Abstract
This review summarizes the biology of the proton-coupled folate transporter (PCFT). PCFT was identified in 2006 as the primary transporter for intestinal absorption of dietary folates, as mutations in PCFT are causal in hereditary folate malabsorption (HFM) syndrome. Since 2006, there have been major advances in understanding the mechanistic roles of critical amino acids and/or domains in the PCFT protein, many of which were identified as mutated in HFM patients, and in characterizing transcriptional control of the human PCFT gene. With the recognition that PCFT is abundantly expressed in human tumors and is active at pHs characterizing the tumor microenvironment, attention turned to exploiting PCFT for delivering novel cytotoxic antifolates for solid tumors. The finding that pemetrexed is an excellent PCFT substrate explains its demonstrated clinical efficacy for mesothelioma and non-small cell lung cancer, and prompted development of more PCFT-selective tumor-targeted 6-substituted pyrrolo[2,3-d]pyrimidine antifolates that derive their cytotoxic effects by targeting de novo purine nucleotide biosynthesis.
Introduction
Proliferating tumor cells have unique metabolic requirements characterized by enhanced cell-autonomous nutrient uptake and reorganization of metabolic pathways to support the biosynthesis of macromolecules needed for cell growth and division. This includes the folate-dependent de novo synthesis of purine nucleotides and thymidylate.
Early observations by Farber and colleagues established the importance of folates to cancer progression.Citation1 It was hypothesized that folic acid antagonists may inhibit or arrest the proliferation of cancer cells. In the late 1940s, a series of folic acid analogs including aminopterin (AMT) () was synthesized. When administered to children with acute lymphoblastic leukemia (ALL), AMT became the first drug to induce remissions in this malignancy.Citation2 This was followed by the synthesis of additional antifolates, including methotrexate (MTX).Citation3 Remarkably, MTX continues to achieve widespread clinical use as an essential component of multidrug regimens for treating ALL, lymphomas, and solid tumors worldwide.Citation4
Figure 1. Structures of clinically relevant antifolates. Structures are shown for aminopterin (AMT), methotrexate (MTX), and pralatrexate (PDX), all dihydrofolate reductase (DHFR) inhibitors, and thymidylate synthase inhibitors raltitrexed (RTX) and pemetrexed (PMX).
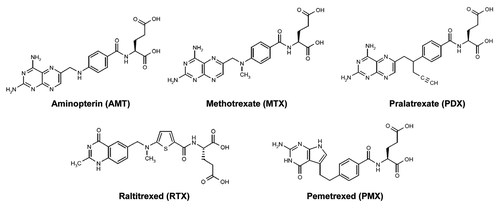
While targeting folate metabolism and nucleotide biosynthesis is a well established therapeutic strategy for cancer, for MTX, clinical efficacy is limited by a lack of tumor selectivity and the presence of de novo and acquired drug resistance.Citation5 These challenges led to decades of drug discovery efforts to identify more effective antifolates with improved pharmacology over MTX. Prominent examples include pemetrexed (PMX; Alimta), FDA-approved for treating malignant pleural mesothelioma in 2004Citation6 and non-small cell lung cancer (NSCLC) in 2008,Citation7 and pralatrexed (PDX; Folotyn), FDA-approved in 2009 for treating refractory peripheral T-cell lymphomaCitation8 (). Additional efforts were directed toward developing more tumor-selective folate-based therapies, reflecting their cellular uptake by folate receptors (FRs) or the proton-coupled folate transporter (PCFT).Citation9-Citation17 Collectively, these efforts have fostered a new paradigm, namely the rational development of tumor-targeted therapies based on tumor-specific expression and/or function of the major folate transporters.
This review will focus on the emerging biology of PCFT and its therapeutic potential for cancer.
Folate Metabolism
Folates designate the family of B9 vitamins that are essential cofactors for a wide spectrum of one-carbon transfer reactions in intermediary metabolism.Citation18 Since mammalian cells are devoid of the metabolic enzymes necessary for folate biosynthesis, all folate requirements must be acquired through the diet.
Tetrahydrofolate (THF) cofactors exist in cells as unsubstituted and N5- and/or N10-substituted THFs, the latter with one-carbon units at the oxidation levels of methanol, formaldehyde, or formate. Folate cofactors are metabolized by folylpoly-γ-glutamate synthetase (FPGS) to polyglutamyl conjugates in which 2 to 8 linked glutamyl residues are added to the γ-carboxyl on the terminal glutamate.Citation19 Folate polyglutamates are polyanions which are impermeable to plasma membranes and are poor substrates for drug efflux by the major folate efflux pumps (ABCC1, ABCG2).Citation20 This preserves high intracellular levels of reduced folate cofactors for biosynthetic reactions.Citation19 Substituted THFs donate one-carbon units in critical steps in de novo pathways for thymidylate and purine nucleotide biosynthesis, regeneration of methionine from homocysteine, and interconversion of serine and glycine ().
Figure 2. Folate transporters, folate metabolic pathways and intracellular enzyme targets of antifolates. Folate and antifolate transport across biological membranes is mediated by the reduced folate carrier (RFC), the proton-coupled folate transporter (PCFT) and folate receptors (FR). While RFC and PCFT are facilitative transporters, FRs mediate transport by a non-classical endocytosis involving formation of endosomes that migrate to the cytoplasm. Exit of the folate ligand from the endosome involves diffusion or a mediated process. PCFT has been proposed to facilitate endosomal exit,Citation38 however this does not appear to be obligatory.Citation16 Intracellular folates including tetrahydrofolate (THF), dihydrofolate (DHF), 10-formyl tetrahydrofolate (10-CHO-THF), 5, 10-methylene tetrahydrofolate (5,10-CH2-THF), 5,10-methenyl tetrahydrofolate (5,10-CH+-THF), and 5-methyl tetrahydrofolate (5-CH3-THF) participate in folate interconverting and biosynthetic reactions. Reactions 1–4 are in both the cytoplasmic and mitochodrial compartments. Reactions 1 and 9 are also present in the nucleus. Reaction 1 designates serine hydroxymethyltransferase. In the mitochondria, reactions 2 and 3 are catalyzed by bifunctional 5,10-CH2-THF dehydrogenase (MTHFD) 2 or MTHFD2L and 4 is catalyzed by monofunctional MTHFD1L. In the cytoplasm, reactions 4, 3 and 2 are catalyzed by the 10-CHO-THF synthetase, 5,10-CH+-THF cyclohydrolase and MTFD activities, respectively, of the trifunctional C1-THF synthase (MTHFD1). Other steps shown are catalyzed by β-glycinamide ribonucleotide formyltransferase (GARFTase; reaction 5), 5-amino-4-imidazolecarboxamide ribonucleotide formyltransferase (AICARFTase; reaction 6), thymidylate synthase (TS; reaction 7), dihydrofolate reductase (DHFR; reaction 8), 5,10-methyleneTHF reductase (MTHFR; reaction 9), and methionine synthase (MS; reaction 10). Antifolates inhibit folate biosynthetic reactions as shown and include aminopterin (AMT), methotrexate (MTX), pralatrexate (PDX), raltitrexed (RTX), lometrexol (LMX), pemetrexed (PMX), ONX-0801 (ONX), and compounds 3, 16 and 17.
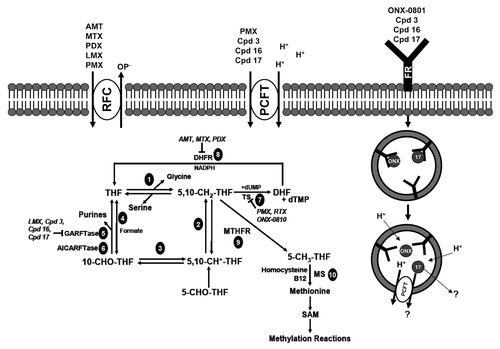
10-Formyl THF is the source of the 2 and 8 carbons of the purine ring. Thus, 10-formyl THF donates its one-carbon to β-glycinamideribonucleotide (GAR) and 5-amino-4-imidazolecarboxamide ribonucleotide (AICAR or ZMP) in steps catalyzed by GAR formyltransferase (GARFTase) and AICAR formyltransferase (AICARFTase), respectively (reactions 5 and 6, respectively, in ).
Thymidylate synthase (TS) converts dUMP to dTMP, using 5,10-methylene THF as a carbon donor (reaction 7). This results in conversion of 5,10-methylene THF to dihydrofolate (DHF), which is reduced to THF in a NADPH-dependent step catalyzed by DHF reductase (DHFR) (reaction 8). 5,10-Methylene THF is reduced by 5,10-methylene THF reductase to 5-methyl THF (reaction 9), the one-carbon donor in vitamin B12-dependent synthesis of methionine from homocysteine by methionine synthase (reaction 10). In addition to its role as constituent of proteins, methionine supports synthesis of S-adenosyl methionine (SAM) required for biological methylation reactions, including methylation of DNA, RNA, neurotransmitters, small molecules, phospholipids, and proteins including histones.Citation21
Membrane Transport of Folates
Three genetically distinct and functionally diverse transport systems have evolved to facilitate membrane transport of folates into mammalian cells, including the reduced folate carrier (RFC), PCFT, and FRs.Citation22-Citation25 While there are other transporters that mediate folate uptake (e.g., OATPB1)Citation26 and efflux (e.g., ABCG2, ABCC1),Citation20 for the purpose of this review, we focus on the major transport systems relevant to delivery of cytotoxic folate analogs for cancer, RFC, FRα and β, and PCFT.
Reduced folate carrier
RFC (SLC19A1) is a member of the Major Facilitator Superfamily (MFS) of solute carriers.Citation22 RFC is a secondary active anionic exchanger which transports reduced folates via counter-transport with organic anions.Citation22 RFC is the major transport system for reduced folates in mammalian cells and tissues and its physiologic substrate is 5-methyl THF, the major circulating folate form.Citation22 RFC has a much lower (~50–100-fold) affinity for folic acid than for reduced folates. Transport by RFC is characterized by a neutral pH optimum and markedly decreased transport activity below pH 7.Citation22,Citation24,Citation25 RFC can transport classic antifolates including MTX, AMT, PDX, raltitrexed (RTX), and PMX with high (micromolar) affinities.Citation22 While these analogs are also substrates for PCFT (see below), the δ-hemiphthaloylornithine antifolate PT523 and the benzoquinazoline antifolate GW1843U89 are selective RFC substrates with no apparent transport activity for PCFT.Citation11,Citation14,Citation15,Citation25
Human RFC (hRFC) is comprised of 591 amino acids and is 64–66% conserved with rodent RFCs. RFC is an integral membrane protein with 12 transmembrane domains (TMDs) and cytoplasmic-oriented amino and carboxyl termini.Citation22 hRFC is N-glycosylated at Asn58 in the first extracellular loop (EL) between TMDs 1 and 2.Citation27 While hRFC, like other MFS transporters, exists as a homo-oligomer,Citation28 each hRFC monomer functions independently.Citation29 However, homo-oligomeric hRFC appears to be critical to intracellular trafficking and surface expression of the functional transporter.Citation28
hRFC is ubiquitously expressed in tissues and tumors.Citation22 RFC is the major folate transporter in mammals and transports folates from blood into cells of peripheral tissues.Citation22,Citation24,Citation30 In human tissues, the highest hRFC transcript levels were recorded in placenta and liver, with significant hRFC levels in leukocytes, kidney, lung, bone marrow, intestine, and portions of the central nervous system (CNS) and brain.Citation31 By immunohistochemistry of mouse tissues probed with antibody to mouse RFC, RFC was identified at the apical brush border membrane of small intestine and colon, hepatocyte membranes, the apical surface of the choroid plexus, the basolateral membrane of renal tubular epithelium, and the apical membrane of cells lining the spinal canal.Citation32 RFC is essential for development since targeting both RFC alleles is embryonic lethal.Citation33 In at least some tissues (e.g., small intestine), mouse RFC is responsive to dietary folates such that increased RFC transcripts and proteins were detected under conditions of dietary folate deficiency.Citation34 However, the significance of this result in intestine is unclear since RFC is unlikely to be active at the acid pH of the gut and PCFT is the major intestinal transporter for absorption of dietary folates (see below).
