Abstract
The state of cancer stem cells (CSC) under reversible fluctuations, which has been revealed in breast cancer cells most recently, suggests that subpopulations with distinct phenotypes and functions within cancer cells can undergo inter-conversion. To investigate the possibility in colon cancer cells, we employed CD133 as the CSC marker, and characterized CD133 expression pattern and the biological features of the CD133+ and CD133- subsets. Flow cytometry revealed that CD133 was bimodally expressed in SW620 cells among eight colon cancer cell lines. The CD133+ clonal SW620 cells displayed a differential gene expression profile, higher cellular reactive oxygen species (ROS), enhanced tumorigenesis and resistance to 5-fluorouracil. The conversion in term of the CD133 phenotype of the sorted cells was observed in vitro and in vivo. The fraction of the CD133+ cells decreased from 99% to 80% in the sorted CD133+ population while rising from 5 to 10% in the sorted CD133- population during the first 20-day cultivation and then stayed almost unchanged. A fraction (about 20%) of the CD133+ clonal cells lost their CD133 marker while about 10% of the CD133- clonal cells acquired the CD133 marker. 5-Azacytidine enhanced the fraction of the CD133+ cells in both of the CD133+ and CD133- clonal cells. Our data demonstrate that CD133 expression is dynamic and reversible, and reveal the inter-conversion between the CD133+ and the CD133- SW620 cells, suggesting that the CD133 phenotype of SW620 cell population is retained by the conversion between the two cell subsets.
Introduction
Cancer stem cells (CSCs) are considered to play a central role in tumorigenesis, which have been experimentally defined by their capacity to initiate tumors and to spawn non-CSC progeny lacking tumorigenic ability in immuno-deficient mice. Current models of CSCs hypothesized that the stem-like cells unidirectionally differentiate into non-stem cells or generate non-stem progeny via asymmetrical division, which is hierachically or stochastically organized within tumors. This concept is challenged most recently by the findings that the non-stem cancer cells can dynamically convert into the stem-like stateCitation1 and the phenotypic equilibrium is maintained by stochastic state transitions between the subsets within cancer cells.Citation2 These studies revealed the possibility that the cell subpopulations with distinct phenotypes and functions undergo reversible and dynamic conversion within breast tumors, which may significantly influence the diagnostic and therapeutic concerns about the CSCs. However, such observations in breast cancer have not been extended in other cancers yet, and less is known about the alternative phenotypes undergoing transitions between CSCs and non-CSCs.
The glycoprotein CD133 is the most widely used marker for isolating and characterizing cancer stem-like cells in various cancers, including brain, pancreatic, lung, liver, colon cancers, etc.Citation3-Citation7 In colon cancers, it is reported that several hundred CD133+ colon cancer cells are capable of initiating tumors in immuno-deficient mice and can maintain their CD133 phenotype by consecutive in vivo passages,Citation8 together with the evidence that CD133 is expressed in stem cells susceptible to neoplastic transformation,Citation9 collectively supporting the hypothesis that CD133 is the marker for colon cancer stem cells. However, its role was argued because CD133- colon cancer cells could equally or even more effectively initiate tumors in comparison with CD133+ cells.Citation10 The central issue of the debates focused on the differential tumorigenicity between the sorted CD133+ and CD133- colon cancer cells, but the alternative possibility was not excluded that there might be a conversion between the two cell subsets with distinct CD133 phenotype, which resulted in contradictive data.
In this study, we employed CD133 phenotype to investigate the inter-conversion between CSCs and non-CSCs within cell populations of colon cancers. We identified the tumorigenicity of CD133+ and CD133- cells in vivo and in vitro, compared the gene expression profile of the two subsets, examined their sensitivity to anticancer drugs and tracked the CD133 phenotype of the sorted and clonal cells for an extended period of time. Our detailed characterization revealed that the spontaneously conversion between the CD133+ cells and the CD133- cells, maintained the phenotypic equilibrium in SW620 cell line.
Results
Human colon cancer cells differentially express CD133, which clearly divides SW620 cells into two subpopulations
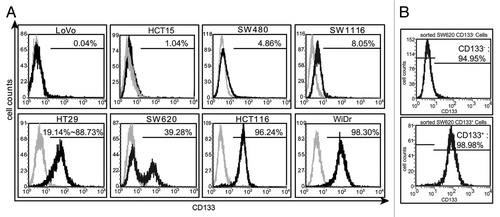
To study the conversion between cell subpopulations with distinct CD133 phenotype in colon cancer, we detected the CD133 expression in eight human colon cancer cell lines by flow cytometry. The results () revealed the following prominent features: (1) The levels of CD133 varied very significantly among those cell lines, and the percentage of the cells expressing CD133 ranged from 0.04% (LoVo) to 98.3% (WiDr); (2) Except the HT29 cell line, all others expressed CD133 at relatively stable levels. The levels of CD133 in HT29 cells were in constant change within a range from 19.1% to 88.7%, which was also reflected in other differential reported resultsCitation11,Citation12; (3) The flow cytometry histogram (CD133 vs. cell counts) was a unimodal curve for the majority of the examined cell lines, indicating that each of those cell lines has a single cell population although the cells may express CD133 at varied levels. The only exception was SW620, the histogram of which was bimodal, manifesting that there existed two cell subpopulations marked by CD133– or CD133+ expression; (4) In the SW620 cell line, the CD133- subpopulation was always a little larger than the CD133+ one with the percentage of about 60% vs. about 40%. These features indicate that the SW620 cell line could be the most suitable one among all the examined cell lines for investigating the characteristics of cell subpopulations with distinct CD133 phenotype. To further characterize these subpopulations, we employed magnetic cell sorting and obtained subpopulations with high purity of CD133+ cells (99%) and CD133- cells (95%), respectively ().
