3D8 at 2.5 μg/ml for 3 days (). These data indicate that A3D8-induced apoptosis is initiated principally through caspase-8 activation that is consistent with a previous report.Citation14
Figure 1. A3D8 treatment induces apoptosis in NB4 cells through activation of caspase-8. (A) Apoptosis induction. NB4 cells were treated with A3D8 at the indicated concentrations for 1 to 3 days. The percentage of apoptotic cells were determined by FACS after staining with annexin-V. The data shown are the mean plus SE of three independent experiments. (B) The levels of cleaved PARP, caspase-3, -8 and -9. NB4 cells were treated with 2.5 μg/ml A3D8 for 1 to 3 days and the relative levels of the indicated proteins were analyzed by Western blotting using specific antibodies. GAPDH levels were used as loading controls. (C) Inhibition of A3D8-induced apoptosis by caspase inhibitors. NB4 cells were pretreated with the pancaspase inhibitor Z-VAD (50 μM), the caspase-9 inhibitor Z-LETD (50 μM), the caspase-8 inhibitor Z-IETD (50 μM) for 4 h and then with 2.5 μg/ml A3D8 for 72 h. The percentage of apoptotic cells were detected by FACS after staining with annexin V. The data shown are the mean plus SE of three independent experiments.
Lipid rafts are clustered in A3D8-treated NB4 cells
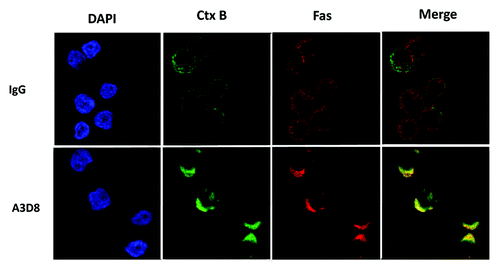
Lipid raft clustering has been found to be involved in apoptosis induction through the activation of caspase-8 probably due to causing Fas aggregation.Citation19 Lipid rafts and the distribution of Fas were determined in NB4 cells after treatment with A3D8 at 2.5 μg/ml for 3 days using a confocal microscopy. Lipid rafts were assessed by lipid raft marker FITC-Ctx B according to the reported method.Citation21 NB4 cells treated with a mouse IgG1 at the same concentration as that of A3D8 were used as a control. FITC-Ctx B distributed evenly on the cell membrane with weak fluorescence strength in NB4 cells treated with the mouse IgG1 (). Compared to cells treated with the mouse IgG, in cells treated with A3D8 FITC-CTx B staining formed a “cap” like structure with stronger fluorescence strength at one pole of the cells, suggesting that lipid rafts are clustered in cells treated with A3D8. Fas aggregated to the lipid raft domain in NB4 cells treated with A3D8, but not in cells treated with IgG1, which was merged with FITC-Ctx B staining (). These data suggest that A3D8 treatment leads to lipid raft clustering and Fas aggregation.
Figure 2. Fas is clustered into membrane lipid rafts in NB4 cells after A3D8 treatment. NB4 cells were treated with or without 2.5 μg/ml A3D8 or mouse IgG for 72 h. The cells were fixed and stained with the FITC-Ctx B subunit to identify lipid rafts (green fluorescence) and with an anti-Fas antibody to identify Fas (red fluorescence). Areas of colocalization between membrane rafts and Fas are yellow.
Lipid raft clustering contributes to A3D8-induced apoptosis and caspase-8 activation
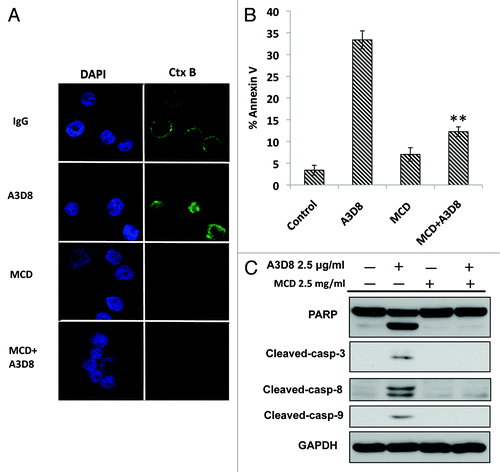
To test the role of lipid raft clustering in A3D8-induced apoptosis, NB4 cells were treated with MCD, an agent disrupting lipid raft clustering, for a short time period during A3D8 treatment. Lipid rafts, apoptotic cells and caspase-8 cleavage were determined. Short time treatment with MCD depleted the Ctx B staining in mouse IgG-treated and A3D8-treated cells indicating that A3D8-induced lipid raft clustering is abrogated with MCD treatment (). MCD at the concentration used for a short time treatment alone did not induce apoptosis, but significantly inhibited A3D8-induced apoptosis (). Western blot analysis showed that the cleavage of PARP, caspase-3 and caspase-8 in NB4 cells treated with A3D8 was blocked by MCD transient treatment (). These data suggest that lipid raft clustering by A3D8 treatment leads to apoptosis induction and caspase-8 activation.