Folate receptor
FRs bind folic acid, reduced folates, many antifolates and folate conjugates with high (low nanomolar) affinities. The three major isoforms of human FR, α, β, and γ, are encoded by distinct genes localized to chromosome 11q13.3-q13.5.Citation23 Human FR isoforms are homologous, with 68~79% identical amino acid sequences and two (β and γ) or three (α) N-glycosylation sites. FRα and β are cell surface glycosyl phosphatidylinositol (GPI)-anchored glycoproteins, while FRγ lacks a signal for GPI-anchor attachment and is a secretory protein of unknown function.Citation23
Membrane-bound FRs mediate cellular uptake of folates via a non-classical endocytotic mechanism whereby folate ligands bind FRs at the cell membrane, followed by invagination and the formation of cytoplasmic vesicles (endosomes)Citation35,Citation36 (). Release of bound ligands occurs upon endosomal acidification which facilitates dissociation of the ligand-FR complex, and exit of the folate ligand from the endosome to the cytoplasm by diffusion or a transport-mediated process that operates at acidic pH.Citation37 PCFT has been implicated in endosomal efflux of folates.Citation38,Citation39
FRα is predominantly expressed on the apical (luminal) surface of polarized epithelial cells where it is not in contact with circulating folate.Citation40 Among normal tissues, FRα is expressed in the choroid plexus, retinal pigment epithelium, proximal tubules in kidney, fallopian tubes, uterus and placenta.Citation23 The polarized expression of FRα appears to protect normal tissues from FR-targeted cytotoxic agents in the circulation.Citation41 FRβ is detected in placenta and hematopoeitic cells.Citation23 In normal bone marrow and peripheral blood cells, expression of FRβ is restricted to the myelomonocytic lineage such as mature neutrophils and was reported to be non-functional.Citation42
Overexpression of FRα has been reported in malignant tissues, such as non-mucinous adenocarcinomas of ovary, uterus and cervix, and ependymal brain tumors.Citation23 FRα levels positively correlate with tumor grades and stages.Citation43-Citation46 FRβ has been reported to involve a substantial fraction of chronic myelogenous leukemia and acute myelogenous leukemia (AML) cells, but not ALL.Citation39,Citation47 Both FRα and FRβ in malignant tissues seem to be functional,Citation41,Citation47 prompting use of folic acid and pteroyl moieties for tumor targeting of toxins, liposomes, imaging and cytotoxic agents.Citation10
Proton-coupled folate transporter
A low pH folate transporter in mammalian cells was reported more than 3 decades ago,Citation48 however, the responsible carrier system remained elusive. In 2006, a protein previously reported to be a low-affinity heme transporterCitation49 was identified as the proton-coupled folate transporter or PCFT (SLC46A1).Citation50 PCFT is a proton-folate symporter that functions optimally at acidic pH by coupling the flow of protons down an electrochemical concentration gradient to the uptake of folates into cells.Citation25,Citation50-Citation52 Like RFC, PCFT is a MFS protein. Human PCFT (hPCFT) shares only ~14% amino acid identity with hRFC.Citation24,Citation25 Although PCFT can transport heme, its primary role involves intestinal absorption of dietary folates and as such it plays a major role in in vivo folate homeostasis.Citation24,Citation53,Citation54
Biology of the Proton-Coupled Folate Transporter
PCFT structure
The hPCFT gene is localized to chromosome 17q11.2 and consists of five exons.
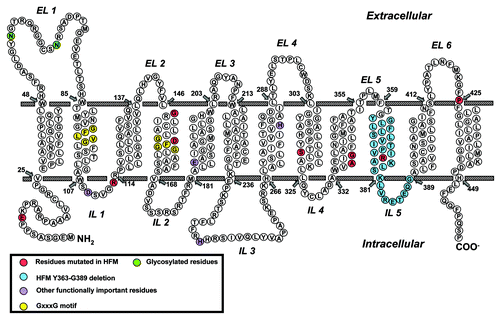
hPCFT is comprised of 459 amino acids with a molecular mass of 49.8 kDa and is predicted to include 12 TMDs with N- and C-termini oriented to the cytoplasm (). This structure has been validated by immunofluorescence analysis of hemagglutin (HA)-tagged hPCFT moleculesCitation55 and scanning cysteine-accessibility methods.Citation56 The EL domain between TMDs 1 and 2 contains two N-glycosylation sites (Asn58, Asn68). On SDS gels, hPCFT migrates broadly with a molecular mass centered at ~50–55 kDa. Digestion with N-glycosidase F, pretreatment of cells with tunicamycin, or mutagenesis of Asn58 and Asn68 to Gln converts this diffuse migrating species to a sharply banding protein at ~35–45 kDa.Citation55,Citation57 While expression or transport function was not appreciably impacted by loss of N-glycosylation in individual Gln58 and Gln68 mutants, activity decreased to ~40% for the double Gln58/Gln68 mutant.Citation55 In MDCK and Caco2 cells, C-terminal-tagged yellow fluorescent protein hPCFT was expressed at the apical membrane, distinct from the basolateral localization for hRFC.Citation58 Truncation of the hPCFT C-terminus (to position 449) does not affect apical membrane targeting or transport activity.Citation58 Although the detailed tertiary structure has not been determined for hPCFT, Cys66 in the first EL forms a disulfide bond with Cys298 in the fourth EL.Citation56
Figure 3. Schematic structure of PCFT topology. Structurally or functionally important amino acids, as determined from published mutagenesis studies, are shown as purple circles. Amino acids mutated in patients with hereditary folate malabsorption (HFM) are shown as red and blue circles. GxxxG putative oligomerization motifs are shown as yellow circles and glycosylated residues Asn58 and Asn68 are shown as green circles.
Transport characteristics
A distinguishing characteristic of PCFT involves its acidic pH optimum.Citation24,Citation25,Citation50,Citation53 For PCFT, transport is maximal at pH 5–5.5. As the pH increases from pH 5.5, transport decreases dramatically; above pH 7, activity is scarcely detectable. Transport conforms to Michaelis-Menten kinetics such that decreased transport activity with increasing pH is due to prominent effects on both Kt and Vmax, although the impact on these parameters varies for different substrates.Citation12-Citation15,Citation25,Citation59 PCFT has similar Kts for reduced folates (5-methyl THF, 5-formyl THF) and folic acid and is stereospecific for 5-formyl THF.Citation25 Direct measurements confirmed transport of [3H]MTX by PCFT.Citation16,Citation54,Citation59 Further, cytotoxicity and transport inhibition experiments indicate that RTX and lometrexol (LMX) are all transported by PCFT.Citation14,Citation16,Citation59 PDX also appears to be transported by PCFT (C. Cherian, L.H. Matherly, unpublished). PMX has been widely considered to be the best PCFT substrate.Citation25,Citation59 However, a series of 6-substituted pyrrolo[2,3-d]pyrimidine antifolates were recently reported with affinities for PCFT at least comparable to those for PMX (see below).Citation12,Citation13,Citation15,Citation60
PCFT activity is not affected by removal of extracellular Na+, K+, Ca+2, Mg+2 or Cl-.Citation50 Dissipation of the transmembrane proton-gradient by carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP; a proton ionophre)Citation50 and nigericin (a K+/H+-exchanging ionophore)Citation53 in Xenopus oocytes and HEK293 cells, respectively, reduced transport by PCFT. Studies in Xenopus oocytes indicate that folate transport by PCFT is electrogenic and there is a net translocation of a positive charge for each negatively charged folate molecule. Since folates are bivalent anions, this means that more than two protons must be co-transported with each folate molecule.Citation50,Citation54 Cellular acidification accompanies folate transport into Xenopus oocytes, confirming proton coupling.Citation61 In HEK293 cells, PCFT transport was insensitive to membrane potential, suggesting potential-independent, non-electrogenic transport in this model, although there was still a requirement for an inwardly directed proton gradient.Citation53 PCFT can function in the absence of a transmembrane pH gradient. Under these conditions, folate transport is driven by membrane potentials.Citation50,Citation51 At low pH, PCFT exhibits channel-like activities; i.e., protons can flow uncoupled from the flow of folates.Citation61,Citation62
Pharmacologically relevant anionic compounds, including indomethacin, sulfasalazine and pyrimethamine, are weak inhibitors of PCFT-mediated cellular uptake.Citation52,Citation53,Citation63 These medications could interfere with intestinal absorption of dietary folates or oral medications (e.g., MTX) when co-administered.
PCFT tissue expression
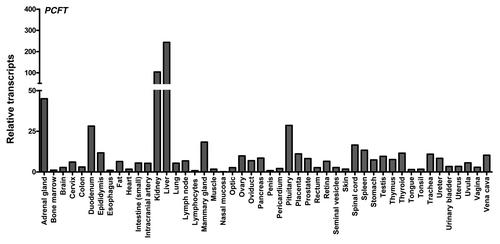
High levels of PCFT are expressed in apical brush-border membranes in the proximal jejunum and duodenum; however, PCFT levels decrease markedly in other segments of the intestine and colon.Citation50,Citation53,Citation54,Citation63 PCFT is also detected in the choroid plexus.Citation38,Citation64 In normal human tissues, elevated hPCFT transcripts were measured in kidney and liver, with modest levels in most tissues and undetectable levels in bone marrow and colon (). For a number of human tissues, including small intestine, hPCFT proteins were detected by immunohistochemistry with hPCFT-specific antibody (not shown). These results substantiate those of Qui et al.Citation50 and demonstrate that while hPCFT is expressed in normal human tissues, its levels are more limited than for hRFC. While PCFT in the upper gastrointestinal tract is involved in absorption of dietary folates, the physiologic role of PCFT in tissues not normally associated with low pH microenvironments is less obvious. PCFT may conceivably still contribute to folate internalization in such tissues by virtue of localized acidification or at sufficiently elevated levels to transport 5-methyl THF and related folates.Citation24 However, at comparatively neutral pHs characterizing most tissues, RFC is far more efficient at delivering reduced folates than PCFT.Citation59
Figure 4. PCFT and RFC transcript expression in human normal tissues. PCFT transcripts were measured by real-time RT-PCR using an Origene cDNA array prepared from 48 pathologist-verified human normal tissues as previously described.Citation12 Transcript levels were normalized to GAPDH transcripts.
Regulation of PCFT gene expression
Studies have begun to explore the transcriptional regulation of hPCFT. The hPCFT minimal transcriptional regulatory region is localized between positions -42 and +96 relative to the transcriptional start site.Citation65,Citation66 The hPCFT promoter is G/C rich and includes a large (1085 bp) CpG island which spans the transcriptional start site and is hypermethylated in human leukemia (CCRF-CEM, Jurkat) and MTX-resistant (R1–11) HeLa cells.Citation65,Citation67 In both cases, treatment with the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine resulted in substantial restoration of hPCFT mRNA expression and pH 5.5 transport. Given the role of folate in supporting SAM levels required for methylation reactions, regulation of hPCFT via promoter methylation is particularly apropos. Interestingly, mouse PCFT transcript levels increased ~13-fold in the proximal small intestine in mice fed folate-deficient vs. folate-replete diets,Citation54 consistent with a self-regulating mechanism of PCFT gene expression by folates.
Nuclear respiratory factor 1 (NRF-1) can bind and transactivate the hPCFT gene, leading to an increase in hPCFT mRNA levels via three NRF-1 binding sites in the PCFT minimal promoter (positions -108 to -97, -93 to -82, and -10 to +1).Citation68 NRF-1 is an important regulator of mitochondrial biogenesis.Citation69 Folates play an important role in mitochondrial integrity, and folate deficiency causes mitochondrial chromosomal deletions, cytochrome c dysfunction, membrane depolarization and superoxide overproduction.Citation70 NRF-1 also regulates de novo purine nucleotide biosynthesis at the level of glutamine phosphoribosyl pyrophosphate amidotransferase (GPAT) and phosphoribosyl aminoimidazole carboxylase/phosphoribosyl aminoimidazole succinocarboxamide synthetase (PAICS).Citation71,Citation72 Thus, NRF-1 can be envisaged to function in coordinating mitochondrial respiration-biogenesis with folate uptake and de novo purine biosynthesis.