Figure 1. The expression of CD133 in eight human colon cancer cell lines. (A) The expression of CD133 was detected by flow cytometry in eight human colon cancer cell lines. The gray histograms represented the isotype control. The values were the percentage of the CD133+ cells in each cell line. (B) The CD133+ cells and the CD133- cells were sorted by magnetic cell sorting. The values evaluated by flow cytometry were the percentage of the CD133+ cells and the CD133- cells before and after being sorted. The results were representative of three independent experiments.
The CD133+ clonal cells and the CD133- clonal cells display differential gene expression profiles
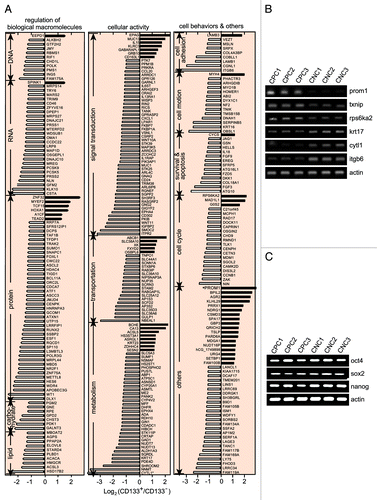
Using Affymetrix U133-plus-2-Genechips that contain the probe sets against the transcripts of 17,942 different genes with their Human Genome Organization (HuGO) names,Citation13 we detected mRNA levels in the purified CD133+ clonal cells and the purified CD133- clonal cells. A list of 326 candidate genes was produced by taking the changes common to two independent experiments (). The changes in the mRNA levels of the 6 detected genes by the cDNA microarray were validated by RT-PCR (), while stem cell-related genes including oct4, sox2 and nanog showed the comparable mRNA levels in the CD133+ and CD133- clonal SW620 cells (). Among those 326 genes, 52 were upregulated (16%) and 274 were downregulated (84%). One hundred and three of the genes are related to regulation of biological macromolecules including DNA, RNA, protein, carbohydrates and lipids, 122 genes to cellular functions involved in cellular signal transduction, cellular substance transport and metabolism, and 58 to the behaviors of the cell including adhesion, motion, survival, apoptosis and cell cycle progression (). Notably, the expression of the genes encoding for the most reported CSC markers (except CD133) was not differential between the CD133+ and CD133- SW620 cells at either the mRNA or protein levels. The above gene expression profiles revealed that over 80% of the expression-altered genes were downregulated in the CD133+ SW620 cells, suggesting that the CD133+ phenotype could be associated with a global inhibition of gene expression. Additionally, the change in the expression of some genes such as nf2, vsnl1, stk11ip and txnip were paid special attention to in the CD133+ cells due to a potential relation to tumorigenicity. nf2, vsnl1 and stk11ip have been reported as candidates of tumor suppressor genes,Citation14-Citation16 and txnip is involved in regulation of cellular ROS.Citation17 The mRNA levels of nf2, vsnl1 and stk11ip were shown to be downregulated more than 2 times while that of txnip was increased about 1.5 times in the CD133+ cells (vs. the CD133- clonal cells).
Figure 2. The differential gene expression profiles of the CD133+ SW620 cells and the CD133- counterparts. (A) Microarray analyses were performed to identify the differentially expressed genes in the purified CD133+ clonal SW620 cells and the purified CD133- counterparts. The results were expressed as the mean of two independent experiments. The genes with more than 2-fold change in their mRNA levels were listed and roughly classified according to their biological functions. (B) The mRNA levels of 6 genes in the microarray data were validated by RT-PCR in three CD133+ (CPC1, CPC2, CPC3) and three CD133- (CNC1, CNC2, CNC3) clonal cell populations. (C) The mRNA levels of the indicated genes were validated as in (B).
The CD133+ SW620 cells reveal more potent tumorigenicity
To confirm whether CD133 phenotype is associated with the tumorigenic potential of the cells, we tested the in vitro colony-formation capability and the in vivo tumorigenicity of both the purified CD133+ clonal cells and the purified CD133- clonal cells. The result showed that the colony formation rate of the CD133+ cells was about 1.6 times as high as that of the CD133- cells (). The in vivo experiments were conducted as the diagram shown in . The CD133+ SW620 cells formed more tumors within a shorter latent period than the CD133- cells after being injected subcutaneously into nude mice at the same number of the cells. Notably, as many as 105 injected CD133- cells failed to initiate tumor formation, while CD133+ cells effectively formed tumors at less cell number ( and ). The tumors were also successfully made 5 consecutive passages in nude mice. The data revealed that CD133+ SW620 cells are more tumorigenic than CD133- cells and maintain their tumor-initiating capacity during in vivo passages.