Figure 3. Disruption of lipid rafts with MCD abrogates A3D8-induced apoptosis in NB4 cells. NB4 cells were treated with A3D8 at 2.5 μg/ml for 2 days and then with 2.5 mg/ml MCD for 30 min. MCD was washed out and cells were treated with or without A3D8 2.5 μg/ml for another 24 h. Lipid rafts were determined with a confocal microscopy (A). Cells were fixed and stained with the FITC-Ctx B subunit to identify rafts (green fluorescence) and nuclei were identified by staining with DAPI. The percentage of apoptotic cells in NB cells treated with A3D8 and/or MCD was measured by FACS after staining with Annexin-V (B). The relative levels of cleaved caspase-3, -8 and PARP in NB cells treated with A3D8 and/or MCD were analyzed by Western blotting (C).
High molecular weight hyaluronic acid (HMWHA) and the antibody J173 do not induce apoptosis and lipid raft clustering in NB4 cells
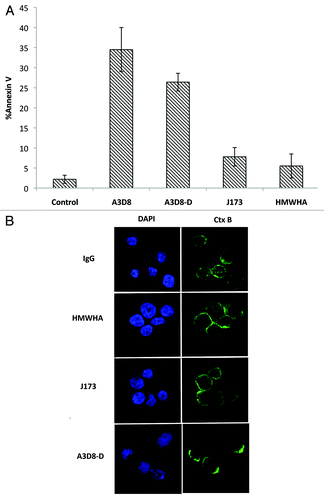
HMWHA is a ligand of CD44. The abilities of HMWHA and J173, another CD44 antibody, to induce apoptosis and lipid clustering were determined in NB4 cells and compared to cells treated with A3D8. Neither HMWHA nor J173 induced apoptosis in NB4 cells as determined with FACS analysis (). Consistent with apoptosis resistance of NB4 cells to HMWHA and J173 treatments. Lipid rafts were not clustered in NB4 cells treated with J173 and HMWHA as determined with a confocal microscopy after staining with FITC-CTx B (). Since both A3D8 and HMWHA can bind CD44 through different mechanims we treat NB4 cells with A3D8 and HMWHA together. Co-treatment of NB4 cells with A3D8 and HMWHA did not show decreased apoptosis compared to cells treated with A3D8 alone (data not shown), suggesting that A3D8-induced apoptosis is not due to blockade of HMWHA binding to CD44. The A3D8 is provided with a solution containing 0.1% sodium azide, but J173 is provided with freeze dried powder without sodium azide. To test if sodium azide has a role in the lipid raft clustering, A3D8 was dialyzed against phosphate buffered saline (PBS) for 24 h. Dialyzed A3D8-D is able to induce apoptosis and lipid raft clustering (). These data suggest that the different responses of NB4 cells to A3D8 and J173 may be resulted from their different binding abilities to CD44, but not due to the presence of sodium azide in the preparation.
Figure 4. HMWHA and J173 neither induce apoptosis nor induce clustering of lipid rafts in NB4 cells. NB4 cells were treated with HMWHA 350 μg/ml, J173 2.5 μg/ml, A3D8 2.5 μg/ml and dialyzed A3D8 (A3D8-D) 2.5 μg/ml for 72 h. The percentage of apoptotic cells was determined by FACS after staining with annexin-V (A). Lipid raft clustering was determined by confocal microscopy after staining with the FITC-CtxB subunit to identify lipid rafts (green fluorescence) and to identify nuclei by staining with DAPI (B).