Expression of PCFT is increased in a dose-dependent fashion in Caco-2 cells in vitro and in duodenal rat biopsies ex vivo treated with 1,25-dihydroxyvitamin D3 (vitamin D3).Citation73 Induction of PCFT is accompanied by enhanced transport at pH 5.5. The vitamin D receptor (VDR) heterodimerizes with retinoid X receptor-α in response to vitamin D3 and binds a VDR response element in the hPCFT promoter (positions -1694 to -1680), resulting in transactivation. These results suggest that vitamin D3 supplementation can affect bioavailability of dietary folates and toxicity of PCFT-targeted therapeutics for cancer (see below).
Finally, PCFT expression may be regulated in response to clinically used proton pump inhibitors (omeprazole, pantroprazole, lansoprazole).Citation63 For instance, PCFT transcript levels were decreased by nearly 50% in duodenal biopsies from patients treated with proton-pump inhibitors in comparison with untreated controls. Although it is unclear whether this is a direct or indirect response to these widely used medications, these results raise the possibility that changes in the tissue microenvironmental pHs may regulate PCFT levels.
Hereditary folate malabsorption
Direct evidence that PCFT is responsible for low pH intestinal transport activity and absorption of dietary folates involved the discovery that homozygous mutations in the hPCFT gene were associated with a rare autosomal recessive disorder, hereditary folate malabsorption (HFM).Citation50,Citation62,Citation74-Citation83 Clinically relevant mutations include base insertions, deletions, or substitutions, manifesting as exon skipping, frame shifts, premature translation terminations and amino acid substitutions.
HFM is characterized by an onset of macrocytic folate-deficiency, anemia, and failure to thrive within the first months of life. This may be accompanied by hypoimmunoglobulinemia, associated with infectious complications, most frequently Pneumocyctis jiroveci pneumonia. HFM is characterized by developmental delays, gait disorders, peripheral neuropathies, and seizures.Citation77 Loss of hPCFT function leads to impaired intestinal folate absorption, resulting in severe systemic folate deficiency and impaired transport of folates across the choroid plexus into the CNS.Citation38,Citation64 These findings establish the important role of PCFT in folate transport across the gastrointestinal epithelium and into the CNS, and indicate that RFC does not significantly contribute to intestinal folate absorption.
Functionally important residues in hPCFT
Structural insights into PCFT transport function have resulted from characterization of clinically relevant loss-of-function hPCFT mutations in HFM cases, and mutagenesis of conserved amino acids implicated as functionally important from considerations of PCFT homologies, charge properties, and TMD localization ().
Functionally important residues include Glu185 (TMD5) (required for proton coupling),Citation82 His281 (TMD7) (important for substrate binding)Citation61 and Arg376 (TMD10) (impacts proton and substrate binding).Citation62 Amino acids mapping to a highly conserved stretch between TMDs 2 and 3 (DXXGRR; positions 109–114) including a β-turn were also implicated as important for hPCFT transport.Citation74,Citation76,Citation78 Asp109 is essential for transport since regardless of charge or polarity, amino acid replacement abolishes substate binding and membrane translocation.Citation78 From the loss of transport activity for Arg113Cys mutant hPCFT, a molecular model (based on the GlpT template) was proposed in which Arg113 is buried in a hydrophobic cavity made up of TMDs 1, 3, 4 and 6.Citation74,Citation76 However, this has not been experimentally confirmed. Arg113 may directly participate in substrate binding and/or membrane translocation of negatively charged transport substrates.Citation76
For His247, mutation (Ala, Arg, Gln, Glu) resulted in markedly decreased rates of transport (decreased Vmax) and increased substrate affinities (decreased Kt) for folate substrates compared with wild-type hPCFT.Citation61 By homology modeling, His247 was localized in a highly electropositive region at the cytoplasmic opening to the water-filled translocation pathway and interacted with Ser172, limiting substrate access to the putative folate-binding pocket (thus determining substrate selectivity). As expected, the Ser172Ala mutant hPCFT showed a similar transport phenotype to that for His247Ala hPCFT and enhanced proton transport in the absence of folate substrate (“slippage”).Citation61
Other residues implicated as functionally important include Glu232 (TMD6), Leu161 (TMD4), Ile304 (TMD8), and Pro425 (EL6, flanking TMD12).Citation84 Loss of transport was associated with a decreased rate of carrier translocation (Glu232Gly mutant) or decreased substrate affinities (Ile304Phe and Leu161Arg mutants). For Pro425, mutation to Arg resulted in loss of binding for MTX and other substrates, but substantial preservation of PMX binding, presumably reflecting a conformation change induced by the Arg substitution.Citation85
Oligomerization of hPCFT
MFS proteins including hRFC often exist as oligomers (e.g., dimers, tetramers, etc.).Citation28,Citation86 By protein cross-linking and blue native gel electrophoresis of ectopically-expressed hPCFT, hPCFT species were identified with molecular masses approximating those of oligomeric hPCFT.Citation87 Physical associations between HA- and His10-tagged hPCFT monomers were established by co-expression in hPCFT-null HeLa cells and co-binding to nickel affinity columns, and by fluorescence resonance energy transfer between co-expressed YPet- and ECFP*-tagged hPCFT monomers in transfected cells. Wild-type and inactive mutant Pro425Arg hPCFTs were co-expressed and exhibited a “dominant-positive” functional phenotype, consistent with positive cooperativity between monomers and suggesting a functional “rescue” of mutant hPCFT by wild-type carrier. Interestingly, hPCFT primary sequence includes GXXXG motifs in TMD 2 (amino acids 93–97) and TMD 4 (amino acids 155–159), analogous to “dimerization motifs” in other amphipathic proteins.Citation88,Citation89 While mutation of Gly93 and Gly97 to Ala preserved hPCFT oligomerization, as assessed by thiol-reactive (MTS-1-MTS) protein cross-linking, when the 7 native Cys residues in wild-type hPCFT were invidually replaced with Ser, only Cys229Ser abolished cross-linking.Citation90 This suggests that TMD6 represents an interface between individual hPCFT monomers.
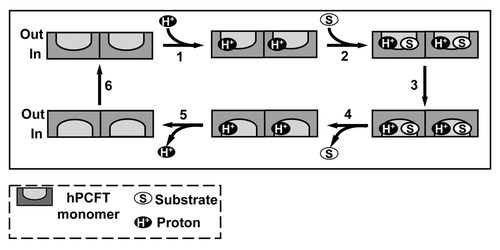
An “alternate access” model for hPCFT, analogous to that suggested for LacYCitation91 and adapted from that for monomeric hPCFT,Citation82 was proposedCitation87 which includes the notion of a functional impact for hPCFT oligomerization (). The model assumes that hPCFT monomers occur as hPCFT homo-dimers which undergo the transport cycle in tandem and a functional cooperativity between hPCFT monomers which permits ordered loading and release of both substrates and protons.
Figure 5. Proposed reaction scheme for hPCFT-mediated cellular uptake involving cooperative interactions between hPCFT monomers. Based on the “alternate access model” for secondary transporters such as Lac Y,Citation91 adapted from that of Unal et al. for monomeric PCFT,Citation82 an analogous reaction scheme is depicted for hPCFT-mediated transport which incorporates the functional impact of hPCFT oligomerization. The model starts from the outward-facing unloaded dimer, followed by the ordered binding of the co-transported protons (step 1) and (anti)folate substrates (step 2), which triggers a conformational change resulting in simultaneous transition of the two hPCFT monomers to an inward-facing state (step 3). This is followed by an ordered release of substrates (step 4) and then protons (step 5) into the cytoplasm. The unloaded homo-oligomeric unit then returns to the outward-facing state (step 6) to complete the transport cycle. In this model, the two hPCFT monomers are suggested to function cooperatively in facilitating substrate and proton binding, conformational changes, and substrate and proton release. From Hou et al.Citation87
The Role of Antifolates in Cancer Biology
Antifolates continue to occupy an important niche in the chemotherapy of a number of cancers, including pediatric ALL, osteogenic sarcoma, lymphoma, breast cancer, non-small cell lung cancer, and malignant pleural mesothelioma.Citation4,Citation92 While MTX had origins during the late 1940s,Citation2,Citation3 this agent remains an extraordinarily important drug for both cancer and non-malignant diseases.Citation4,Citation93,Citation94 In recent years, newer antifolates have been introduced that were subsequently approved for patients with cancer, including PDX,Citation6,Citation7 RTX,Citation95,Citation96 and PMX.Citation8 Although numerous other antifolates have been synthesized and tested pre-clinically or even advanced to clinical trials, only to later fail, these were nonetheless invaluable prototypes, fostering development of clinically important agents or tumor-targeted antifolates still in development, typified by FR-selective ONYX 0810Citation9 and PCFT-selective 6-substituted pyrrolo[2,3-d]pyrimidine antifolates (see below).
As classical antifolates, like their folate cofactor counterparts, are all anions at physiologic pH, membrane transport is essential to drug efficacies. RFC has long been considered the primary transport route for antifolate drugs into both tumors and normal tissues,Citation22 even though their cellular uptake by other routes (i.e., FRs and/or PCFT) can occur. For MTX, membrane transport is requisite to generating adequate intracellular drug concentrations to maximize inhibition of DHFR and for synthesis of polyglutamyl forms required for drug retention and sustained antitumor effects.Citation97 For non-DHFR inhibitors such as PMX, RTX, or LMX, polyglutamate derivatives typically show substantially higher binding affinities for intracellular enzyme targets than non-polyglutamylated drugs.Citation98-Citation100 Finally, impaired membrane transport by RFC is a major mode of resistance to classical antifolates such as MTX with in vitro and in vivo preclinical models, and is also implicated in clinical resistance to MTX.Citation5,Citation22
Antifolate inhibitors of dihydrofolate reductase and the de novo thymidylate biosynthesis pathway
Antifolates that inhibit DHFR include AMT, MTX, and PDX (). Inhibition of DHFR blocks synthesis of THF from DHF, generated during synthesis of thymidylate by TS (). The effect is a build-up of DHF, a “depletion” of unsubstituted and C1-substituted THF pools, resulting in suppression of biosynthesis of purines, thymidylate, serine, and methionine. The extent of THF depletion varies for different cofactor forms and cell types,Citation101-Citation103 likely due to folate sequestration in organelles and binding to cellular proteins.Citation104-Citation106
AMT preceded MTX in use for pediatric ALL.Citation3 AMT is a better substrate than MTX for both hRFC and FPGS and is therefore transported into cells and metabolized to polyglutamates much more rapidly.Citation107 This increases its antitumor potency but may also contribute to its increased toxicity over MTX. While there has been renewed clinical interest of AMT for cancer and inflammatory diseases,Citation108,Citation109 MTX remains the most extensively used and widely-studied antifolate and is the standard by which newer agents of this class are compared.