Figure 3. Tumorigenicity, drug sensitivity and cellular ROS levels of the CD133+ cells and the CD133- cells. (A) The differential colony-formation rate of the CD133+ clonal cells (CPC1) and the CD133- clonal cells (CNC1). The asterisks denoted statistical significance by the Student’s t test (p < 0.05). (B) The schedule for the in vivo tumor formation assays. At day 0, the clonal CD133- (CNC1) cells and CD133+ (CPC1) cells and the unsorted SW620 cells were subcutaneously injected into nude mice. In following 74 d, tumor volume was measured twice a week. At day 74, the mice bearing primary tumors were euthanized and the fresh tumors were transplanted into nude mice for sequential passages in vivo for 130 d and the 5th passage of xenografts were taken out for the detection of CD133. (C) Growth curves of the primary tumors generated from the CNC1 and CPC1 cells and the unsorted SW620 cells. The tumor volume was recorded up to 74 d post the cell injection and expressed as mean ± SD (n = 6) if applicable. The single values were from the groups with less than 3 xenografts formed or mice died of tumor burden (after 60 d post the cell injection). The differences between tumor volumes of CPC1 and those of CNC1 were analyzed by the Student’s t test and marked with asterisks if there was statistical significance (p < 0.05). Solid squares, CPC1 cells; solid triangles, CNC1 cells; blank circles, unsorted SW620 cells. (D) The growth inhibition of anti-tumor drugs on CD133+ and CD133- cells was evaluated by SRB assays and the results were statistically analyzed by the Student’s t test (asterisks, p < 0.05). (E) The mRNA (upper panel) and protein (lower panel) levels of VDUP1 were detected by RT-PCR and western blotting, respectively. (F) Intrtacellular ROS levels in the indicated clonal cells (CPC, CD133+; CNC, CD133-; number, different clones) were detected by flow cytometry. Grey, blank control (right panel). All the data were representative of three independent experiments.
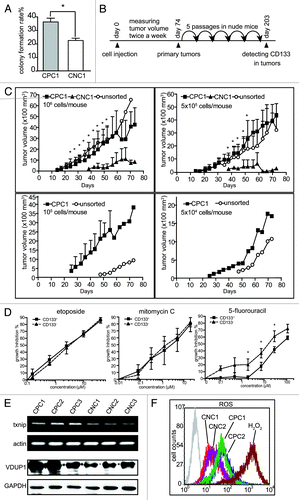
Table 1. Tumor incidence in nude mice for limiting dilution assays
The CD133+ cells are resistant to 5-fluorouracil
To investigate whether the sorted cells differentially responded to chemotherapeutic treatment, we exposed the CD133+ clonal cells and the CD133- clonal cells to antitumor agents, including adriamycin, etoposide, chimmitecan, vincristine, taxol, rapamycin, cytarabine, 5-fluorouracil, mitomycin C and cisplatin. The results showed that both the CD133+ clonal cells and the CD133- clonal cells displayed comparable drug sensitivity to all the tested agents except 5-fluorouracil (Table S1). The CD133+ clonal cells were relatively resistant to 5-fluorouracil with the reduced inhibition rate of about 10% and 20% at 10 μM and 100 μM, respectively. The results were then validated by using the sorted CD133+ and CD133- SW620 cells. The sorted CD133+ cells were approximately 2-fold resistant to 5-fluorouracil as compared with their CD133- counterparts, but both types of the cells were equally sensitive to etoposide or mitomycin C ().
The CD133+ cells display an increased level of cellular ROS
The expression of txnip gene was upregulated in CD133+ cells as shown in the microarray data, which was validated by RT-PCR at the mRNA level and by western blotting at the protein level (). As txnip encodes VDUP1 protein which interacts with thioredoxin and inhibits its activity of ROS elimination, further comparison revealed that the CD133+ clonal cells had relatively higher levels of ROS than their CD133- counterparts ().
CD133 phenotype spontaneously changes in the sorted CD133+ and CD133- SW620 cell populations
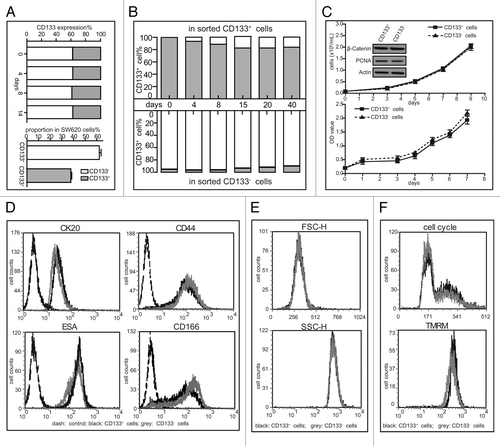
Although unsorted SW620 cells maintained an apparently stable CD133 phenotype even following consecutive 2-week cultivation (Figs. One and 4A), we found that the counterparts emerged in the sorted subpopulations during the cultivation of sorted CD133+ and CD133- SW620 cells. After the CD133+ cells and the CD133- cells were isolated with the purity of above 95% for continuous cultivation up to 40 d, it showed that the fraction of CD133+ cells rapidly decreased from 99% to 80% in the purified CD133+ population while rising from 5% to 10% in the purified CD133- population during the first 20-d cultivation. Then the proportion of the CD133+/CD133- cells stayed almost unchanged (). Previous reports indicate that CD133+ cells are more proliferative than their negative counterparts, and the CD133 phenotype is dependent on cell cycle progression, or influenced by energy metabolism or correlated with differentiation degree.Citation6,Citation8,Citation11,Citation18,Citation19 However, our results showed that the very similar growth curves detected either by cell counting or by SRB assays, together with the equal levels of PCNA and β-catenin (), collectively revealed the comparable proliferative potential of the cells between those two subpopulations. Our results also indicated that there was no obvious difference between the CD133+ and CD133- subpopulations of the SW620 cell line in their cell size, complexity of intracellular structures, cell cycle progression, mitochondria membrane potential, levels of some cell surface markers ().