A3D8 binds to CD44s, but J173 does not, in NB4 cells
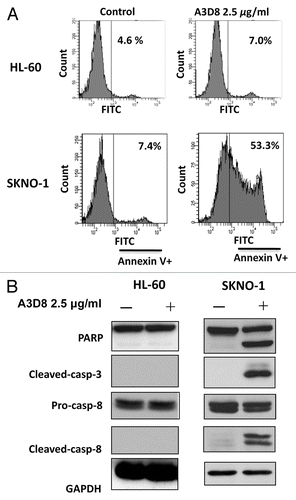
It has been found that HL-60 cells did not respond to A3D8-induced apoptosis.Citation14 Using Western blot analysis we analyzed the CD44 protein levels in HL-60 and NB4 cells by probing with A3D8 and J173. As shown in , probing with A3D8 detected a single protein band with a MW 81 kDa in NB4 cells, but not in HL-60 cells. However, probing with J173 detected multiple bands with MW higher than 90 kDa. Based on the sizes of CD44 proteins detected with A3D8 and J173, it indicates that A3D8 binds to CD44s while J173 does not bind to it. CD44 is reported to be induced by fusion protein AML1-ETO in leukemia cells.Citation20 We took advantage of SKNO-1 cells which contain AML1-ETO fusion protein and compared the levels of CD44 protein. SKNO-1 cells contained higher levels of CD44s and CD44v than NB4 cells as probed with A3D8 and J173, respectively (). The binding abilities of A3D8 and J173 on cell surface CD44 were determined by FACS in HL-60, SKNO-1 and NB4 cells. Consistent with the protein levels of CD44s and CD44v detected with Western blot analysis, HL-60 cells were weakly bond by both A3D8 and J173. A3D8 bond more strongly to cell surface than does J173 in NB4 cells. Both A3D8 and J173 have strong binding ability to cell surface of SKNO-1 cells (). SKNO-1 cells were more sensitive than NB4 cells to A3D8-induced apoptosis. Treatment with A3D8 at 2.5 μg/ml for two days induced more than 50% of SKNO-1 cells undergoing apoptosis () with activated caspase-8 based on the detection of cleaved fragment (). HL-60 cells do not respond to A3D8-induced apoptosis which is correlated with the lack of detection of CD44s. Although J173 has a strong binding ability to SKNO-1 cell surface (), it does not induce apoptosis in SKNO-1 cells (data not shown). These data suggest that the apoptosis induction ability of A3D8 in leukemia cells is mediated with its strong binding to CD44s.
Figure 5. A3D8 and J173 antibodies have different binding abilities to HL-60, SKNO-1 and NB4 cells. (A) Western blot analysis of CD44 protein levels. Cellular lysates were isolated from HL-60, SKNO-1 and NB4 cells, subjected to 8% SDS-gel electrophoresis and then probed with either A3D8 or J173 antibody. (B) Cell surface CD44 binding of A3D8 and J173. HL-60, SKNO-1 and NB4 cells were incubated with mouse IgG, A3D8 and J173 first and then FITC labeled secondary antibody. The fluorescence strength was determined by FACS.
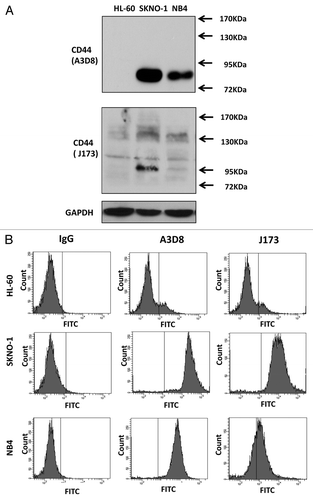
Figure 6. SKNO-1 cells, but not HL-60 cells, are sensitive to A3D8-induced apoptosis and caspase-8 activation. SKNO-1 cells were treated with or without 2.5 μg/ml A3D8 for 2 days and HL-60 cells were treated with or without 2.5 μg/ml A3D8 for 3 days. Apoptotic cells were determined by FACS after staining with annexin-V (A). The relative levels of cleaved PARP, caspase-3 and -8 were analyzed by Western blotting analysis (B).
