Abstract
It has been suggested that paclitaxel and gemcitabine modulate the immune system. This paper reports the safety and efficacy of paclitaxel plus gemcitabine followed by interleukin-2 (IL-2)and granulocyte macrophage colony-stimulating factor (GM-CSF), the PGIG chemobiotherapy, for patients with metastatic melanoma. All patients received 175 mg/m2 paclitaxel on day 1 and 800 mg/m2 gemcitabine on day two. IL-2 and GM-CSF were administered from day 4 to day 8 at a dosage of 2 MIU/m2 and 100 μg, respectively. The PGIG chemobiotherapy was repeated every 21 d. Serum cytokine levels at baseline and at the end of the second cycle were measured via flow cytometry. Twenty-seven patients with metastatic melanoma accepted PGIG chemobiotherapy from August 2009 to March 2011. There were five patients that exhibited a partial response, 14 patients that exhibited a stable response and eight that displayed progressive disease. Therefore, the response rate was 18.5%, and the disease control rate was 70.4%. The median time to progression and median survival were 4 mo and 8 mo, respectively. The one-year and two-year survival rates were 25.9% and 18.5%, respectively. Frequent side effects included chills, fever, arthralgia, rash and pruritus. Among the 13 patients who experienced a rash and pruritus and the 14 patients who did not suffer from this side effect, the response rates and disease control rates were 30.8% vs 7.1% and 77% vs 64.2%, respectively. No relationship between serum IL-6 levels, clinical response, and either skin side effect was observed. The PGIG chemobiotherapy is safe and effective for the treatment of patients with advanced melanoma, but randomized trials are necessary to validate this effect.
Introduction
Malignant melanoma is one of the most fatal malignancies in the world. The standard therapy consists of dacarbazine or temozolomide, but high-dose interleukin-2 (HDI), ipilimumab and vemurafenib have demonstrated success in specific subtypes.Citation1-Citation9
The success of HDI and ipilimumab demonstrated that melanoma is an immunotherapy-sensitive tumor. Modulating the immune response could thus improve the efficacy of treatment. In addition to cytotoxicity, paclitaxel and gemcitabine have immune-modulating effects.Citation10,Citation11 Paclitaxel reduces regulatory T cell numbers and inhibitory function, as well as inducing antigen release and dendritic cell (DC) maturation.Citation12-Citation19 Gemcitabine acts as a powerful anticancer immune-adjuvant agent that is useful in immunotherapeutic strategies. Its mechanism is most likely related to its selective ability to target immunosuppressive T regulatory cells and promote a Th1 cytotoxic response.Citation20-Citation24 Biweekly treatment of non-small cell lung cancer patients with gemcitabine, docetaxel, GM-CSF and low-dose IL-2 resulted in a partial response in 58.3% of patients and stable disease in 16.7% of patients.Citation25 In addition, gemcitabine plus FOLFOX 4 followed by subcutaneous administration of GM-CSF and IL-2 also induce strong immunological and antitumor activity in patients with metastatic colorectal cancer.Citation26,Citation27
In the present study, we hypothesized that the biological properties of paclitaxel and gemcitabine in metastatic melanoma could be enhanced and sustained by immunoadjuvant cytokines such as GM-CSF (which induces and activates peripheral DCs) and low dose IL-2 (which is one of the most powerful NK- and antigen-specific T-cell growth factors), which are able to generate immunostimulating effects with potential therapeutic applications. Thus, the aim of this study was to investigate the antitumor activity and toxicity of PGIG agents in patients with metastatic melanoma.
Results
Patient characteristics
There were 27 patients with metastatic melanoma that accepted PGIG biochemotherapy between August 2009 and March 2011, including 18 males and 9 females. The characteristics of the 27 patients enrolled are shown in .