Figure 4. Changes of the CD133 levels in sorted SW620 cells. (A) The proportion of the CD133+ cells and the CD133- cells was measured by flow cytometry in the SW620 cell line during 2-week cultivation (left panel) and the data were statistically analyzed as shown in right panel. (B) The levels of CD133 in the sorted SW620 cells were examined at the indicated time points during about 6-week cultivation after cell sorting. (C) The growth rate of the sorted populations was evaluated by cell counting (upper panel) and SRB assays (lower panel). Bars represent the standard deviation. The indicated proteins were tested by western blotting. (D) The expression of CK20, CD44, ESA and CD166 was examined by flow cytometry. (E) Comparison between the two sorted subpopulations in the cell size (FSC-H) and complexity of intracellular structure (SSC-H) by flow cytometry. (F) Assays for cell cycle progression (top) and mitochondria membrane potential (bottom) of the CD133+ cells and the CD133- cells by flow cytometry. All the data were representative of three independent experiments.
Cell populations derived from single CD133+ and CD133- SW620 cells developed differential phenotypic equilibrium in vitro and in vivo
To validate the above observations, we established the CD133+ and CD133- clonal SW620 cells to monitor their CD133 expression in a much longer period of cultivation. The day was counted as the first day when starting to grow single clones from the purified CD133+ or CD133- SW620 cells. After about a two-week cultivation, the proper single clones were selected and propagated to produce enough cells for their CD133 expression assays by flow cytometry at the indicated time up to the 160th day. Although the cells were derived from single cells, either CD133+ or CD133-, they were not homogeneous in terms of the CD133 expression. A fraction (about 20%) of the CD133+ clonal cells lost their inherent CD133 marker while about 10% of the CD133- clonal cells acquired the CD133 marker (). We consecutively detected the CD133 expression in 18 clonal cells every 10 or 20 d and all of them displayed the similar phenotypic alteration as in CPC1 and CPC2 or CNC1 and CNC2. The diagram of the observation was shown in . Within the single CD133+ cell-derived populations, about 80% CD133+ cells retained their CD133 expression and the other 20% cells converted into CD133- cells; in contrast, about 90% CD133- cells retained their phenotype and about the rest of 10% cells converted into CD133+ cells within the single CD133- cell-derived populations. This conversion may give rise to the phenotypic equilibrium in the whole SW620 cell population with about 40% CD133+ and 60% CD133- cells.
Figure 5. Cell populations derived from single CD133+ and CD133- SW620 cells developed differential phenotypic equilibrium in vitro and in vivo. (A) The levels of CD133 in the clonal cells established from the sorted SW620 cells were measured at the indicated time points during 160-d cultivation. Single cell suspension from the sorted CD133+ subpopulation and the CD133- counterpart was plated. After 16 h, the medium was changed and the positions of adherent single cells were marked. Two weeks later, 18 marked clones were randomly picked out, expanded for another 3 to 4 weeks and two of them were taken to examine the levels of CD133. CPC and CNC represent the clonal cells established from sorted CD133+ subpopulation and the CD133- counterpart, respectively. (B) The diagram illustrates the conversion between CD133+ and CD133- cells and the phenotypic equilibrium in SW620 cells. The values represent the proportion within the cell population. Grey cirle, CD133+ cells; blank circle, CD133- cells. (C) The cells from the 5th passaged xenografts (as described in ) were labeled with CD133/2-PE and ESA-FITC to examine the CD133 levels. The ESA+ cells represented SW620 cells while ESA- cells represented the cells from mice. The value in each quadrant was the proportion of the corresponding cells in the whole population. (D) The CD133+ clonal cells and the CD133- clonal cells were treated with 5-azacytidine (5-azaCR) for 48 h. The levels of CD133 were examined by flow cytometry. All the data were representative of three independent experiments.
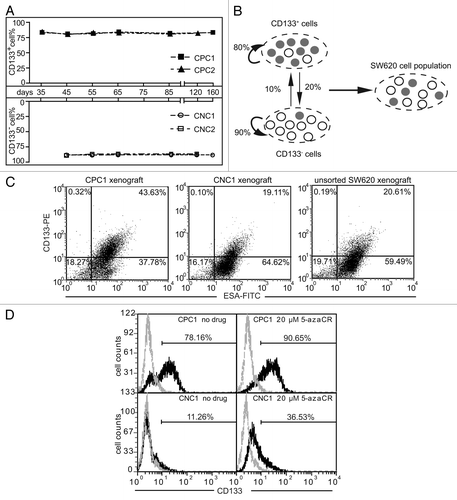
The similar observation was also confirmed by measuring the expression of CD133 in the tumors from the xenografts in the nude mice at the 203-d time point following the cell injection as shown in . The results revealed that the CD133+ human tumor cells accounted for 43.6%, 19.1% and 20.6% of all cells in the tumors derived from the CD133+ clonal cells, the CD133- clonal cells and the unsorted SW620 cells, respectively ().