Discussion
Apoptosis induction has been found to be an important requirement in inducing malignant cell death by chemotherapeutic agents.Citation22 Although AML cells respond to chemotherapeutic agents which induce apoptosis (mainly through the mitochondrial-mediated pathway),Citation23 resistance and relapse due to failure of targeting leukemic stem cells are barriers to the cure of this disease.Citation24 CD44 is not only used as a marker to isolate leukemic stem cells but also is found to be one reason for resistance to chemotherapy.Citation1 Targeting CD44 should be helpful for the eradication of leukemic stem cells and cure of leukemia.Citation25
CD44 is a receptor for HA. CD44 ligation by HA has been found to increase the levels of anti-apoptotic proteins Mcl-1 and Bcl-xL which protect cells from chemotherapy-induced apoptosis.Citation26,Citation27 CD44 ligation by HA also increases the level of MDR1 which lead to chemotherapy resistance.Citation28 Recently it has been shown that CD44 expression is associated with Fas-mediated apoptosis. CD44s enhances Fas L-induced apoptosisCitation7 while CD44v inhibits Fas L-induced apoptosis.Citation8 The approached to overcome chemotherapy resistance by blocking HA binding to CD44 has been developed, but limited progress has been made so far.
Ligation of CD44 with the A3D8 antibody, but not with the J173 antibody, induces apoptosis in NB4 cells ().Citation9,Citation14 HL-60 cells did not respond to A3D8-induced apoptosis (). Although both A3D8 and J173 were generated against human antigen CD44 standard form, using Western blot analysis we found that A3D8 binds to CD44s, not to CD44v (). The binding ability of J173 to CD44 proteins is different from that of A3D8 based upon the Western blot analysis. J173 binds to high MW CD44v, but not to CD44s (). These data indicate that A3D8 has higher affinity than J173 to CD44s. A3D8 has been used to examine the expression of CD44s in ovarian carcinoma.Citation29 Since J173 does not bind to CD44s and does not induce apoptosis in NB4 cells, it seems that A3D8-induced apoptosis in NB4 cells is mediated through its selective binding to CD44s. Using Western blot analysis it has been shown that breast cancer MDA-MB-231 cells express CD44s.Citation30 We used MDA-MB-231 cells as a control to confirm the CD44s expression in NB4 cells by probing with A3D8 antibody (data not shown). The expression of CD44 in HL-60 cells is controversial.Citation31 We did not detect any band of CD44 protein by probing with A3D8 in HL-60 cells, but detected multiple weak bands of high MW CD44 by probing with J173 (). Using FACS we further confirm that HL-60 cells do express very low levels of cell surface CD44 stained with both A3D8 and J173 (). The fusion protein, AML1-ETO, increases the expression of CD44 in AML cells.Citation20 SKNO-1 cells, which contain AML1-ETO, express higher levels of CD44s and CD44v than NB4 cells as determined by probing with A3D8 and J173, respectively (). SKNO-1 cells were more sensitive than NB4 and HL-60 cells to A3D8-induced apoptosis (). Therefore, the insensitivity of HL-60 cells to A3D8-induced apoptosis is due to defect of CD44s expression. Since J173 does not induce apoptosis in both NB4 and SKNO-1 cells even it has a strong binding ability to cell surface CD44, it suggests that binding to CD44s, but not to CD44v, leads to apoptosis induction.