PDX () was discovered through collaboration between F.M. Sirotnak (Memoral Sloan Kettering Cancer Center) and J.I. Degraw (Southern Research Institute) to identify antifolates with improved cellular pharmacology over MTX. 10-Deaza-AMT was more potent than MTXCitation110 toward preclinical models and its 10-ethyl derivative (edatrexate) was even more potent.Citation111,Citation112 A 3rd generation analog, 10-propargyl-10-deaza-AMT (PDX), was a less potent DHFR inhibitor than AMT, MTX, or edatrexate but was more active for RFC transport and polyglutamylation than these analogs.Citation113 This resulted in substantially increased cytotoxicity toward leukemia, breast cancer, and NSCLC cell lines in vitro and in vivo. PDX showed efficacy and safety in phase I and in phase II trials, including patients with NSCLCCitation114 and peripheral T-cell lymphoma.Citation107,Citation115 In September 2009, the FDA approved use of PDX for the treating relapsed, refractory peripheral T-cell lymphoma.Citation8
The quinazoline antifolate RTX (Tomudex, ZD1694) () is a potent TS inhibitor that grew from rational drug design efforts of scientists at the Institute for Cancer Research and Astra Zeneca.Citation98,Citation116 RTX was an outgrowth of N10-propargyl-5,8-dideazafolic acid (CB3717) which in phase I/II clinical trials showed activity against ovarian, liver, and breast cancers along with hepatic toxicity and dose-limiting nephrotoxicity. To ameliorate toxicity, the 2-amino group was replaced with a 2-desamino-2-methyl, a thiophene was substituted for p-aminobenzoate, and the N10-propargyl was replaced with a methyl, producing RTX.Citation117 RTX is a less potent TS inhibitor than CB3717 but exhibits substantially greater RFC uptake and metabolism to polyglutamates, leading to more potent anti-tumor effects in vitro and in vivo. RTX was approved for advanced colorectal cancer outside the USCitation96 and shows efficacy toward malignant pleural mesothelioma combined with cisplatin.Citation118
Jackman and colleagues extended their search for a new generation of TS-targeted therapeutics to include the FRα-targeted agents BGC638 and BGC945, both cyclopenta[g]quinazoline analogs, neither of which are RFC or FPGS substrates.Citation9,Citation119 BGC945 showed superior in vitro efficacy over BGC638 with FRα-expressing tumors. BGC945 was tested in vivo in mice bearing human KB tumor xenografts and treated with 5-[125I]-iodo-2’-deoxyuridine.Citation9,Citation119 The results established that BGC945 was a selective TS inhibitor toward FRα-expressing tumors. BGC945 was licensed by Onyx Pharmaceuticals and renamed ONYX0801, and in 2009 a phase I clinical trial was initiated in the UK.
Antifolate inhibitors of de novo purine nucleotide biosynthesis
Purines serve as building blocks of RNA and DNA, and as components of ATP, cyclic AMP, NADH, and coenzyme A. Differentiated adult cells often satisfy their purine requirements through purine salvage.Citation120,Citation121 Conversely, proliferating cells require de novo synthesis to meet their greater demands for purine nucleotides for DNA and RNA synthesis.
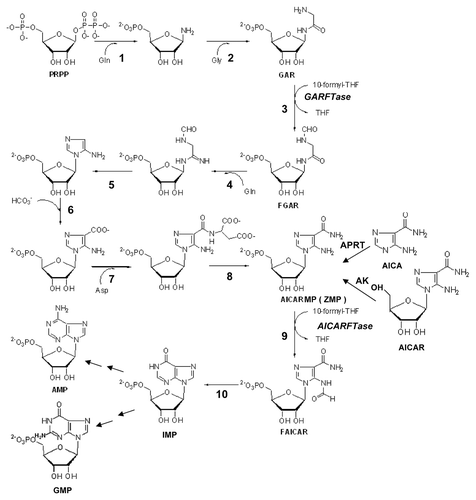
Both purine salvage and de novo biosynthetic pathways use phosphoribosyl pyrophosphate (PRPP). In purine salvage, hypoxanthine phosphoribosyl transferase (HPRT) converts guanine and hypoxanthine to GMP and IMP, respectively, and adenine phosphoribosyl transferase (APRT) converts adenine to AMP. The de novo purine nucleotide biosynthetic pathway consists of 10 reactions catalyzed by 6 distinct enzymes including multifunctional proteins, eventually generating IMP (). The two folate-dependent steps are catalyzed by GARFTase and AICARFTase.
Figure 6. De novo purine nucleotide biosynthesis pathway. The de novo purine nucleotide biosynthetic pathway from phosphoribosyl pyrophosphate (PRPP) to IMP is shown. The numbered reactions are catalyzed by the following monofunctional enzymes: 1, glutamine phosphoribosylpyrophosphate amidotransferase (GPAT); 4, formylglycinamide ribonucleotide synthase (FGAM synthetase); and 8, adenylosuccinate lyase (ASL). Reactions 2, 3 and 5 are catalyzed by the trifunctional glycinamide ribonucleotide (GAR) formyltransferase (GARFTase) which contains GAR synthase (GARS; reaction 2), GAR formyltransferase (GARFTase; reaction 3) and 5-amino-4-imidazole ribonucleotide synthase (AIRS; reaction 5) activities. Reactions 6 and 7 are catalyzed by the bifunctional phosphoribosylaminoimidazole carboxylase/ phosphoribosylaminoimidazole succinocarboxamide synthetase (PAICS) enzyme, which contains carboxyaminoimidazole ribonucleotide synthase (CAIRS; reaction 6) and 5-aminoimidazole-4-(N-succinylocarboxamide) ribonucleotide synthase (SAICARS; reaction 7) activities. Reactions 9 and 10 are catalyzed by a bifunctional enzyme, 5-amino-4-imidazolecarboxamide ribonucleotide (AICAR) formyltransferase (AICARFTase)/IMP cyclohydrolase (ATIC) that sequentially catalyzes the last two steps in the pathway for de novo synthesis of IMP. Folate-dependent reactions (reactions 3 and 9) in which 10-CHO-THF serves as the one-carbon donor are catalyzed by GARFTase and AICARFTase. 5-Aminoimidazole-4-carboxamide (AICA) and AICAR can be metabolized to AICAR monophosphate (ZMP) by adenine phosphoribosyl transferase (APRT) and adenosine kinase (AK), respectively, thus circumventing the reaction catalyzed by GARFTase.
In an effort to develop new inhibitors of folate metabolism at targets other than DHFR, E.C. Taylor at Princeton and Chuan (Joe) Shih at Eli Lilly collaborated to synthesize the (6R) diastereomer of 5,10-dideazatetrahydrofolate or LMX ().Citation122,Citation123 LMX is structurally analogous to THF except that the 5 and 10 nitrogens are replaced by carbons. While LMX is an excellent substrate for FRs,Citation124 it is primarily transported into tumors by hRFC, whereupon it is extensively polyglutamylated by FPGS and inhibits GARFTase, leading to ATP and GTP depletion and potent antitumor activity with preclinical models in vitro and in vivo.Citation99,Citation122,Citation123,Citation125 In CCRF-CEM cells with impaired RFC function, polyglutamylation of LMX is so extensive as to effectively negate the impact of loss of RFC and the resistant phenotype.Citation126 In a phase I study without folic acid co-adminstration, LMX caused severe cumulative toxicity, with dose-limiting myelosuppression (anemia, thrombocytopenia and neutopenia) and mucositis.Citation127 When LMX was administered with folic acid, there was a reduction in clinical toxicity, permitting a 10-fold dose escalation over that without folate supplementation.Citation128
Figure 7. Structures of GARFTase inhibitors. Structures are shown for Lometrexol (LMX) and LY309887 and AG2034.
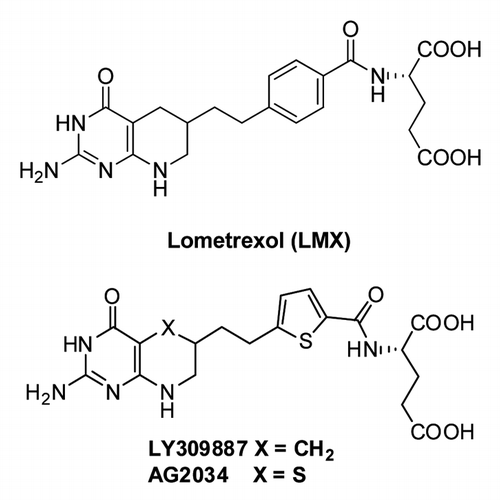
To reduce toxicity, second generation GARFTase inhibitors were synthesized (). LY309887 had a lower affinity for FRs, reduced polyglutamylation and a 9-fold increased affinity for GARFTase compared with LMX.Citation99 AG2034 () and AG2037 were based on X-ray crystal structures of E. coli GARFTase and the GARFTase domain of the bifunctional human enzyme.Citation129 AG2034 differed from AG2037 in its lower substrate activity toward FRs, although AG2034 and AG2037 were both transported by RFC and were potent GARFTase inhibitors.Citation129 Clinical evaluation of AG2034 and LY309887 showed similar cumulative toxicities to that for LMX.Citation130,Citation131
Development of a multitargeted antifolate
To meet FDA requirements of purity and to eliminate the chirality at the 6-position of LMX, the 5-deazapteridine ring was replaced with a pyrrolo[2,3-d]pyrimidine, resulting in LY231514 or PMX (Alimta) ().Citation132 Cell culture experiments with nucleoside (e.g., thymidine, hypoxanthine) additions confirmed that PMX was primarily a TS inhibitor, although secondary targets were suggested, including folate-dependent enzymes in de novo purine biosynthesis.Citation132,Citation133 Enzymology studies confirmed potent inhibition of TS and weaker inhibition of DHFR, GARFTase and AICARFTase.Citation132,Citation133 Inhibitions for PMX polyglutamates were especially potent, with a Ki for PMX pentaglutamate (PMX plus 4 glutamates) of 1.3 nM at TS, compared with 109 nM for the parent drug. For GARFTase and AICARFTase, Kis were substantially increased, suggesting decreased inhibitory potencies. Reflecting this multi-targeted enzyme inhibition, PMX was originally designated “multi-targeted antifolate” or “MTA.”
PMX is an excellent transport substrate for both RFCCitation22 and PCFT,Citation25 and is among the best substrates for FPGS.Citation133,Citation134 Polyglutamylation of PMX is highly sensitive to cellular folate status, such that its in vitro efficacy is enhanced in RFC deficient cells in the presence of physiologic concentrations of THF cofactors, as long as PCFT is present.Citation134-Citation137 For antifolates such as MTX or RTX that are poorer substrates for PCFT and are primarily transported by RFC, loss of RFC results in drug resistance. It is its PCFT transport which is a defining characteristic of PMX, as PMX is among the best known substrates for PCFT and shows reduced pH sensitivity for transport compared with other (anti)folate substrates.Citation25,Citation59
Although GARFTase was originally suggested to be an important secondary target of PMX, R.G. Moran and colleagues suggested that the 2nd folate-dependent step in de novo purine nucleotide biosynthesis, AICARFTase, was likely a more important target for PMX than originally envisaged. They showed that treatment of CCRF-CEM ALL and a number of solid tumor cell lines with PMX resulted in marked accumulations of the AICARFTase substrate, ZMP,Citation138,Citation139 even though ATP pools were not depleted.Citation138 ZMP is an AMP mimetic and activator of AMP-activated protein kinase (AMPK). AMPK activation causes phosphorylation of AMPK target proteins involved in initiation of cap-dependent translation, lipid synthesis, and energy metabolism. AMPK phosphorylates tuberous sclerosis complex 2 and raptor (component of mTORC1 complex) proteins, leading to inhibition of mTOR signaling. Interestingly, PMX synergizes with sorafenib, enhancing tumor killing via a toxic form of autophagy and activation of the intrinsic apoptosis pathway.Citation140 PMX was FDA approved in 2004 for use (with cisplatin) in treating malignant pleural mesotheliomaCitation6 and in 2008 as a first-line treatment for non-squamous NSCLC in combination with cisplatin.Citation7 In 2009, PMX was approved for maintenance therapy of patients with locally advanced or metastatic non-squamous NSCLC.Citation141
Hijacking The Acidic Tumor Microenvironment For Solid Tumor Targeting
Cancer cells often have a greater need for energy and metabolic precursors (e.g., nucleotides) than normal differentiated cells. This increased biosynthetic demand can be, at least in part, met by an altered metabolic program known as the “Warburg effect” or aerobic glycolysis in which cancer cells become highly glycolytic even in the presence of normal oxygen tension.Citation142 To avoid intracellular acidification, glycolytically-produced acid must be extruded. This is achieved by increased expression and/or activity of plasma membrane ion pumps and transporters, including H+-ATPases or vacuolar ATPases,Citation143 the Na+/H+ exchanger (NHE1) of the SLA9A family,Citation144 monocarboxylate-H+ efflux symporters (MCT1 and MCT4) of the SLC9A family,Citation145,Citation146 carbonic anhydrases (CAIX and CAXII),Citation147 the Cl-/HCO3- exchanger (CBE),Citation148 and the Na+/HCO3- co-transporter (NBC).Citation149 The net result is reversal of intra-to-extracellular pH gradients, such that tumors generate significant acidification of their extracellular environments. The extracellular pH (pHe) in the vicinity of tumor cells can be as low as pH ~6.7 to ~7.1, while tumors maintain a normal to slightly alkaline intracellular pH (pHi) of ≥ 7.4. This compares to pHi of ~7.2 and pHe of ~7.3 in normal differentiated cells.Citation150-Citation152 Acidification of the tumor extracellular environment is exacerbated by limited removal of glycolytic waste products due to poor perfusion, which can be affected by tumor size and abnormal vascularization.Citation152 The resulting H+ electrochemical gradient favors passive weak acid uptake at the tumor plasma membrane by pH partition and also acts as a driving force for H+-coupled membrane transport of solutes including chemotherapy drugs used for cancer.