The expression of CD133 was shown to be epigenetically regulated by DNMT and histone deacetylase (HDAC) in tumor cells.Citation20 To investigate the possible mechanisms by which the state of CD133 expression was changed, we exposed the CD133+ or CD133- clonal cells to the DNMT inhibitor 5-azacytidine for 48 h or the HDAC inhibitor trichostatin A for 96 h. The results revealed that 5-azacytidine enhanced the fraction of the CD133+ cells (between 10% and 30%), in both the CD133+ clonal cells and the CD133- clonal cells (). However, trichostatin A did not elicit any discernable alteration (Fig. S1). The results indicate that the change in the state of DNA methylation play an important role in regulating the expression of CD133 in SW620 cells, while histone acetyl-modification may not get involved.
Discussion
The SW620 cell line was shown to have a CD133+ cell subset in a fraction of 39% that displayed CSC properties.Citation21 In the present study, we investigated the possibility of inter-conversion between the two cell subsets in colon cancer SW620 cells. Our findings disclosed two important features of the two cell subpopulations with distinct CD133 expression: (1) CD133- cells can spontaneously convert into CD133+ cells and vice versa, while they display differential potential in tumor initiation, gene expression profile and sensitivity to anticancer agents; (2) the CD133 phenotype of the whole SW620 cell population is dynamically maintained in a phenotypic equilibrium by the two subsets.
The cell model was clearly defined by flow cytometry that in striking contrast to one cell population of the other seven tested colon cancer cell lines, the SW620 cell line had two distinct subpopulations with negative and positive CD133 expression. The two subpopulations were maintained at an apparently stable ratio of about 40% to 60% (CD133+ vs. CD133-) under continuous in vitro cultivation. Both the CD133+ or CD133- cells could grow into single clones, from which we established CD133+ and CD133- clonal cells. Interestingly, CD133 disappeared from part (about 20%) of both the CD133+ sorted cells and the CD133+ clonal cells while appearing in part (about 10%) of both the CD133- sorted cells and the CD133- clonal cells when consecutively incubated in vitro. Similarly, in the tumors derived from the CD133+ or CD133- clonal cells, after being passaged 5 times in nude mice, the fraction of the tumor cells expressing CD133 also decreased or increased correspondingly. The CD133+ SW620 cells and the CD133- SW620 cells were shown to have equal growth rate. So the above change in the proportion of the CD133+ cells to the CD133- cells could not result from their different growth rate. Therefore, our results indicate the possibility that the CD133+ tumor cells lose their CD133 while the CD133- cells acquire CD133. In other words, the CD133+ cells and the CD133- cells could convert mutually.
By carefully looking over literature, we found out the data suggesting that the CD133- cells could convert into the CD133+ cells.Citation18,Citation22,Citation23 For instance, the detectable CD133 with its specific antibody (AC133) was shown to vary in a cell cycle dependent manner in colon cancer and melanoma cell linesCitation18; the closest data to ours are those showing that CD133 re-emerged in the purified CD133- SW480 cells when persistently incubated in vitro.Citation23 In addition, the level of CD133 in cancer cells could be upregulated in hypoxia.Citation19 Those data, together with ours, collectively indicate that the CD133- cells could indeed convert into the CD133+ cells, which has long been neglected. DNMT regulates the methylation levels of CpG in DNA, which are closely associated with the transcription activity of genes. Our data showed that the DNMT inhibitor enhanced the proportion of the CD133-expressed cells in both the CD133+ clonal cells and the clonal CD133- cells, strongly suggesting that the mutual conversion between the CD133+ cells and the CD133- cells could be under epigenetic control. Once such control is altered by some yet-to-be-identified conditions, the conversion could be initiated or stopped.
The mutual conversion between the CD133+ cells and the CD133- cells may have important implications because the two types of the SW620 cells are distinct in several aspects. Although both CD133+ and CD133- SW620 cells are very similar in their cell size, cell cycle progression, mitochondria membrane potential, levels of some cell surface markers and growth rate, they display differential gene expression profiles, tumorigenesis and drug sensitivity. As compared with the CD133- clonal cells, the CD133+ clonal cells exhibited the altered expression of 326 genes, including some tumor suppressor genes and ROS regulation genes. Such differential gene expression profiles could thus be finally reflected in enhanced tumorigenic potential and drug resistance of the CD133+ clonal cells in this study. More importantly, such conversion between the CD133+ cells and the CD133- cells may reasonably explain why the CD133- cells form tumor xenografts in nude mice or SCID mice but requiring for more seeded cell numbers, as in our current study and in other previous reports.Citation6,Citation11 Moreover, the conversion of the CD133- cells to the CD133+ cells may lead to the increase in their tumorigenicity and the decrease in their drug sensitivity, suggesting the equal importance of eliminating both the CD133- cells and the CD133+ cells in treating corresponding cancers.