CD44s enhances Fas L-induced apoptosis through activation of Fas.Citation7 Fas-mediated apoptosis can be initiated by several agents through lipid raft clustering without participation of Fas L.Citation32 Treatment with either edelfosine or resveratrol has been found to induce translocation of Fas and FADD into lipid rafts followed by activation of caspase-8.Citation32 We and other group have found that A3D8-induced apoptosis in NB4 cells is correlated with the activation of caspase-8 (),Citation14 suggesting that lipid raft-mediated Fas activation could be induced by A3D8 treatment. By measuring lipid raft clustering with FITC-Ctx B and Fas distribution in NB4 cells treated with A3D8, we observed lipid raft clustering with aggregated Fas (). Interruption of lipid rafts by MCD inhibited A3D8-induced apoptosis and caspase-8 activation (). These data indicate that A3D8-induced apoptosis in NB4 cells is mediated though lipid raft clustering due to binding to CD44s. NB4 cells have been found to be sensitive to apoptosis induction by many agents including arsenic trioxide through a mitochondrial-mediated pathway.Citation33 Since A3D8 is provided in a solution containing sodium azide, we tested if sodium azide in the preparation induces apoptosis in NB4 cells. Sodium azide at a concentration existed in the A3D8 solution induced minimal (<10%) apoptosis. We found that sodium azide could induce evident apoptosis at much higher concentration in NB4 cells through a mitochondrial-mediated pathway (data not shown). Sodium azide was dialyzed against PBS from A3D8 and we found that the dialyzed A3D8 is effective to induce apoptosis and lipid raft clustering in NB4 cells (). These data imply that the apoptosis induction by A3D8 in NB4 cells is due to its binding to CD44s, but not due to the existence of sodium azide. However, the existence of sodium azide in the preparation may enhance A3D8-induced apoptosis. Overall, our data suggest that the levels of CD44s expression is an important factor in determining cell sensitivity to A3D8-induced apoptosis and the A3D8 antibody may have beneficial therapeutic effects in AML cells which express high levels of CD44s such as those containing AML1-ETO fusion protein.
Materials and Methods
Reagents
Monoclonal antibody (mAb) clone A3D8, methyl-β-cyclodextrin (MCD) and fluorescein isothiocyanate (FITC)-cholera toxin B subunit (FITC-Ctx B) were purchased from Sigma. mAb J173 was purchased from Beckman Coulter. Normal mouse IgG was obtained from Santa Cruz Biotechnology, Inc. Antibodies to poly-ADP-ribose polymerase (PARP), caspase-3 and caspase-8 were obtained from BD Biosciences (San Diego, CA), to cleaved-caspase-3, caspase-9 and cleaved-caspase-9 from Cell Signaling (Boston, MA); To Fas (clone CH11) from Millipore; to GAPDH from Calbiochem. The pancaspase inhibitor Z-VAD, the caspase-8 inhibitor Z-IETD and the caspase-9 inhibitor Z-LEHD were obtained from Calbiochem.
Cell culture and CD44 ligation
Human myeloid leukemia cell lines NB4 (provided by Dr. M. Lanotte),Citation34 HL-60 (obtained from ATCC, VA) and SKNO-1 (provided by Dr. Y. Honma)Citation35were cultured in RPMI 1640 medium supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L L-glutamine and 10% (v/v) heat-inactivated fetal bovine serum (FBS). All cells were cultured by initially seeding 1 × 105 cells/ml and treated with 1.25, 2.5, or 5 μg/ml A3D8 antibody or J173 antibody for 1 to 3 days.
Quantitation of apoptotic cells
Levels of apoptotic cells were determined by the annexin V assay. The annexin V assay was done according to the manufacturer’s instructions in the annexin V–FITC Apoptosis Detection Kit (BD Biosciences). In general, 106 cells were washed twice with phosphate buffered saline (PBS) and then exposed to annexin V-FITC and prodidum iodide (PI) in binding buffer for 15 min in the dark at room temperature. The analysis was done by fluorescence-activated cell sorting (FACS). Fluorescence signals were detected at 518 and 620 nm for FITC and PI detection, respectively. For each analysis, 10,000 events were recorded. Data were analyzed using CELLQuest (BD Biosciences) software.
Western blot analysis.
Protein extracts (50 µg) prepared with radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.1% SDS, 1% NP40, 0.5% sodium deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride, 100 μmol/L leupeptin and 2 μg/mL aprotinin (pH, 8.0)] were separated on 8% or 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were stained with 0.2% Ponceau S red to assure equal protein loading and transfer. After blocking with 5% nonfat milk, the membranes were incubated with specific antibodies overnight at 4ºC. Immunocomplexes were visualized using enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences Inc.).