Table 1. Patient characteristics (n = 27)
Toxicity
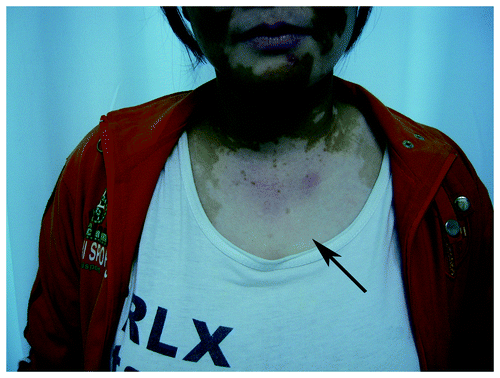
A total of 83 biochemotherapy cycles were administered (a median of 3 cycles per patient; range from 2 to 6 cycles). The most frequent adverse events were grade 1 to 2 arthralgia, rash and pruritus as well as chills and fever (). Arthralgia occurred after 24 h to 48 h of paclitaxel administration but could be treated with non-steroidal anti-inflammatory drugs. Only one patient discontinued the treatment at the fourth cycle due to intolerable arthralgia. Rash and pruritus occurred from day3 to day 8. In all circumstances, the rash was maculopapular, which mostly spread on the skin of the belly and back, but there were some cases where it appeared on the skin of arms and legs. The rash could be treated with antihistamine agents. Three patients stopped IL-2 treatment at the second cycle because of grade 3–4 rash and pruritus, and the rash faded 24 h to 48 h after IL-2 discontinuation. One of these three patients experienced vitiligo after 2 mo of PGIG administration ().
Table 2. Adverse events of PGIG agents (n = 27)
Figure 1. The patient that suffered from rash and pruritus and experienced vitiligo after 2 mo of PGIG treatment (shown by the arrow).
Chills and fever occurred on the day of IL-2 administration. The fever could be reduced after 4 h to 5 h. Cardiovascular toxicity occurred in 18.5% of all patients, and most of them were grade 1–2. One patient suffered from atrial fibrillation during the fifth cycle and recovered after IL-2 discontinuation. None of the patients died during the PGIG treatment.
Clinical Response
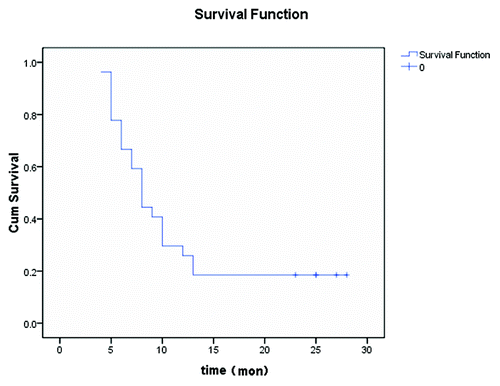
All of the 27 patients were assessable for response. Five patients achieved a partial response (PR), 14 exhibited stable disease (SD) and eight displayed progressive disease (PD). The overall response rate was 18.5%, and the disease control rate (PR+SD) was 70.4%. Five patients were still alive after follow-up from 4 to 28 mo. The median time to progression and median survival were 4 mo (range from 1 to 28 mo) and 8 mo (range from 4 to 28 mo), respectively. The 1-y and 2-y survival rates were 25.9% and 18.5%, respectively (). The two patients that achieved a PR remained progression-free for 23 and 28 mo, and one of the patients that achieved SD remained progression-free for 25 mo. All five patients who were still alive achieved a PR or SD due to administration of the PGIG regimen. The main response site was unresectable metastatic lymph nodes.
Relationship between the clinical response and skin side effect
Among the 13 patients who developed a rash and pruritus and the 14 patients who did not have this side effect, the response rates and disease control rates were 30.8% vs. 7.1% and 77% vs. 64.2%, respectively (). Two patients who experienced a grade 3–4 rash and pruritus achieved a PR, but one of them developed vitiligo after 2 mo of PGIG administration. However, this patient only received 2 cycles of PGIG and has continued to achieve a PR after 28 mo (). However, the relationship between the incidence of a skin side effect and clinical response failed to achieve statistical significance (p = 0.286).