Our finding of the conversion between the CD133+ cells and the CD133- cells seems to make the study employing CD133 as a CSC marker more complicated due to the following two causes. First, the data of ours and the reportedCitation23 showed that only a small fraction of the CD133+ cells or the CD133- cells could change into the opposite and that there appear to exist some yet-to-be-clarified balance mechanisms to maintain those two types of the cells at a proper, apparently stable proportion according to different kinds of tumor cells. The expression of CD133 in a mixed population of the CD133+ cells and the CD133- cells is likely to be in a dynamic, changeable state though with an apparently stable proportion. This means that one cell at a given time point is a CD133+ cell, but at another given time point, it may be a CD133- cell, suggesting that the state of CD133 for a given cell is likely to be changeable over the time. Second, the expression of CD133 is not certain to lead to the differential expression of other CSC markers or transcription factors related to “stemness.” Our results did not reveal any significant difference in the expression of the stemness-related genes oct4, sox2 and nanog at the mRNA levels in the CD133+ and CD133- SW620 cells. Thus, it could be reasonably referred that the expression of CD133 and the expression of the other so-called CSC markers are regulated by different mechanisms. Probably, the variability of CD133 in the cell and the poor correlation between the CD133 expression and the expression of the other so-called CSC markers are the two important reasons why there are a lot of contradictory reports about CD133 as a CSC marker.Citation24
Nevertheless, there are open questions about the conversion between cell subsets within tumors. For example, our findings in colon cancer and previous observations on breast cancerCitation1,Citation2 were still limited to established cell lines, and primary tumor samples are needed for further exploration. The detailed molecular mechanisms of the conversion are obscure. And the determinants of the phenotypic equilibrium have not been identified yet. However, accumulating evidence supports the point that in certain contexts, the state of cancer cells is unstable and under dynamic conversion rather than stays static.Citation25,Citation26
This study reveals that there are two cell subsets with distinct CD133 expression undergoing inter-conversion and retaining the CD133 phenotypic equilibrium of the SW620 cell population in vitro and in vivo, highlighting the dynamic conversion between the CD133+ cells and the CD133- cells in colon cancer SW620 cells.
Materials and Methods
Cell lines and cell culture
The colon cancer HT29, SW1116, WiDr, HCT116, HCT15, LoVo, SW480 and SW620 cell lines were from the American Type Culture Collection (ATCC) and maintained in appropriate medium supplemented with 10% fetal bovine serum (Gibco), 100 µg/ml penicillin and 100 µg/ml streptomycin. SW620 and SW480 were incubated in 100% air and the other cells in humidified air with 5% CO2 at 37°C. Mycoplasma infection was ruled out by routine detection every two weeks.
Anticancer drugs
Etoposide, adriamycin, taxol, vincristine, rapamycin, mitomycin C, cytarabine, cisplatin and 5-fluorouracil were purchased from Sigma-Aldrich (St. Louis, MO). Chimmitecan was obtained from Knowshine Pharmachemicals (Shanghai, China). All compounds were dissolved at 10−2 M in 100% dimethyl sulfoxide and stored at −20°C as stock solutions that were thawed and diluted with normal saline immediately before each experiment.
Cell proliferation assays
Cell proliferation was measured by standard sulforhodamine B (SRB) assaysCitation27 and the optical density (OD) value was read at 515 nm using a multi-well spectrophotometer (VERSAmax, Molecular Devices). The growth inhibition rate was calculated as (OD515control − OD515agents)/OD515control × 100%.
Colony formation assays
Cells were seeded at a density of 800 cells per well in 6-well plates. After 14-d cultivation, the cells were fixed with 90% alcohol for 30 min at 4°C and stained with crystal violet. The colonies containing more than 50 cells were counted to calculate the colony formation rate as (the number of colonies/800) × 100%.
Flow cytometry and cell sorting
The following antibodies were used: anti-CD133/1, anti-CD133/2-PE (Miltenyi Biotec, Inc.), anti-CD44 (Santa Cruz Biotec), anti-CD166-PE (R&D System Inc. Minneapolis, MN) and anti-ESA-FITC (Biomeda). Cells were incubated with the indicated antibodies for 20 min, washed once and labeled with the Alexa Fluor® 488 Dye Conjugated secondary antibody (for CD133/1 and CD44) followed by flow cytometry analyses as described previously.Citation28 For the detection of CK20, cells were fixed with 4% paraformaldehyde, permeabilized by 0.1% Triton X-100, and incubated with the anti-CK20 antibody (Dako) before being labeled with the secondary antibody. The isotype antibody labeled sample served as the control in flow cytometry analyses.
SW620 cells were sorted by using the Miltenyi Biotec CD133 Cell Isolation Kit according to manufacturer’s instructions. The purity of the sorted cells was evaluated by flow cytometry.
Cell cycle analyses
Cells (4 × 105/well) were seeded into six-well plates overnight, then harvested and washed with PBS, fixed with 70% ethanol at 4°C and incubated with 40 μg/mL RNase A for 15 min at 37°C. The samples were stained with 10 μg/mL propidium iodide in the dark for 30 min and analyzed at least 10000 events using a FACSCalibur flow cytometerCitation29 (Becton Dickinson).
Reverse transcription PCR (RT-PCR)
Total RNA of the cells was extracted with Trizol. One microgram of total RNA was reverse-transcribed. The primer sequences used were as follows: 5′-TGGATGCAGAACTTGACAACGT-3′ (forward) and 5′-ATACCTGCTACGACAGTCGTGGT-3′ (reverse) for prominin1Citation22; 5′-GGTGGATGTCAATACCCCTG-3′ (forward) and 5′-ATTGGCAAGGTAACTGTGGC-3′ (reverse) for txnip; 5′-AAGGGCAAGCGATCAAGC-3′ (forward); and GGAAAGGGACCGAGGAGTA-3′ (reverse) for oct4; 5′-GCCGAGTGGAAACTTTT
GTCG-3′ (forward); and 5′-GCAGCGTGTACTTATCCTTCTT-3′ (reverse) for sox2; 5′-ATGCCTGTGATTTGTGGGCC-3′ (forward) and GCCAGTTGTTTTTCTGCCAC-3′ (reverse) for nanog; 5′-CTCCATCCTGGCCTCGCTGT-3′ (forward); and 5′-GCTGTCACCTTCACCGTTCC-3′ (reverse) for β-actin. cDNA was amplified for 30 cycles (for prominin1, nanog, sox2 and oct4), 28 cycles (for txnip) or 25 cycles (for β-actin) by PCR as described previously.Citation30 Ten microliters of each RT-PCR product were electrophoresed on a 1.5% agarose gel and visualized with ethidium bromide staining.