Confocal microscopic analysis
NB4 cells (1 × 106) treated with mouse IgG or A3D8 were cyto-spun to a microscope slide with a Shandon CytoSpin III Cytocentrifuge. Then cells were fixed in 4% neutral paraformaldehyde for 30 min, washed in PBS, permeabilized in a solution containing 0.1% Triton X-100/0.05% NP40/PBS and blocked with 1% BSA. Cells on the slides were incubated with the anti-human Fas monoclonal mouse antibody (diluted 1:150 in PBS) at 4°C for overnight, washed with PBS for 3 times and then incubated for 1 h at 4°C with Alexa Fluor® 594 goat anti-mouse IgG antibody (diluted 1:150 in 1% BSA). The slides were washed with PBS and then were incubated with FITC-Ctx B at 4°C for 2 h, following by staining DNA with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min. The immunofluorescence of the stained cells was examined by Leica SP5 DMI confocal microscope.Citation36
Cholesterol depletion
For cholesterol depletion, NB4 cells were seeded at 1 × 105 cells and then treated with or without 2.5 μg/ml A3D8 for 48 h. These cells were washed with serum-free medium three times and treated with 2.5 mg/ml MCD for 30 min at 37°C in serum-free medium. Cells were washed three times with PBS, resuspended in culture medium with serum containing with or without 2.5 μg/ml A3D8 and cultured for another 24 h.Citation37
Detection of cell surface CD44
Cells (2 × 105) were incubated in 100 μl diluent [PBS with 1% BSA and 0.1% NaN3] with 5 μl A3D8, J173 and mouse IgG for 30 min at room temperature. Cells were washed twice with 2 ml PBS. Cells were stained with FITC-labeled anti-mouse antibody (1:1000, Millipore) in 100 μl diluents and kept in dark for 30 min. Cells were washed 3 times with PBS and analyzed with a FACS Calibur. Data were analyzed using CELLQuest (BD Biosciences) software.
Statistical analysis
Data were analyzed for statistical significance using the Student’s t test (Microsoft Excel, Microsoft Corp.). A p-value of less than 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interest to disclose.
Acknowledgments
This work was supported by The Samuel Waxman Cancer Research Foundation. Critical reading of the manuscript by William Scher is appreciated.
Contributors: H.Q. designed and performed the research, analyzed data and wrote the paper; Y.J. designed the research, analyzed the data and wrote the paper; P.L. provided the agents and analyzed data; S.W. analyzed the data and wrote the paper; L.X. performed research.
References
- Hertweck MK, Erdfelder F, Kreuzer KA. CD44 in hematological neoplasias. Ann Hematol 2011; 90:493 - 508; http://dx.doi.org/10.1007/s00277-011-1161-z; PMID: 21258793
- Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A 1992; 89:12160 - 4; http://dx.doi.org/10.1073/pnas.89.24.12160; PMID: 1465456
- Ghaffari S, Dougherty GJ, Eaves AC, Eaves CJ. Altered patterns of CD44 epitope expression in human chronic and acute myeloid leukemia. Leukemia 1996; 10:1773 - 81; PMID: 8892681
- Bendall LJ, Bradstock KF, Gottlieb DJ. Expression of CD44 variant exons in acute myeloid leukemia is more common and more complex than that observed in normal blood, bone marrow or CD34+ cells. Leukemia 2000; 14:1239 - 46; http://dx.doi.org/10.1038/sj.leu.2401830; PMID: 10914548
- Legras S, Günthert U, Stauder R, Curt F, Oliferenko S, Kluin-Nelemans HC, et al. A strong expression of CD44-6v correlates with shorter survival of patients with acute myeloid leukemia. Blood 1998; 91:3401 - 13; PMID: 9558399