Table 3. Response to PGIG chemobiotherapy
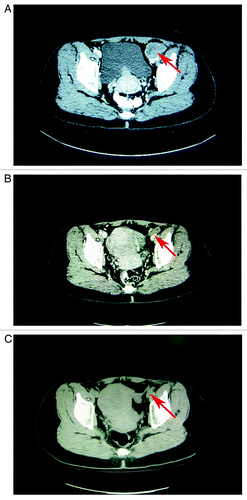
Figure 3. (A) The patient that suffered from unresectable melanoma metastasis to the lymph node beside the iliac blood vessels (shown by the arrow) (B) Shrinking of the lymph node (shown by the arrow), which contained unresectable melanoma metastases following 2 cycles of PGIG treatment (C) The lymph node that had contained metastatic melanoma is hardly visible (shown by the arrow) at a follow-up time of 28 mo.
Relationship between the clinical response and serum cytokines
Nineteen pairs of patient serum samples were acquired for immunological analysis. The analyzed cytokines included IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ and IL-17A. All of the cytokines except IL-6 were nearly undetectable during PGIG treatment. Compared with the baseline, IL-6 levels increased in eight patients, declined in six patients and remained constant in the remaining five patients. Serum IL-6 levels increased in the two patients that achieved a PR (2/4, 50%) as well as in 2 patients that achieved SD (5/10, 50%) but increased in only one patient with PD (1/5, 20%). There were no statistically significant relationships between either serum IL-6 levels and the clinical response (p = 0.267) or serum IL-6 levels and the incidence of the skin side effect (p = 0.395).
Discussion
The most common toxicities due to PGIG treatment were arthralgia after paclitaxel administration; chills and fever, which were associated with cytokine administration; and rash, pruritus and moderate neutropenia. These toxicities could be easily controlled by nonsteroidal anti-inflammatory-drugs, antihistamine agents or G-CSF. Cardiac toxicity occurred in 18.5% (5/27) of patients. Only one patient suffered from atrial fibrillation but recovered after discontinuing IL-2 treatment. It was reported that the cardiac toxicity of paclitaxel was approximately 20–30% and could be enhanced by other drugs with cardiac toxicity.Citation28 Furthermore, rare cardiovascular toxicity such as hypotension and vascular leak syndrome was observed in cases of IL-2 treatment.Citation29-Citation31 This study showed that the cardiac toxicity induced by PGIG agents was moderate and no different from that reported due to paclitaxel alone.
Our clinical results showed high rates of disease control (70.4%). The median time to progression and median survival were 4 mo and 8 mo, respectively. The 1-y and 2-y survival rates were 25.9% and 18.5%, respectively. Although the response rate was only 18.5%, the two patients that achieved PR remained progression-free for 23 and 28 mo, and the one patient who achieved SD remained progression-free for 25 mo. All five patients who were still alive achieved a PR or SD due to administration of the PGIG regimen. This result indicated that the anti-tumor activity of PGIG chemobiotherapy could be sustained for a long time period following the effective induction of the immune response. Clinical trials showed that the response rate was rather low in melanoma and renal carcinoma patients treated with high-dose IL-2; however, CRs were observed in 5% of all patients treated with high doses of IL-2 alone.Citation5,Citation32,Citation33 It has been reported that paclitaxel and gemcitabine have immune-modulating effects. The antitumor effect of cytokines might be enhanced when combined with these immune-modulators.