Establishment of clonal cell populations
CD133+ and CD133- cells were isolated with the purity of over 95% for clonal cell establishment. The isolated CD133+ or CD133- cells (500–1000 cells) were plated onto 100 mm dishes. Following 4–6 h cultivation to allow the cells to adhere to the dishes, the single cells were, under a microscope, marked on the bottom of the dishes. After about 2 weeks cultivation, the marked clones (18 clones for each sorted CD133+ or CD133- subpopulation were selected to exclude possible contamination by the remaining rare counterparts) were carefully transferred to 96-well plates. After serial expansions in 96-well plates, 24-well plates and finally 60 mm dishes, the clonal cells were examined for their CD133 expression every 10–20 d.
Tumorigenesis assays in nude mice
Cells were suspended in 150 μl L15 medium and subcutaneously injected into female BALB/c nude mice (4–6 weeks old). Tumors were routinely measured with a slide gauge twice a week and tumor volume was calculated as length × width × width ÷ 2. Tumor volume was recorded and mice were euthanized by cervical dislocation 74 d post transplantation. The xenografts were transplanted into fresh nude mice for in vivo passages. All experimental procedures were approved and performed according to the guidelines of Animal Ethics Committee of Shanghai Institute of Materia Medica.
Determination of reactive oxygen species (ROS) and mitochondria membrane potential
Cells were seeded at 4 × 105/well in 6-well plates overnight. ROS and mitochondria membrane potential were measured by flow cytometry with 10 μM 2′,7′-dichlorodi-hydrofluorescein diacetate and 25 nM tetramethylrhodamine methyl ester, respectively.
Western blotting
Standard western blotting was done with antibodies including anti-PCNA, anti-β-Catenin, anti-VDUP1 (Santa Cruz Biotec), anti-GAPDH antibody (KangChen Bio-tech, Shanghai, China) and anti-β-actin antibody (Sigma). Proteins were visualized with peroxidase-coupled secondary antibodies (Calbiochem) using an ECL-plus kit from Amersham Biosciences (Buckinghamshire, UK).
cDNA microarray assays
Clonal SW620 cells were further purified by using the Miltenyi Biotec CD133 Cell Isolation Kit. Total RNA of the purified CD133+ cells and the purified CD133- cells was extracted with the miRNeasy kit (Qiagen) and amplified with the IVT express kit (Affymetrix) according to manufacturer’s instructions. The fragmented, biotin-labeled cDNA was hybridized to the Affymetrix Human Genome U133 plus 2.0 Genechip microarrays (Affymetrix). The data of two independent experiments were normalized by using the RMA analysis and compared between the CD133+ and CD133- samples. The results were expressed as Log2 (CD133+/CD133-). The microarray data are accessible through accession number GSE23295 in the NCBI’s Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?Acc=GSE23295).
Statistical analyses
Data were statistically analyzed by Student's t test. P values of < 0.05 were considered as significant difference.
| Abbreviations: | ||
| CSC | = | cancer stem cell |
| SRB | = | sulforhodamine B |
| ROS | = | reactive oxygen species |
| DNMT | = | DNA methyltransferase |
| HDAC | = | histone deacetylase |
Additional material
Download Zip (127 KB)Acknowledgments
We sincerely thank Mrs. Li-Juan Lu, Mr. Yong Xi and Miss Yan-Yan Shen for their technical supports in animal experiments. This study was supported by grants from the National Natural Science Foundation of China (No. 81025020 and No. 81021062), the National Basic Research Program of China (No. 2012CB932502), the National Science and Technology Major Project of China (No. 2012ZX09301–001–002), the “Interdisciplinary Cooperation Team” Program for Science and Technology Innovation of the Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai Municipality (STCSM) (No. 09540704100). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A 2011; 108:7950 - 5; http://dx.doi.org/10.1073/pnas.1102454108; PMID: 21498687
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 2011; 146:633 - 44; http://dx.doi.org/10.1016/j.cell.2011.07.026; PMID: 21854987
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63:5821 - 8; PMID: 14522905
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007; 1:313 - 23; http://dx.doi.org/10.1016/j.stem.2007.06.002; PMID: 18371365
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008; 15:504 - 14; http://dx.doi.org/10.1038/sj.cdd.4402283; PMID: 18049477
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445:106 - 10; http://dx.doi.org/10.1038/nature05372; PMID: 17122772
- Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007; 132:2542 - 56; http://dx.doi.org/10.1053/j.gastro.2007.04.025; PMID: 17570225