- Yokota A, Ishii G, Sugaya Y, Nishimura M, Saito Y, Harigaya K. Expression of exon v6-containing CD44 isoforms is related to poor prognosis of acute myelocytic leukemia. Hematol Oncol 1998; 16:131 - 41; http://dx.doi.org/10.1002/(SICI)1099-1069(199812)16:4<131::AID-HON631>3.0.CO;2-K; PMID: 10414233
- Mielgo A, Brondani V, Landmann L, Glaser-Ruhm A, Erb P, Stupack D, et al. The CD44 standard/ezrin complex regulates Fas-mediated apoptosis in Jurkat cells. Apoptosis 2007; 12:2051 - 61; http://dx.doi.org/10.1007/s10495-007-0115-3; PMID: 17726647
- Mielgo A, van Driel M, Bloem A, Landmann L, Günthert U. A novel antiapoptotic mechanism based on interference of Fas signaling by CD44 variant isoforms. Cell Death Differ 2006; 13:465 - 77; http://dx.doi.org/10.1038/sj.cdd.4401763; PMID: 16167069
- Artus C, Maquarre E, Moubarak RS, Delettre C, Jasmin C, Susin SA, et al. CD44 ligation induces caspase-independent cell death via a novel calpain/AIF pathway in human erythroleukemia cells. Oncogene 2006; 25:5741 - 51; http://dx.doi.org/10.1038/sj.onc.1209581; PMID: 16636662
- Gadhoum Z, Delaunay J, Maquarre E, Durand L, Lancereaux V, Qi J, et al. The effect of anti-CD44 monoclonal antibodies on differentiation and proliferation of human acute myeloid leukemia cells. Leuk Lymphoma 2004; 45:1501 - 10; http://dx.doi.org/10.1080/1042819042000206687; PMID: 15370200
- Charrad RS, Gadhoum Z, Qi J, Glachant A, Allouche M, Jasmin C, et al. Effects of anti-CD44 monoclonal antibodies on differentiation and apoptosis of human myeloid leukemia cell lines. Blood 2002; 99:290 - 9; http://dx.doi.org/10.1182/blood.V99.1.290; PMID: 11756184
- Bourcier S, Sansonetti A, Durand L, Chomienne C, Robert-Lézénés J, Smadja-Joffe F. CD44-ligation induces, through ERK1/2 pathway, synthesis of cytokines TNF-alpha and IL-6 required for differentiation of THP-1 monoblastic leukemia cells. Leukemia 2010; 24:1372 - 5; http://dx.doi.org/10.1038/leu.2010.100; PMID: 20508620
- Charrad RS, Li Y, Delpech B, Balitrand N, Clay D, Jasmin C, et al. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat Med 1999; 5:669 - 76; http://dx.doi.org/10.1038/9518; PMID: 10371506
- Maquarre E, Artus C, Gadhoum Z, Jasmin C, Smadja-Joffe F, Robert-Lézénès J. CD44 ligation induces apoptosis via caspase- and serine protease-dependent pathways in acute promyelocytic leukemia cells. Leukemia 2005; 19:2296 - 303; http://dx.doi.org/10.1038/sj.leu.2403944; PMID: 16208414
- Onoda M, Nakaseko C, Yokota A, Saito Y. Ligation of CD44 with low-molecular-weight hyaluronan and a monoclonal antibody leads to inhibition of drug-induced apoptosis in a human myeloid cell line. Hematology 2009; 14:213 - 9; http://dx.doi.org/10.1179/102453309X426236; PMID: 19635184
- Kruidering M, Evan GI. Caspase-8 in apoptosis: the beginning of “the end”?. IUBMB Life 2000; 50:85 - 90; PMID: 11185963
- Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta 2008; 1785:182 - 206
- Gajate C, Gonzalez-Camacho F, Mollinedo F. Involvement of raft aggregates enriched in Fas/CD95 death-inducing signaling complex in the antileukemic action of edelfosine in Jurkat cells. PLoS One 2009; 4:e5044; http://dx.doi.org/10.1371/journal.pone.0005044; PMID: 19352436
- Gajate C, Mollinedo F. Lipid rafts and Fas/CD95 signaling in cancer chemotherapy. Recent Pat Anticancer Drug Discov 2011; 6:274 - 83; http://dx.doi.org/10.2174/157489211796957766; PMID: 21762074
- Peterson LF, Wang Y, Lo MC, Yan M, Kanbe E, Zhang DE. The multi-functional cellular adhesion molecule CD44 is regulated by the 8;21 chromosomal translocation. Leukemia 2007; 21:2010 - 9; http://dx.doi.org/10.1038/sj.leu.2404849; PMID: 17657222