The most common and interesting toxicities were rash and pruritus, which occurred in 48.1% of all patients and seemed to associate with a favorable clinical response; the response rates among patients who did and did not suffer from this side effect were 30.8% vs. 7.1%. Two patients that exhibited a grade 3–4 rash and pruritus and achieved PR had to discontinue IL-2 administration, and one patient experienced vitiligo after two months of PGIG treatment. However, this patient only received two cycles of PGIG and has continued to maintain a PR for over 28 mo. It has been reported that the rate of rash and pruritus was approximately 7%~30% in patients treated with gemcitabine and 27% in patients treated with IL-2 or GM-CSF.Citation34,Citation35 However, rash and pruritus occurred in 72% of patients with non-small cell lung cancer undergoing docetaxel plus gemcitabine treatment followed by IL-2 and GM-CSF.Citation25 These side effects are most likely associated with a marked increase in the abundance of mononuclear cells, eosinophils and basophils, which is indicative of an immunological response. Cutaneous allergic reactions to medications might be associated with humoral and cellular components of the innate immune response, including antibodies of different classes and/or T lymphocytes. The manifestation of side effects on the skin might also be the result of direct chemical effects of the drug, which may induce mast cell degranulation or the release of inflammatory mediators such as histamines and leukotrienes.Citation36-Citation38 The fact that one of the patients that suffered from a grade 3–4 rash and pruritus experienced vitiligo and maintained a PR for more than 28 mo is suggestive of the participation of the immune system. In brief, the manifestation of side effects on the skin, which seemed to associate with a favorable clinical response in this group of patients, indicated that the PGIG treatment might effectively induce an immune response and enhance anti-tumor activity among metastatic melanoma patients. The reason underlying this phenomenon remains unknown. One potential mechanism is that the antigen release induced by paclitaxel and gemcitabine may lead to the generation of a more active CTLs response and contribute to the achievement of a more effective antitumor response when integrated with GM-CSF + IL-2 immunoadjuvant treatment. Another potential mechanism is that the immune microenviroment may be modulated by paclitaxel or gemcitabine, which might effectively enhance and sustaine the antitumor effect of cytokines. Two similar models have already been investigated in advanced colorectal carcinoma patients and non-small cell lung cancer patients.Citation25-Citation27 Both have indicated an effective antitumor activity of chemotherapy combined with GM-CSF and IL-2 cytokines, as well as the activation of CTLs or increased number of immune cells in blood. However, no statistically significant relationship between serum cytokine levels and the clinical response was seen in our study, which may due to the small number of samples or the incorrect timing of blood collection. Further immunological studies should be designed to verify this hypothesis.
In conclusion, the PGIG chemobiotherapy is safe and effective for advanced melanoma patients, but randomized trials are necessary to validate this effect.
Materials and Methods
Study design
The inclusion criteria consisted of a histological diagnosis of melanoma, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, a life expectancy of more than three months, normal renal and hepatic function, a white blood cell count of more than 3,500/mm3, a hemoglobin concentration of more than 9 g/dl, a platelet cell count of more than 100,000/mm3 and normal cardiac function. The exclusion criteria were any major organ failure, central nervous system involvement, second tumors, active infectious disease, major autoimmune diseases or significant immunosuppression due to Acquired Immune Deficiency Syndrome (AIDS) or medical treatment with major immunosuppressive agents (such as cyclosporine for organ transplantation).
The patients received 175 mg/m2 paclitaxel in a 3-h intravenous (IV) infusion on day 1. Then, they received 800 mg/m2 gemcitabine in a 30 min IV infusion on day 2. IL-2 was administered at a dosage of 2 MIU/m2 in an IV infusion from day 4 to day 8. GM-CSF was administered subcutaneously (SC) at a dosage of 100 μg from day 4 to day 8. The treatment was repeated every 21 d. Standard assessments (clinical history, physical examination and hematochemical analysis) and CT scans were taken at baseline and repeated every two cycles. All of the patients were evaluated for response and toxicity. Response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Toxicity was assessed according to the National Cancer Institute common toxicity criteria (NCI-CTC 2.0). This study was approved by the institutional ethical review committee of Sun Yat-Sen University Cancer Center, and all of the patients gave their informed written consent.
Immunological analysis
Peripheral blood samples for immunological assessment were drawn from all of the patients at baseline and at the end of the second cycle. The serum samples were prepared by simple centrifugation. These samples were immediately frozen at –80°C until their final examination. Cytokine protein levels including interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor (TNF), interferon-γ (IFN-γ) and interleukin-17A (IL-17A) were measured using the BD Cytometric Bead Array Human Th1/Th2/Th17 Cytokine Ki(Becton, Dickinson and Company, Franklin Lakes, NJ, USA)via flow cytometry.