A targeted drug strategy for cancer founded on the selective uptake of therapeutics into tumors by H+-coupled transporters is novel but not unprecedented.Citation153 Aberrant H+-coupled di-/tripeptide (PepT1 and PepT2) transport can be used for targeting experimental and clinical anticancer substrates to tumor cells, including the photodynamic therapy and imaging agent 5-aminolevulinic acidCitation154 and the aminopeptidase inhibitor bestatin.Citation155 Prodrugs of floxuridine and cytarabine can also be transported by PepT1.Citation156 The H+-coupled amino acid transporter PAT1 and the pH-dependent OATPs also facilitate uptake of anticancer drugs. PAT1 can transport 5-aminolevulinic acidCitation154 and L-cycloserineCitation157 and OATP1A2 shows MTX transport activity at low pH.Citation158 Recently, OATP2B1 was identified as a low affinity/low pH transporter for classic antifolates.Citation26
Therapeutic Targeting Of Solid Tumors With Pcft-Selective Antifolates
Expression of the major folate transporters in human tumors
Following reports of a low pH transport activity in solid tumor cells linesCitation136 and PCFT expression in a small cohort of human tumors,Citation67 studies were performed to establish a comprehensive expression profile for hPCFT compared with hRFC and FRs in 80 established cell lines derived from human solid tumors (n = 53) and leukemias (n = 27).Citation12 Transcript levels for hPCFT, hRFC, and FRs α and β were measured by real-time RT-PCR. The results confirmed substantial levels of hPCFT transcripts in the majority of tumor cell lines of different origins (e.g., breast, prostate, ovarian, etc.) and very low-to-undetectable hPCFT levels in leukemias. hPCFT levels were highest in Caco-2 (colorectal adenocarcinoma), SKOV3 (ovarian carcinoma), HepG2 (hepatoma), and H69 (small cell lung cancer) cells, with substantial hPCFT in numerous other tumor sublines. hRFC transcripts were detected in all tumor and leukemia cell lines with the exception of MDA-MB-231 breast cancer cells. FRα was detected in a small cohort of ovarian, cervical, and breast cancer cell lines and FRβ was detected in a few AML and T-cell ALL cell lines. In ten tumor cell lines, hPCFT protein was measured on western blots and [3H]MTX transport was measured at pH 5.5,Citation60 thus corroborating the original findings of Zhao et al.Citation136 and identifying hPCFT as the transporter responsible for low pH transport activity.
Development of hPCFT-selective antifolates
The clinical successes with PMX, combined with evidence that hPCFT may offer a tumor-selective mode of antifolate delivery, led to an intensive effort to develop novel cytotoxic antifolates with transport specificity for PCFT over RFC. Analogs were initially tested in engineered CHO sublines derived from RFC-, FR-, and PCFT-null cells (RIIMTXROua2–4) to express individually hRFC (PC43–10) or hPCFT (R2/hPCFT4).Citation16,Citation17,Citation159 While tissue culture media is at pH 7.2–7.4, during cell outgrowth the pH of the culture media decreases to pH ~6.7.Citation11,Citation14
PMX is a 5-substituted 2-amino-4-oxo-pyrrolo[2,3-d]pyrimidine antifolate with a 2 carbon bridge attached to a p-aminobenzoyl glutamate (). While the 6-regioisomer of PMX was inert toward tumor cells in culture,Citation160 when the bridge region between the heterocycle and p-aminobenzoate was elongated to 3 (compound 3) or 4 (compound 4) carbons to provide greater conformational flexibility, the resulting analogs () were modest inhibitors of proliferation of CCRF-CEM ALL cells at elevated (micromolar) drug and folic acid concentrations.Citation161,Citation162 Later studies with the engineered PC43–10 and R2/hPCFT4 CHO sublines convincingly showed that compounds 3 and 4 were highly selective inhibitors of proliferation of hPCFT-expressing cells (but not hRFC-expressing cells) at nanomolar concentrations.Citation11,Citation14 Compound 3 was ~9-fold more active than compound 4 (IC50s of 23 and 213 nM) toward hPCFT-expressing R2/hPCFT4 cells. These results contrast with those for antifolates such as MTX, PMX, or RTX which showed no selectivity for hPCFT over hRFC. Compounds 3 and 4 were also inhibitors of FR-expressing CHO cells.
Figure 8. 6-Substituted pyrrolo- and thieno[2,3-d]pyrimidine antifolates with hPCFT and FR specificity over hRFC. Structures are shown for 6-substituted pyrrolo[2,3-d]pyrimidine benzoyl antifolates with carbon bridge length variations of 1- to 6-carbons (compounds 1–6, respectively), 6-substituted thieno[2,3-d]pyrimidine benzoyl antifolates with bridge length variations from 2–8 carbons (compounds 7–13, respectively), 6-substituted pyrrolo[2,3-d]pyrimidine antifolates with a thienoyl replacement for the benzoyl moiety and bridge length variations from 1–6 carbons (compounds 14–19, respectively), and 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers of compound 17 (compounds 20–24).
![Figure 8. 6-Substituted pyrrolo- and thieno[2,3-d]pyrimidine antifolates with hPCFT and FR specificity over hRFC. Structures are shown for 6-substituted pyrrolo[2,3-d]pyrimidine benzoyl antifolates with carbon bridge length variations of 1- to 6-carbons (compounds 1–6, respectively), 6-substituted thieno[2,3-d]pyrimidine benzoyl antifolates with bridge length variations from 2–8 carbons (compounds 7–13, respectively), 6-substituted pyrrolo[2,3-d]pyrimidine antifolates with a thienoyl replacement for the benzoyl moiety and bridge length variations from 1–6 carbons (compounds 14–19, respectively), and 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers of compound 17 (compounds 20–24).](/cms/asset/e36ad4fd-29ac-44ca-8418-d10223e01df7/kcbt_a_10922020_f0008.gif)
Additional studies established structure-activity profiles for PCFT transport, using the analogous 6-substituted pyrrolo[2,3-d]pyrimidine antifolates with one (compound 1), two (compound 2), five (compound 5), or six (compound 6) bridge region methylenesCitation14,Citation17(). Further, replacement of the pyrrolo ring of compounds 3 and 4 with an isosteric thieno ring (compounds 8 and 9, respectively), resulted in a larger ring more closely approximating the 6–6 fused ring system of the THF cofactor, and replaced the hydrogen bond (NH) donor with an S.Citation16 None of these analogs were active for hPCFT- or hRFC-expressing CHO cells, although compounds 8 and 9 preserved substantial anti-proliferative activities toward FR-expressing cells.
The synthesis of 6-substituted pyrrolo[2,3-d]pyrimidines based on compounds 3 and 4 with a thienoyl-for-benzoyl replacement afforded the most potent PCFT-selective antifolates (compounds 16 and 17; )Citation12,Citation13,Citation15 This modification was partly based on the previous GARFTase inhibitors LY309887Citation99 and AG2034Citation129 (). Compounds 16 and 17 with 3 and 4 bridge carbons, respectively, showed substantial PCFT-targeted activity for R2/hPCFT4 CHO cells, with IC50s of 3.34 and 43 nM, respectively.Citation13 Activity for this series declined dramatically for analogs with carbon bridge lengths shorter than 3 or greater than 4.
A series of pyrrolo[2,3-d]pyrimidine thienoyl regioisomers of 17 with a 4 carbon bridge and thienoyl ring substitutions, 4’,5′ (compound 20), 2’,3′ (compound 21), 3′,4’ (compound 22), 2’,4’ (compound 23), and 3′,5′ (compound 24) (), were synthesized and tested, as a means of forcing the bicyclic scaffold and L-glutamate closer together than in the parent 3-atom bridge compound 16.Citation11 While the analogs were all inactive toward hRFC-expressing CHO cells, compounds 23 and 24 were quite inhibitory toward hPCFT-expressing R2/hPCFT4 CHO cells, essentially equivalent to compound 17. This establishes the selective cellular uptake of compounds 23 and 24 by hPCFT. Like compounds 3 and 4, compounds 16, 17, 23 and 24 were also active toward cell lines expressing FRs.Citation11-Citation13,Citation15
Tumor-targeting by PCFT-mediated uptake of novel antifolates
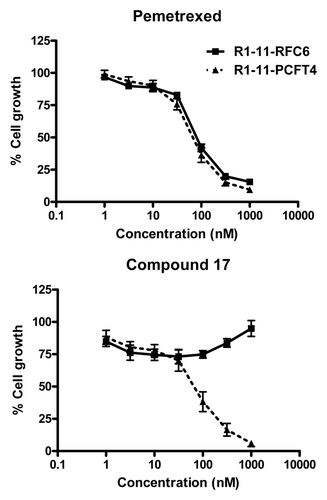
The anti-proliferative effects of 6-substituted pyrrolo[2,3-d]pyrimidine antifolates 3, 16, 17, 23 and 24 toward hPCFT-expressing CHO cells provided strong impetus for additional studies to explore the therapeutic potential of hPCFT for the chemotherapy of human tumors.Citation11-Citation13,Citation15 With compounds 16 and 17, selective inhibition of proliferation was confirmed in hPCFT-expressing HeLa cells (R1–11-PCFT4), derived from a hRFC- and hPCFT-null HeLa subline (R1–11), whereas activity was nominal in the isogenic R1–11-RFC6 subline which expresses only hRFC ( shows results for compound 17). Compound 17 was a potent inhibitor of R1–11-PCFT4 colony formation during intermittent drug exposures and showed dose- and time-dependence.Citation12 hPCFT transport of compounds 16 and 17 was inferred from competition assays in R1–11-PCFT4 HeLa and R2/hPCFT4 CHO cells incubated with [3H]MTX in their presence and from induced currents in Xenopus oocytes microinjected with hPCFT cRNA in the presence of PCFT-substrates.Citation12,Citation13,Citation15 Direct transport measurements of [3H] compounds 16 and 17 established kinetics and pH dependencies consistent with the known properties of hPCFT.Citation12,Citation60 Substrate activities with compounds 16 and 17 were at least equivalent to those of PMX. In HeLa cells treated at pH 6.8 with [3H] compounds 16 and 17, polyglutamates were detected, with ~6-fold higher levels for 16 over 17.Citation60 Polyglutamyltion of compound 17 was also reported in HepG2 hepatoma cells.Citation12
Figure 9. Characterization of compound 17 and PMX growth inhibition in R1–11 sublines differing in hPCFT and hRFC expression. Cell proliferation inhibition was measured for R1–11-PCFT4 (expresses hPCFT but not hRFC) and -RFC6 (expresses hRFC but not hPCFT) cells treated with PMX (A) or compound 17 (B) over a range of concentrations in complete folate-free RPMI1640 in the presence of 5-CHO-THF at 25 nM for 96 h. Cell densities were measured with CellTiter BlueTM fluorescence dye and a fluorescence plate reader. Results were normalized to cell densities in the absence of drug. From Kugel Desmoulin et al.Citation12
In assorted cell lines treated with compounds 3, 16, 17, 23 and 24, growth inhibition protection assays were performed with adenosine and thymidine, and de novo purine biosynthesis was implicated as the targeted pathway.Citation11-Citation13,Citation15 Since de novo purine biosynthesis involves folate-dependent reactions catalyzed by GARFTase and AICARFTase (), additional protection experiments used AICA, a precursor of ZMP, which circumvents the GARFTase step. In these experiments, AICA was completely protective, establishing GARFTase as the principal cellular target. GARFTase inhibition was confirmed by an in situ GARFTase assay in R2/hPCFT4 CHO and R1–11-PCFT4 HeLa cells which measures accumulation of the GARFTase product [14C]formyl GAR in the presence of [14C]glycine, and by direct assay of recombinant murine GARFTase in the presence of the monoglutamyl drugs.Citation11-Citation15 For compounds 3, 16 and 17, GARFTase inhibition was accompanied by a dramatic drop in ATP levels, to an extent approximating that seen with LMX but far exceeding the modest impact of PMX on ATP pools.Citation11-Citation15 For compound 17, treatment of R1–11-PCFT4 HeLa cells resulted in time- and dose-dependent accumulation in late S-phase, accompanied by cell death, apparently by a non-apoptotic mechanism.Citation12
These compelling in vitro results with compounds 16 and 17 were extended in vivo in SCID mice bearing human tumor xenografts (HepG2, HeLa), and provide proof-of-concept of in vivo tumor targeting of these novel analogs via hPCFT.Citation12,Citation60
Considerations for selectivity and efficacy of current generation PCFT-targeted therapeutics
The 6-substituted pyrrolo[2,3-d]pyrimidine antifolates 16 and 17, with 3- or 4-carbon bridge lengths, respectively, are lead compounds for hPCFT-targeted chemotherapy of human solid tumors. While thienoyl-for-benzoyl ring replacement was comparatively neutral within the context of the 4-carbon bridge platform, the pyrrolo[2,3-d]pyrimidine thienoyl 3-carbon bridge analog 16 is the most potent of the hPCFT-selective antifolates thus far identified.Citation13,Citation60 At both pH 5.5 and pH 6.8, compound 16 is at least comparable to PMX as a PCFT transport substrate, yet it shows greater tumor selectivity than does PMX due to its near complete absence of RFC transport. Compound 16 is efficiently converted to polyglutamates, to levels exceeding those for compound 17, resulting in substantially increased GARFTase inhibition and ATP depletion at lower drug concentrations, thus explaining differences in drug sensitivities.Citation60
Previous generation GARFTase-targeted drugs include LMX, LY309887 and AG2034, all of which entered into clinical trials and resulted in significant toxicity.Citation127,Citation130,Citation131 In retrospect, toxicities of these agents were most likely due, at least in part, to their membrane transport by hRFC in normal tissues. Indeed, GARFTase inhibitors with hPCFT selectivity such as compound 16 would seem to afford substantial advantages over not only classic antifolates such as MTX and PMX, but also previous iterations of GARFTase-targeted drugs which are primarily transported by hRFC.