- Mollinedo F, de la Iglesia-Vicente J, Gajate C, Estella-Hermoso de Mendoza A, Villa-Pulgarin JA, de Frias M, et al. In vitro and In vivo selective antitumor activity of Edelfosine against mantle cell lymphoma and chronic lymphocytic leukemia involving lipid rafts. Clin Cancer Res 2010; 16:2046 - 54; http://dx.doi.org/10.1158/1078-0432.CCR-09-2456; PMID: 20233887
- Schimmer AD. Novel therapies targeting the apoptosis pathway for the treatment of acute myeloid leukemia. Curr Treat Options Oncol 2007; 8:277 - 86; http://dx.doi.org/10.1007/s11864-007-0037-x; PMID: 18004514
- Del Poeta G, Bruno A, Del Principe MI, Venditti A, Maurillo L, Buccisano F, et al. Deregulation of the mitochondrial apoptotic machinery and development of molecular targeted drugs in acute myeloid leukemia. Curr Cancer Drug Targets 2008; 8:207 - 22; http://dx.doi.org/10.2174/156800908784293640; PMID: 18473734
- Roboz GJ, Guzman M. Acute myeloid leukemia stem cells: seek and destroy. Expert Rev Hematol 2009; 2:663 - 72; http://dx.doi.org/10.1586/ehm.09.53; PMID: 21082958
- Misaghian N, Ligresti G, Steelman LS, Bertrand FE, Bäsecke J, Libra M, et al. Targeting the leukemic stem cell: the Holy Grail of leukemia therapy. Leukemia 2009; 23:25 - 42; http://dx.doi.org/10.1038/leu.2008.246; PMID: 18800146
- Herishanu Y, Gibellini F, Njuguna N, Hazan-Halevy I, Farooqui M, Bern S, et al. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma 2011; 52:1758 - 69; http://dx.doi.org/10.3109/10428194.2011.569962; PMID: 21649540
- Lakshman M, Subramaniam V, Jothy S. CD44 negatively regulates apoptosis in murine colonic epithelium via the mitochondrial pathway. Exp Mol Pathol 2004; 76:196 - 204; http://dx.doi.org/10.1016/j.yexmp.2003.12.009; PMID: 15126101
- Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem 2009; 284:2657 - 71; http://dx.doi.org/10.1074/jbc.M806708200; PMID: 19047049
- Ross JS, Sheehan CE, Williams SS, Malfetano JH, Szyfelbein WM, Kallakury BV. Decreased CD44 standard form expression correlates with prognostic variables in ovarian carcinomas. Am J Clin Pathol 2001; 116:122 - 8; http://dx.doi.org/10.1309/KUK0-1M3D-LGNE-THXR; PMID: 11447742
- Afify A, Purnell P, Nguyen L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration and invasion. Exp Mol Pathol 2009; 86:95 - 100; http://dx.doi.org/10.1016/j.yexmp.2008.12.003; PMID: 19167378
- Morimoto K, Robin E, Le Bousse-Kerdiles MC, Li Y, Clay D, Jasmin C, et al. CD44 mediates hyaluronan binding by human myeloid KG1A and KG1 cells. Blood 1994; 83:657 - 62; PMID: 7507730
- Mollinedo F, de la Iglesia-Vicente J, Gajate C, Estella-Hermoso de Mendoza A, Villa-Pulgarin JA, Campanero MA, et al. Lipid raft-targeted therapy in multiple myeloma. Oncogene 2010; 29:3748 - 57; http://dx.doi.org/10.1038/onc.2010.131; PMID: 20418917
- Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 1999; 94:2102 - 11; PMID: 10477740
- Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991; 77:1080 - 6; PMID: 1995093
- Miyoshi H, Ohki M, Nakagawa T, Honma Y. Glucocorticoids induce apoptosis in acute myeloid leukemia cell lines with A t(8;21) chromosome translocation. Leuk Res 1997; 21:45 - 50; http://dx.doi.org/10.1016/S0145-2126(96)00089-6; PMID: 9029185
- Xu ZX, Ding T, Haridas V, Connolly F, Gutterman JU. Avicin D, a plant triterpenoid, induces cell apoptosis by recruitment of Fas and downstream signaling molecules into lipid rafts. PLoS One 2009; 4:e8532; http://dx.doi.org/10.1371/journal.pone.0008532; PMID: 20046832
- Reis-Sobreiro M, Gajate C, Mollinedo F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene 2009; 28:3221 - 34; http://dx.doi.org/10.1038/onc.2009.183; PMID: 19561642