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445:111 - 5; http://dx.doi.org/10.1038/nature05384; PMID: 17122771
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009; 457:603 - 7; http://dx.doi.org/10.1038/nature07589; PMID: 19092805
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest 2008; 118:2111 - 20; PMID: 18497886
- Ieta K, Tanaka F, Haraguchi N, Kita Y, Sakashita H, Mimori K, et al. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann Surg Oncol 2008; 15:638 - 48; http://dx.doi.org/10.1245/s10434-007-9605-3; PMID: 17932721
- Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, et al. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol 2008; 15:2927 - 33; http://dx.doi.org/10.1245/s10434-008-0074-0; PMID: 18663533
- Miao ZH, Player A, Shankavaram U, Wang YH, Zimonjic DB, Lorenzi PL, et al. Nonclassic functions of human topoisomerase I: genome-wide and pharmacologic analyses. Cancer Res 2007; 67:8752 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-06-4554; PMID: 17875716
- Golovnina K, Blinov A, Akhmametyeva EM, Omelyanchuk LV, Chang LS. Evolution and origin of merlin, the product of the Neurofibromatosis type 2 (NF2) tumor-suppressor gene. BMC Evol Biol 2005; 5:69; http://dx.doi.org/10.1186/1471-2148-5-69; PMID: 16324214
- Mahloogi H, González-Guerrico AM, Lopez De Cicco R, Bassi DE, Goodrow T, Braunewell KH, et al. Overexpression of the calcium sensor visinin-like protein-1 leads to a cAMP-mediated decrease of in vivo and in vitro growth and invasiveness of squamous cell carcinoma cells. Cancer Res 2003; 63:4997 - 5004; PMID: 12941826
- Smith DP, Rayter SI, Niederlander C, Spicer J, Jones CM, Ashworth A. LIP1, a cytoplasmic protein functionally linked to the Peutz-Jeghers syndrome kinase LKB1. Hum Mol Genet 2001; 10:2869 - 77; http://dx.doi.org/10.1093/hmg/10.25.2869; PMID: 11741830
- Tonissen KF. Targeting the human thioredoxin system by diverse strategies to treat cancer and other pathologies. Recent Pat DNA Gene Seq 2007; 1:164 - 75; http://dx.doi.org/10.2174/187221507782360227; PMID: 19075930
- Jaksch M, Múnera J, Bajpai R, Terskikh A, Oshima RG. Cell cycle-dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res 2008; 68:7882 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-08-0723; PMID: 18829544
- Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR Jr., et al. CD133 is a marker of bioenergetic stress in human glioma. PLoS One 2008; 3:e3655; http://dx.doi.org/10.1371/journal.pone.0003655; PMID: 18985161
- Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene 2009; 28:209 - 18; http://dx.doi.org/10.1038/onc.2008.374; PMID: 18836486
- Kawamoto H, Yuasa T, Kubota Y, Seita M, Sasamoto H, Shahid JM, et al. Characteristics of CD133(+) human colon cancer SW620 cells. Cell Transplant 2010; 19:857 - 64; http://dx.doi.org/10.3727/096368910X508988; PMID: 20587144
- Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008; 27:1749 - 58; http://dx.doi.org/10.1038/sj.onc.1210811; PMID: 17891174
- Elsaba TM, Martinez-Pomares L, Robins AR, Crook S, Seth R, Jackson D, et al. The stem cell marker CD133 associates with enhanced colony formation and cell motility in colorectal cancer. PLoS One 2010; 5:e10714; http://dx.doi.org/10.1371/journal.pone.0010714; PMID: 20502714
- Yu X, Lin Y, Yan X, Tian Q, Li L, Lin EH. CD133, Stem Cells, and Cancer Stem Cells: Myth or Reality?. Curr Colorectal Cancer Rep 2011; 7:253 - 9; http://dx.doi.org/10.1007/s11888-011-0106-1; PMID: 22131911
- Weinberg R, Fisher DE, Rich J. Dynamic and transient cancer stem cells nurture melanoma. Nat Med 2010; 16:758; http://dx.doi.org/10.1038/nm0710-758; PMID: 20613753
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer 2012; 12:133 - 43; PMID: 22237392
- Liu Q, Chen J, Wang X, Yu L, Hu LH, Shen X. Withagulatin A inhibits hepatic stellate cell viability and procollagen I production through Akt and Smad signaling pathways. Acta Pharmacol Sin 2010; 31:944 - 52; http://dx.doi.org/10.1038/aps.2010.72; PMID: 20644552
- Yi Y, Kamata-Sakurai M, Denda-Nagai K, Itoh T, Okada K, Ishii-Schrade K, et al. Mucin 21/epiglycanin modulates cell adhesion. J Biol Chem 2010; 285:21233 - 40; http://dx.doi.org/10.1074/jbc.M109.082875; PMID: 20388707
- Zhu H, Miao ZH, Huang M, Feng JM, Zhang ZX, Lu JJ, et al. Naphthalimides induce G(2) arrest through the ATM-activated Chk2-executed pathway in HCT116 cells. Neoplasia 2009; 11:1226 - 34; PMID: 19881958
- Feng JM, Zhu H, Zhang XW, Ding J, Miao ZH. Proteasome-dependent degradation of Chk1 kinase induced by the topoisomerase II inhibitor R16 contributes to its anticancer activity. Cancer Biol Ther 2008; 7:1726 - 31; http://dx.doi.org/10.4161/cbt.7.11.6728; PMID: 18787399
- Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 2009; 347:70 - 8; http://dx.doi.org/10.1016/j.jim.2009.06.008; PMID: 19567251