Thus, in normal tissues, the tissue milieu is generally at neutral pH which would greatly favor (anti)folate membrane transport by hRFC. While hPCFT expression is more limited in normal tissues than hRFC, even if hPCFT is present, the decreased electrochemical proton gradient would result in only modest accumulations of hPCFT substrates such as compound 16. Further, the increased capacity of hRFC to transport reduced folates compared with hPCFT should result in elevated intracellular folate pools in normal tissues which compete at FPGS for polyglutamylation (with consequent effects on drug retention and inhibition of folate-dependent enzyme targets), or at intracellular drug targets such as GARFTase, protecting normal cells from drug-induced cytotoxicity. Other compensatory effects in response to elevated intracellular folates are also possible including decreased FPGS activityCitation163 or increased levels and/or altered cellular distributions of ABC transporters.Citation164 In solid tumors, if sufficient hRFC is present, this may transport reduced folates even at slightly acidic pHs to decrease efficacy of hPCFT-targeted drugs. Consistent with this are studies with hRFC-null HeLa cells in which the inhibitory effects of compounds 16 and 17 were exacerbated compared with wild-type HeLa with intact hRFC, as long as hPCFT was present.Citation60 Thus, tumor selectivity of cytotoxic hPCFT-targeted antifolates such as compound 16 is not only determined by differences in hPCFT levels between normal tissues and tumors, but also by the interstitial pH, and the activity of hRFC.
GARFTase inhibitors such as compounds 16 and 17 would seem to have other features that render them uniquely useful for therapy of solid tumors. For instance, based on studies with LMX, GARFTase inhibition resulting in cytotoxicity appears to be independent of p53 status.Citation165,Citation166 This reflects depletion of ATP/GTP pools, resulting in p53 hypophosphorylation and hypoacetylation which, while not impeding nuclear retention and p21 promoter binding, renders p53 transcriptionally inert.Citation165,Citation166
Differences in purine salvage between normal and tumor cells can confer selectivity for antipurine antifolates.Citation120,Citation121 This may explain low rates of de novo purine synthesis in bone marrow and suggests that inhibitors of de novo purine synthesis should exhibit minimal marrow toxicity. Further, methylthioadenosine phosphorylase (MTAP), another salvage enzyme that releases adenine from methylthioadenosine formed during polyamine biosynthesis is abundantly expressed in normal tissues yet is co-deleted with CDKN2A in many tumors.Citation167 This would potentially render tumors deficient in purine salvage especially sensitive to GARFTase inhibitors, while functional purine salvage via MTAP in normal tissues should provide selective protection.Citation168
Conclusions
This review summarizes the biology of PCFT. PCFT was originally identified as a heme transporterCitation49 and in 2006, it was reclassified as the primary folate transporter involved in intestinal absorption of dietary folates.Citation50 Loss of PCFT function, secondary to hypermutation of functionally or structurally important amino acids, was recognized as causal in the rare autosomal recessive condition, HFM.Citation51,Citation62,Citation75-Citation84 Characterization of the causal basis for HFM identified mechanistically important residues and regions involved in substrate binding or proton coupling, prompting further mechanistic studies. While formation of hPCFT homo-oligomers was confirmed, establishing their functional significance relied on studies of HFM mutant/wild-type hetero-oligomers.Citation87 Further studies documented mechanisms of hPCFT transcriptional control including promoter methylation,Citation65-Citation67 regulation by NRF-1,Citation68 and effects of exogenous vitamin DCitation73 or possibly changes in microenvironmental pHCitation63 on PCFT expression.
With the recognition that hPCFT was abundantly expressed in many human solid tumors,Citation12,Citation136 attention soon turned to considerations of how PCFT could be therapeutically exploited for cancer. The demonstration that PMX is an excellent hPCFT transport substrate provided validation of its established clinical efficacy for malignant mesothelioma and NSCLC, and studies of cross-resistance patterns provided a rational explanation for collateral sensitivities to PMX of tumors that are deficient in hRFC.Citation135-Citation137 These successes prompted development of more selective PCFT-targeted 6-substituted pyrrolo[2,3-d]pyrimidine antifolates such as compounds 16 and 17 that, unlike PMX, derive their cytotoxic effects by targeting de novo purine nucleotide biosynthesis and GARFTase.Citation12,Citation13,Citation15 Selectivity for these novel analogs is enhanced by virtue of the acidic pHs of solid tumors which favor hPCFT membrane transport over hRFC, and the preferential membrane transport of THF cofactors into normal tissues at neutral pH that favors hRFC over hPCFT transport.Citation14
The progress in our understanding of the biology and clinical applications of PCFT since 2006 has been nothing short of staggering. With this said, there are significant questions that must be answered should basic science studies of PCFT be further extended to patients with HFM and potentially cancer. For instance, further understanding of hPCFT regulation is essential including both transcriptional and posttranscriptional controls that account for elevated levels of hPCFT in many solid tumors and comparatively modest hPCFT levels in many normal tissues. Of particular interest will be the possible regulatory impact of exogenous folates or microenvironmental factors. The demonstration that hPCFT can form functionally important homo-oligomersCitation87 points to another level of regulation, namely formation and intracellular trafficking of these higher-order hPCFT complexes. Evidence that wild-type/mutant hPCFT mixtures exhibit a dominant-positive phenotype provides an explanation for exclusive homozygosity in HFM but is also relevant to potential resistant phenotypes involving mutant hPCFT in tumors treated with hPCFT-targeted antifolates. It will be especially important to identify the structural determinants of hPCFT oligomerization, as this may eventually lead to approaches for modulating this critical process, i.e., through the use of small molecule chaperones or peptidomimetics based on the peptide interface that can impact membrane trafficking.
Although the 6-substituted pyrrolo[2,3-d]pyrimidine antifolates exhibit near exclusive selectivity for hPCFT over hRFC, it will be important to better identify structure-activity relationships for substrate binding and transport for both these carriers, and also for FRs. Indeed, while it has been possible to identify cytotoxic folate analogs with selective membrane transport by hRFC or FRs over other transporters, to date no compounds have been identified with hPCFT transport selectivity without substantial FR uptake, as well. Indeed, it is not yet certain whether it might be beneficial to develop exclusive hPCFT-selective substrates without FR transport, as long as hRFC transport is nominal. These studies will be undoubtedly facilitated by continued structural and molecular modeling studies of these transporters. It will be especially important to study resistance to novel hPCFT-selective antifolates including alterations involving the hPCFT protein and the potential impact of alterations in hRFC and/or FRs on the development of resistance. Finally, it will be important to identify the activities of hPCFT-specific antifolates for the major efflux pumps, MRP1 and ABCG2, since these could also impact the antitumor efficacies for these novel agents.
The intracellular impact of tumor-targeted hPCFT-selective inhibitors must be further defined, including specificities and binding determinants for FPGS and folate-dependent enzymes such as GARFTase, potential effects of altered purine nucleotide pools on AMPK and mTOR signaling pathways, and effects on the formation and catalytic function of the multienzyme de novo purine biosynthetic complex termed the “purinosome.”Citation169 It will be important to determine the mechanisms by which tumor-targeted hPCFT-selective antifolates that inhibit de novo purine enzymes actually kill tumor cells. This may further validate the therapeutic value of targeting de novo purine biosynthesis and/or imply that developing hPCFT-selective antifolates with different intracellular targets (e.g., TS) may be warranted. Clearly, better understanding of these pathways and the perturbations induced by novel hPCFT-selective antifolates will be essential for optimizing their potential clinical utility for targeting solid tumors.
| Abbreviations: | ||
| AICA | = | 5-amino-4-imidazole carboxamide |
| AICAR | = | 5-amino-4-imidazole carboxamide ribonucleotide |
| AICARFTase | = | 5-amino-4-imidazole carboxamide ribonucleotide formyltransferase |
| ALL | = | acute lymphoblastic leukemia |
| AML | = | acute myeloid leukemia |
| AMPK | = | AMP-activated protein kinase |
| AMT | = | aminopterin |
| APRT | = | adenine phosphoribosyl transferase |
| ATIC | = | 5-amino-4-imidazolecarboxamide ribonucleotide formyltransferase/IMP cyclohydrolase |
| CAIRS | = | carboxyaminoimidazole ribonucleotide synthase |
| CNS | = | central nervous system |
| DHF | = | dihydrofolate |
| DHFR | = | dihydrofolate reductase |
| EL | = | extracellular loop |
| FGAM | = | formylglycinamide ribonucleotide synthase |
| FPGS | = | folylpoly-γ-glutamyl synthetase |
| FR | = | folate receptor |
| GAR | = | β-glycinamide ribonucleotide |
| GARFTase | = | glycinamine ribonucleotide formyl transferase |
| GPAT | = | glutamine phosphoribosyl pyrophosphate amidotransferase |
| GPI | = | glycosylphosphatidylinositol |
| HA | = | hemagglutin |
| HFM | = | hereditary folate maladsorption |
| hPCFT | = | human proton-coupled folate transporter |
| HPRT | = | hypoxanthine phosphoribosyl transferase |
| hRFC | = | human reduced folate carrier |
| IL | = | intracellular loop |
| LMX | = | lometrexol |
| MFS | = | major facilitator superfamily |
| MTAP | = | methylthioadenosine phosphorylase |
| MTX | = | methotrexate |
| NRF-1 | = | nuclear respiratory factor 1 |
| NSCLC | = | non-small cell lung cancer |
| PAICS | = | phosphoribosyl aminoimidazole carboxylase/phosphoribosyl aminoimidazole succinocarboxamide synthetase |
| PCFT | = | proton-coupled folate transporter |
| PDX | = | pralatrexate |
| PMX | = | pemetrexed |
| PPRP | = | phosphoribosylpyrophosphate |
| RFC | = | reduced folate carrier |
| RTX | = | raltitrexed |
| SAM | = | S-adenosylmethionine |
| THF | = | tetrahydrofolate |
| TMD | = | transmembrane domain |
| TS | = | thymidylate synthase |
| VDR | = | vitamin D receptor |
Acknowledgments
This work was supported by grants from the National Cancer Institute, National Institutes of Health (CA53535, CA125153 and CA152316). S.K.D. was supported by a Doctoral Research Award from the Canadian Institute of Health Research.