Statistical analysis
All statistical analyses were conducted using SPSS 13.0 (SPSS Inc.). The correlations between skin side effects or serum cytokine levels with patients’ response to PGIG agent were analyzed with a chi-square test or Fisher’s exact test. Statistical significance was assumed for a two-tailed p < 0.05.
| Abbreviations: | ||
| IL-2 | = | interleukin-2 |
| GM-CSF | = | granulocyte macrophage colony-stimulating factor |
| PGIG | = | paclitaxel plus gemcitabine followed by IL-2 and GM-CSF |
| HDI | = | high dose interleukin-2 |
| FOLFOX-4 | = | oxaliplatin, fluorouracil and folinic acid |
| DC | = | dendritic cell |
| NK | = | natural killer |
| ECOG | = | Eastern Cooperative Oncology Group |
| AIDS | = | Acquired Immune Deficiency Syndrome |
| IV | = | intravenous |
| SC | = | subcutaneously |
| RECIST | = | Response Evaluation Criteria in Solid Tumors |
| NCI-CTC | = | National Cancer Institute common toxicity criteria |
| TNF | = | tumor necrosis factor |
| IFN | = | interferon |
| PR | = | partial response |
| SD | = | stable disease |
| PD | = | progressive disease |
| G-CSF | = | granulocyte colony-stimulating factor |
Acknowledgments
This study was supported by research grants from the National Natural Science Foundation of China (81201772) and the Medical Scientific Research Foundation of Guangdong Province, China (B2011112).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277 - 300; http://dx.doi.org/10.3322/caac.20073; PMID: 20610543
- Yan L, Rosen N, Arteaga C. Targeted cancer therapies. Chin J Cancer 2011; 30:1 - 4; http://dx.doi.org/10.5732/cjc.010.10553; PMID: 21192839
- Abdullah C, Wang X, Becker D. Molecular therapy for melanoma: useful and not useful targets. Cancer Biol Ther 2010; 10:113 - 8; http://dx.doi.org/10.4161/cbt.10.2.12595; PMID: 20574152
- Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res 2000; 19:21 - 34; PMID: 10840932
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000; 18:158 - 66; PMID: 10623706
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000; 6:Suppl 1 S11 - 4; PMID: 10685652
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517 - 26; http://dx.doi.org/10.1056/NEJMoa1104621; PMID: 21639810
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al, BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507 - 16; http://dx.doi.org/10.1056/NEJMoa1103782; PMID: 21639808
- Umansky V, Sevko A. Overcoming immunosuppression in the melanoma microenvironment induced by chronic inflammation. Cancer Immunol Immunother 2012; 61:275 - 82; http://dx.doi.org/10.1007/s00262-011-1164-6; PMID: 22120757
- Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci 2009; 38:283 - 90; http://dx.doi.org/10.1016/j.ejps.2009.08.009; PMID: 19733657
- Zhu Y, Liu N, Xiong SD, Zheng YJ, Chu YW. CD4+Foxp3+ regulatory T-cell impairment by paclitaxel is independent of toll-like receptor 4. Scand J Immunol 2011; 73:301 - 8; http://dx.doi.org/10.1111/j.1365-3083.2011.02514.x; PMID: 21223350
- Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med 2010; 8:71; http://dx.doi.org/10.1186/1479-5876-8-71; PMID: 20653948
- Yuan L, Wu L, Chen J, Wu Q, Hu S. Paclitaxel acts as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Vaccine 2010; 28:4402 - 10; http://dx.doi.org/10.1016/j.vaccine.2010.04.046; PMID: 20434553
- Marin-Esteban V, Charron D, Gelin C, Mooney N. Chemotherapeutic agents targeting the tubulin cytoskeleton modify LPS-induced cytokine secretion by dendritic cells and increase antigen presentation. J Immunother 2010; 33:364 - 70; http://dx.doi.org/10.1097/CJI.0b013e3181cd1094; PMID: 20386470
- Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol 2010; 263:79 - 87; http://dx.doi.org/10.1016/j.cellimm.2010.03.001; PMID: 20346445
- John J, Ismail M, Riley C, Askham J, Morgan R, Melcher A, et al. Differential effects of Paclitaxel on dendritic cell function. BMC Immunol 2010; 11:14; http://dx.doi.org/10.1186/1471-2172-11-14; PMID: 20302610