References
- Farber S, Cutler EC, Hawkins JW, Harrison JH, Peirce EC 2nd, Lenz GG. The Action of Pteroylglutamic Conjugates on Man. Science 1947; 106:619 - 21; http://dx.doi.org/10.1126/science.106.2764.619; PMID: 17831847
- Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948; 238:787 - 93; http://dx.doi.org/10.1056/NEJM194806032382301; PMID: 18860765
- Farber S. Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood 1949; 4:160 - 7; PMID: 18107667
- Monahan BP, Allegra CJ. Antifolates. In: Chabner BA, Longo, D.L., ed. Cancer Chemotherapy and Biotherapy. Philadelphia, PA: Lippincott Williams and Wilkins, 2011:109-38.
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene 2003; 22:7431 - 57; http://dx.doi.org/10.1038/sj.onc.1206946; PMID: 14576850
- Hazarika M, White RM, Johnson JR, Pazdur R. FDA drug approval summaries: pemetrexed (Alimta). Oncologist 2004; 9:482 - 8; http://dx.doi.org/10.1634/theoncologist.9-5-482; PMID: 15477632
- Cohen MH, Justice R, Pazdur R. Approval summary: pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist 2009; 14:930 - 5; http://dx.doi.org/10.1634/theoncologist.2009-0092; PMID: 19737998
- Thompson CA. FDA approves pralatrexate for treatment of rare lymphoma. Am J Health Syst Pharm 2009; 66:1890; http://dx.doi.org/10.2146/news090080; PMID: 19850775
- Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, et al. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res 2005; 65:11721 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-05-2034; PMID: 16357184
- Yang J, Vlashi E, Low P. Folate-linked drugs for the treatment of cancer and inflammatory diseases. Subcell Biochem 2012; 56:163 - 79; http://dx.doi.org/10.1007/978-94-007-2199-9_9; PMID: 22116699
- Wang L, Cherian C, Kugel Desmoulin S, Mitchell-Ryan S, Hou Z, Matherly LH, et al. Synthesis and biological activity of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of de novo purine biosynthesis with selectivity for cellular uptake by high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier. J Med Chem 2012; 55:1758 - 70; http://dx.doi.org/10.1021/jm201688n; PMID: 22243528
- Kugel Desmoulin S, Wang L, Hales E, Polin L, White K, Kushner J, et al. Therapeutic targeting of a novel 6-substituted pyrrolo [2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Mol Pharmacol 2011; 80:1096 - 107; http://dx.doi.org/10.1124/mol.111.073833; PMID: 21940787
- Wang L, Desmoulin SK, Cherian C, Polin L, White K, Kushner J, et al. Synthesis, biological, and antitumor activity of a highly potent 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitor with proton-coupled folate transporter and folate receptor selectivity over the reduced folate carrier that inhibits β-glycinamide ribonucleotide formyltransferase. J Med Chem 2011; 54:7150 - 64; http://dx.doi.org/10.1021/jm200739e; PMID: 21879757
- Desmoulin SK, Wang Y, Wu J, Stout M, Hou Z, Fulterer A, et al. Targeting the proton-coupled folate transporter for selective delivery of 6-substituted pyrrolo[2,3-d]pyrimidine antifolate inhibitors of de novo purine biosynthesis in the chemotherapy of solid tumors. Mol Pharmacol 2010; 78:577 - 87; http://dx.doi.org/10.1124/mol.110.065896; PMID: 20601456
- Wang L, Cherian C, Desmoulin SK, Polin L, Deng Y, Wu J, et al. Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry. J Med Chem 2010; 53:1306 - 18; http://dx.doi.org/10.1021/jm9015729; PMID: 20085328
- Deng Y, Zhou X, Kugel Desmoulin S, Wu J, Cherian C, Hou Z, et al. Synthesis and biological activity of a novel series of 6-substituted thieno[2,3-d]pyrimidine antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors over the reduced folate carrier and proton-coupled folate transporter for cellular entry. J Med Chem 2009; 52:2940 - 51; http://dx.doi.org/10.1021/jm8011323; PMID: 19371039
- Deng Y, Wang Y, Cherian C, Hou Z, Buck SA, Matherly LH, et al. Synthesis and discovery of high affinity folate receptor-specific glycinamide ribonucleotide formyltransferase inhibitors with antitumor activity. J Med Chem 2008; 51:5052 - 63; http://dx.doi.org/10.1021/jm8003366; PMID: 18680275
- Stokstad ELR. Historical Perspective on Key Advances in the Biochemistry and Physiology of Folates. In: Picciano MF, Stokstad, E.L.R., Spector, R., ed. Folic Acid Metabolism in Health and Disease. New York: Wiley-Liss, 1990:1-21.
- Shane B. Folylpolyglutamate synthesis and role in the regulation of one-carbon metabolism. Vitam Horm 1989; 45:263 - 335; http://dx.doi.org/10.1016/S0083-6729(08)60397-0; PMID: 2688305
- Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat 2006; 9:227 - 46; http://dx.doi.org/10.1016/j.drup.2006.09.001; PMID: 17092765
- Lu SC. S-Adenosylmethionine. Int J Biochem Cell Biol 2000; 32:391 - 5; http://dx.doi.org/10.1016/S1357-2725(99)00139-9; PMID: 10762064
- Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev 2007; 26:111 - 28; http://dx.doi.org/10.1007/s10555-007-9046-2; PMID: 17334909
- Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev 2004; 56:1067 - 84; http://dx.doi.org/10.1016/j.addr.2004.01.001; PMID: 15094207
- Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 2009; 11:e4; http://dx.doi.org/10.1017/S1462399409000969; PMID: 19173758
- Zhao R, Goldman ID. The molecular identity and characterization of a Proton-coupled Folate Transporter--PCFT; biological ramifications and impact on the activity of pemetrexed. Cancer Metastasis Rev 2007; 26:129 - 39; http://dx.doi.org/10.1007/s10555-007-9047-1; PMID: 17340171
- Visentin M, Chang MH, Romero MF, Zhao R, Goldman ID. Substrate- and pH-specific antifolate transport mediated by organic anion-transporting polypeptide 2B1 (OATP2B1-SLCO2B1). Mol Pharmacol 2012; 81:134 - 42; http://dx.doi.org/10.1124/mol.111.074823; PMID: 22021325
- Wong SC, Zhang L, Proefke SA, Matherly LH. Effects of the loss of capacity for N-glycosylation on the transport activity and cellular localization of the human reduced folate carrier. Biochim Biophys Acta 1998; 1375:6 - 12; http://dx.doi.org/10.1016/S0005-2736(98)00118-7; PMID: 9767079
- Hou Z, Matherly LH. Oligomeric structure of the human reduced folate carrier: identification of homo-oligomers and dominant-negative effects on carrier expression and function. J Biol Chem 2009; 284:3285 - 93; http://dx.doi.org/10.1074/jbc.M807206200; PMID: 19019821
- Hou Z, Cherian C, Drews J, Wu J, Matherly LH. Identification of the minimal functional unit of the homo-oligomeric human reduced folate carrier. J Biol Chem 2010; 285:4732 - 40; http://dx.doi.org/10.1074/jbc.M109.086033; PMID: 20018840
- Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 2011; 31:177 - 201; http://dx.doi.org/10.1146/annurev-nutr-072610-145133; PMID: 21568705
- Whetstine JR, Flatley RM, Matherly LH. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: identification of seven non-coding exons and characterization of a novel promoter. Biochem J 2002; 367:629 - 40; http://dx.doi.org/10.1042/BJ20020512; PMID: 12144527
- Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta 2001; 1513:49 - 54; http://dx.doi.org/10.1016/S0005-2736(01)00340-6; PMID: 11427193
- Zhao R, Russell RG, Wang Y, Liu L, Gao F, Kneitz B, et al. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem 2001; 276:10224 - 8; PMID: 11266438
- Liu M, Ge Y, Cabelof DC, Aboukameel A, Heydari AR, Mohammad R, et al. Structure and regulation of the murine reduced folate carrier gene: identification of four noncoding exons and promoters and regulation by dietary folates. J Biol Chem 2005; 280:5588 - 97; http://dx.doi.org/10.1074/jbc.M412662200; PMID: 15579899
- Rijnboutt S, Jansen G, Posthuma G, Hynes JB, Schornagel JH, Strous GJ. Endocytosis of GPI-linked membrane folate receptor-alpha. J Cell Biol 1996; 132:35 - 47; http://dx.doi.org/10.1083/jcb.132.1.35; PMID: 8567728
- Sabharanjak S, Mayor S. Folate receptor endocytosis and trafficking. Adv Drug Deliv Rev 2004; 56:1099 - 109; http://dx.doi.org/10.1016/j.addr.2004.01.010; PMID: 15094209
- Kamen BA, Wang MT, Streckfuss AJ, Peryea X, Anderson RG. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem 1988; 263:13602 - 9; PMID: 3417674
- Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem 2009; 284:4267 - 74; http://dx.doi.org/10.1074/jbc.M807665200; PMID: 19074442
- Pan XQ, Zheng X, Shi G, Wang H, Ratnam M, Lee RJ. Strategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acid. Blood 2002; 100:594 - 602; http://dx.doi.org/10.1182/blood.V100.2.594; PMID: 12091353
- Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, et al. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem 2000; 275:20676 - 84; http://dx.doi.org/10.1074/jbc.M002328200; PMID: 10787414
- Weitman SD, Weinberg AG, Coney LR, Zurawski VR, Jennings DS, Kamen BA. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res 1992; 52:6708 - 11; PMID: 1330299
- Reddy JA, Haneline LS, Srour EF, Antony AC, Clapp DW, Low PS. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood 1999; 93:3940 - 8; PMID: 10339503
- Veggian R, Fasolato S, Ménard S, Minucci D, Pizzetti P, Regazzoni M, et al. Immunohistochemical reactivity of a monoclonal antibody prepared against human ovarian carcinoma on normal and pathological female genital tissues. Tumori 1989; 75:510 - 3; PMID: 2481353
- Garin-Chesa P, Campbell I, Saigo PE, Lewis JL Jr., Old LJ, Rettig WJ. Trophoblast and ovarian cancer antigen LK26. Sensitivity and specificity in immunopathology and molecular identification as a folate-binding protein. Am J Pathol 1993; 142:557 - 67; PMID: 8434649
- Buist MR, Molthoff CF, Kenemans P, Meijer CJ. Distribution of OV-TL 3 and MOv18 in normal and malignant ovarian tissue. J Clin Pathol 1995; 48:631 - 6; http://dx.doi.org/10.1136/jcp.48.7.631; PMID: 7560169
- Wu M, Gunning W, Ratnam M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomarkers Prev 1999; 8:775 - 82; PMID: 10498396
- Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 1994; 73:2432 - 43; http://dx.doi.org/10.1002/1097-0142(19940501)73:9<2432::AID-CNCR2820730929>3.0.CO;2-S; PMID: 7513252
- Selhub J, Rosenberg IH. Folate transport in isolated brush border membrane vesicles from rat intestine. J Biol Chem 1981; 256:4489 - 93; PMID: 7217093
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, et al. Identification of an intestinal heme transporter. Cell 2005; 122:789 - 801; http://dx.doi.org/10.1016/j.cell.2005.06.025; PMID: 16143108
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006; 127:917 - 28; http://dx.doi.org/10.1016/j.cell.2006.09.041; PMID: 17129779
- Umapathy NS, Gnana-Prakasam JP, Martin PM, Mysona B, Dun Y, Smith SB, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci 2007; 48:5299 - 305; http://dx.doi.org/10.1167/iovs.07-0288; PMID: 17962486