- Vicari AP, Luu R, Zhang N, Patel S, Makinen SR, Hanson DC, et al. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother 2009; 58:615 - 28; http://dx.doi.org/10.1007/s00262-008-0586-2; PMID: 18802696
- Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, et al. The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother 2009; 32:54 - 65; http://dx.doi.org/10.1097/CJI.0b013e31818b3dad; PMID: 19307994
- Rettig L, Seidenberg S, Parvanova I, Samaras P, Curioni A, Knuth A, et al. Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int J Cancer 2011; 129:832 - 8; http://dx.doi.org/10.1002/ijc.25756; PMID: 21710545
- Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol 2009; 9:900 - 9; http://dx.doi.org/10.1016/j.intimp.2009.03.015; PMID: 19336265
- Soeda A, Morita-Hoshi Y, Makiyama H, Morizane C, Ueno H, Ikeda M, et al. Regular dose of gemcitabine induces an increase in CD14+ monocytes and CD11c+ dendritic cells in patients with advanced pancreatic cancer. Jpn J Clin Oncol 2009; 39:797 - 806; http://dx.doi.org/10.1093/jjco/hyp112; PMID: 19797418
- Suzuki E, Sun J, Kapoor V, Jassar AS, Albelda SM. Gemcitabine has significant immunomodulatory activity in murine tumor models independent of its cytotoxic effects. Cancer Biol Ther 2007; 6:880 - 5; http://dx.doi.org/10.4161/cbt.6.6.4090; PMID: 17582217
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11:6713 - 21; http://dx.doi.org/10.1158/1078-0432.CCR-05-0883; PMID: 16166452
- Correale P, Tindara Miano S, Remondo C, Migali C, Saveria Rotundo M, Macrì P, et al. Second-line treatment of non small cell lung cancer by biweekly gemcitabine and docetaxel +/- granulocyte-macrophage colony stimulating factor and low dose aldesleukine. Cancer Biol Ther 2009; 8:497 - 502; http://dx.doi.org/10.4161/cbt.8.6.7593; PMID: 19242101
- Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol 2005; 23:8950 - 8; http://dx.doi.org/10.1200/JCO.2005.12.147; PMID: 16061910
- Correale P, Tagliaferri P, Fioravanti A, Del Vecchio MT, Remondo C, Montagnani F, et al. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial). Clin Cancer Res 2008; 14:4192 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-07-5278; PMID: 18593999
- Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev 2011; 37:300 - 11; http://dx.doi.org/10.1016/j.ctrv.2010.11.001; PMID: 21126826
- Guan H, Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS One 2012; 7:e35650; http://dx.doi.org/10.1371/journal.pone.0035650; PMID: 22532866
- Hornyak SC, Orentas DM, Karavodin LM, Gehlsen KR. Histamine improves survival and protects against interleukin-2-induced pulmonary vascular leak syndrome in mice. Vascul Pharmacol 2005; 42:187 - 93; http://dx.doi.org/10.1016/j.vph.2005.02.011; PMID: 15820445
- Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 1997; 37:117 - 32; http://dx.doi.org/10.1016/S0162-3109(97)00041-6; PMID: 9403331
- McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer 2009; 115:Suppl 2298 - 305; http://dx.doi.org/10.1002/cncr.24236; PMID: 19402060
- Dutcher JP. High-dose interleukin-2 therapy for metastatic renal cell carcinoma and metastatic melanoma: still the standard. [Williston Park] Oncology (Williston Park) 2011; 25:427 - 8; PMID: 21710841
- Kanai M, Matsumoto S, Nishimura T, Matsumura Y, Hatano E, Mori A, et al. Premedication with 20 mg dexamethasone effectively prevents relapse of extensive skin rash associated with gemcitabine monotherapy. Ann Oncol 2010; 21:189 - 90; http://dx.doi.org/10.1093/annonc/mdp513; PMID: 19889615
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17:2105 - 16; PMID: 10561265
- Bellón T, Blanca M. The innate immune system in delayed cutaneous allergic reactions to medications. Curr Opin Allergy Clin Immunol 2011; 11:292 - 8; PMID: 21659858
- Ardern-Jones MR, Friedmann PS. Skin manifestations of drug allergy. Br J Clin Pharmacol 2011; 71:672 - 83; http://dx.doi.org/10.1111/j.1365-2125.2010.03703.x; PMID: 21480947
- Beer K, Oakley H. Clinical and histopathologic correlation of an eruption secondary to Taxotere. J Drugs Dermatol 2010; 9:1534 - 5; PMID: 21120264