- Nakai Y, Inoue K, Abe N, Hatakeyama M, Ohta KY, Otagiri M, et al. Functional characterization of human proton-coupled folate transporter/heme carrier protein 1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther 2007; 322:469 - 76; http://dx.doi.org/10.1124/jpet.107.122606; PMID: 17475902
- Inoue K, Nakai Y, Ueda S, Kamigaso S, Ohta KY, Hatakeyama M, et al. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am J Physiol Gastrointest Liver Physiol 2008; 294:G660 - 8; http://dx.doi.org/10.1152/ajpgi.00309.2007; PMID: 18174275
- Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, et al. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol 2007; 293:C1669 - 78; http://dx.doi.org/10.1152/ajpcell.00202.2007; PMID: 17898134
- Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta 2008; 1778:1407 - 14; http://dx.doi.org/10.1016/j.bbamem.2008.03.009; PMID: 18405659
- Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry 2010; 49:2925 - 31; http://dx.doi.org/10.1021/bi9021439; PMID: 20225891
- Hou Z, Kugel Desmoulin S, Etnyre E, Olive M, Hsiung B, Cherian C, et al. Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter. J Biol Chem 2012; 287:4982 - 95; http://dx.doi.org/10.1074/jbc.M111.306860; PMID: 22179615
- Subramanian VS, Marchant JS, Said HM. Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am J Physiol Cell Physiol 2008; 294:C233 - 40; http://dx.doi.org/10.1152/ajpcell.00468.2007; PMID: 18003745
- Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol 2008; 74:854 - 62; http://dx.doi.org/10.1124/mol.108.045443; PMID: 18524888
- Kugel Desmoulin S, Wang L, Polin L, White K, Kushner J, Stout M, et al. Functional Loss of the Reduced Folate Carrier Enhances the Antitumor Activities of Novel Antifolates with Selective Uptake by the Proton-coupled Folate Transporter. Mol Pharmacol 2012; In Press
- Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem 2009; 284:17846 - 57; http://dx.doi.org/10.1074/jbc.M109.008060; PMID: 19389703
- Mahadeo K, Diop-Bove N, Shin D, Unal ES, Teo J, Zhao R, et al. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am J Physiol Cell Physiol 2010; 299:C1153 - 61; http://dx.doi.org/10.1152/ajpcell.00113.2010; PMID: 20686069
- Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB. The human proton-coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am J Physiol Gastrointest Liver Physiol 2010; 298:G248 - 54; http://dx.doi.org/10.1152/ajpgi.00224.2009; PMID: 19762432
- Wollack JB, Makori B, Ahlawat S, Koneru R, Picinich SC, Smith A, et al. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J Neurochem 2008; 104:1494 - 503; http://dx.doi.org/10.1111/j.1471-4159.2007.05095.x; PMID: 18086128
- Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther 2009; 8:2424 - 31; http://dx.doi.org/10.1158/1535-7163.MCT-08-0938; PMID: 19671745
- Stark M, Gonen N, Assaraf YG. Functional elements in the minimal promoter of the human proton-coupled folate transporter. Biochem Biophys Res Commun 2009; 388:79 - 85; http://dx.doi.org/10.1016/j.bbrc.2009.07.116; PMID: 19643086
- Gonen N, Bram EE, Assaraf YG. PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun 2008; 376:787 - 92; http://dx.doi.org/10.1016/j.bbrc.2008.09.074; PMID: 18817749
- Gonen N, Assaraf YG. The obligatory intestinal folate transporter PCFT (SLC46A1) is regulated by nuclear respiratory factor 1. J Biol Chem 2010; 285:33602 - 13; http://dx.doi.org/10.1074/jbc.M110.135640; PMID: 20724482
- Scarpulla RC. Nucleus-encoded regulators of mitochondrial function: Integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim Biophys Acta 2011; 1819:1088 - 97; PMID: 22080153
- Chang CM, Yu CC, Lu HT, Chou YF, Huang RFS. Folate deprivation promotes mitochondrial oxidative decay: DNA large deletions, cytochrome c oxidase dysfunction, membrane depolarization and superoxide overproduction in rat liver. Br J Nutr 2007; 97:855 - 63; http://dx.doi.org/10.1017/S0007114507666410; PMID: 17381984
- Brayton KA, Chen Z, Zhou G, Nagy PL, Gavalas A, Trent JM, et al. Two genes for de novo purine nucleotide synthesis on human chromosome 4 are closely linked and divergently transcribed. J Biol Chem 1994; 269:5313 - 21; PMID: 8106516
- Chen S, Nagy PL, Zalkin H. Role of NRF-1 in bidirectional transcription of the human GPAT-AIRC purine biosynthesis locus. Nucleic Acids Res 1997; 25:1809 - 16; http://dx.doi.org/10.1093/nar/25.9.1809; PMID: 9108165
- Eloranta JJ, Zaïr ZM, Hiller C, Häusler S, Stieger B, Kullak-Ublick GA. Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol Pharmacol 2009; 76:1062 - 71; http://dx.doi.org/10.1124/mol.109.055392; PMID: 19666701
- Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, et al. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 2007; 110:1147 - 52; http://dx.doi.org/10.1182/blood-2007-02-077099; PMID: 17446347
- Min SH, Oh SY, Karp GI, Poncz M, Zhao R, Goldman ID. The clinical course and genetic defect in the PCFT gene in a 27-year-old woman with hereditary folate malabsorption. J Pediatr 2008; 153:435 - 7; http://dx.doi.org/10.1016/j.jpeds.2008.04.009; PMID: 18718264
- Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, et al. A novel loss-of-function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood 2008; 112:2055 - 61; http://dx.doi.org/10.1182/blood-2008-04-150276; PMID: 18559978
- Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine (Baltimore) 2002; 81:51 - 68; http://dx.doi.org/10.1097/00005792-200201000-00004; PMID: 11807405
- Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT-SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood 2010; 116:5162 - 9; http://dx.doi.org/10.1182/blood-2010-06-291237; PMID: 20805364
- Shin DS, Mahadeo K, Min SH, Diop-Bove N, Clayton P, Zhao R, et al. Identification of novel mutations in the proton-coupled folate transporter (PCFT-SLC46A1) associated with hereditary folate malabsorption. Mol Genet Metab 2011; 103:33 - 7; http://dx.doi.org/10.1016/j.ymgme.2011.01.008; PMID: 21333572
- Atabay B, Turker M, Ozer EA, Mahadeo K, Diop-Bove N, Goldman ID. Mutation of the proton-coupled folate transporter gene (PCFT-SLC46A1) in Turkish siblings with hereditary folate malabsorption. Pediatr Hematol Oncol 2010; 27:614 - 9; http://dx.doi.org/10.3109/08880018.2010.481705; PMID: 20795774
- Meyer E, Kurian MA, Pasha S, Trembath RC, Cole T, Maher ER. A novel PCFT gene mutation (p.Cys66LeufsX99) causing hereditary folate malabsorption. Mol Genet Metab 2010; 99:325 - 8; http://dx.doi.org/10.1016/j.ymgme.2009.11.004; PMID: 20005757
- Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol 2009; 297:C66 - 74; http://dx.doi.org/10.1152/ajpcell.00096.2009; PMID: 19403800
- Borzutzky A, Crompton B, Bergmann AK, Giliani S, Baxi S, Martin M, et al. Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter. Clin Immunol 2009; 133:287 - 94; http://dx.doi.org/10.1016/j.clim.2009.08.006; PMID: 19740703
- Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. Random mutagenesis of the proton-coupled folate transporter (SLC46A1), clustering of mutations, and the bases for associated losses of function. J Biol Chem 2011; 286:24150 - 8; http://dx.doi.org/10.1074/jbc.M111.236539; PMID: 21602279
- Shin DS, Zhao R, Yap EH, Fiser A, Goldman IDA. A P425R mutation of the proton-coupled folate transporter causing hereditary folate malabsorption produces a highly selective alteration in folate binding. Am J Physiol Cell Physiol 2012; 302:C1405 - 12; http://dx.doi.org/10.1152/ajpcell.00435.2011; PMID: 22345511
- Veenhoff LM, Heuberger EH, Poolman B. Quaternary structure and function of transport proteins. Trends Biochem Sci 2002; 27:242 - 9; http://dx.doi.org/10.1016/S0968-0004(02)02077-7; PMID: 12076536
- Hou Z, Kugel Desmoulin S, Etnyre E, Olive M, Hsiung B, Cherian C, et al. Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter. J Biol Chem 2012; 287:4982 - 95; http://dx.doi.org/10.1074/jbc.M111.306860; PMID: 22179615
- Duan P, Wu J, You G. Mutational analysis of the role of GXXXG motif in the function of human organic anion transporter 1 (hOAT1). Int J Biochem Mol Biol 2011; 2:1 - 7; PMID: 21340049
- Polgar O, Ierano C, Tamaki A, Stanley B, Ward Y, Xia D, et al. Mutational analysis of threonine 402 adjacent to the GXXXG dimerization motif in transmembrane segment 1 of ABCG2. Biochemistry 2010; 49:2235 - 45; http://dx.doi.org/10.1021/bi902085q; PMID: 20088606
- Zhao R, Shin DS, Fiser A, Goldman ID. Identification of a functionally critical GXXG motif and its relationship to the folate binding site of the proton-coupled folate transporter (PCFT-SLC46A1). Am J Physiol Cell Physiol 2012; In Press http://dx.doi.org/10.1152/ajpcell.00123.2012; PMID: 22785121
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science 2003; 301:610 - 5; http://dx.doi.org/10.1126/science.1088196; PMID: 12893935
- Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 2007; 6:404 - 17; http://dx.doi.org/10.1158/1535-7163.MCT-06-0343; PMID: 17308042
- Chládek J, Martínková J, Simková M, Vanecková J, Koudelková V, Nozicková M. Pharmacokinetics of low doses of methotrexate in patients with psoriasis over the early period of treatment. Eur J Clin Pharmacol 1998; 53:437 - 44; http://dx.doi.org/10.1007/s002280050404; PMID: 9551702
- Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, et al, The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children’s Study Group. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. N Engl J Med 1992; 326:1043 - 9; http://dx.doi.org/10.1056/NEJM199204163261602; PMID: 1549149
- Wilson KS, Malfair Taylor SC. Raltitrexed: optimism and reality. Expert Opin Drug Metab Toxicol 2009; 5:1447 - 54; http://dx.doi.org/10.1517/17425250903307455; PMID: 19863453
- Chu E, Callender MA, Farrell MP, Schmitz JC. Thymidylate synthase inhibitors as anticancer agents: from bench to bedside. Cancer Chemother Pharmacol 2003; 52:Suppl 1 S80 - 9; http://dx.doi.org/10.1007/s00280-003-0625-9; PMID: 12819937
- Goldman ID, Matherly LH. The cellular pharmacology of methotrexate. Pharmacol Ther 1985; 28:77 - 102; http://dx.doi.org/10.1016/0163-7258(85)90083-X; PMID: 2414788
- Hughes LR, Stephens TC, Boyle FT, Jackman AL. Raltitrexed (Tomudex), a Highly Polyglutamatable Antifolate Thymidylate Synthase Inhibitor. In: Jackman AL, ed. Anticancer Drug Development Guide: Antifolate Drugs in Cancer Therapy. Totowa, NJ: Humana Press, Inc., 1999:147-65.
- Mendelsohn LG, Worzalla JF, Walling JM. Preclinical and Clinical Evaluation of the Glycinamide Ribonucleotide Formyltransferase Inhibitors Lometrexol and LY309887. In: Jackman AL, ed. Anticancer Drug Development Guide: Antifolate Drugs in Cancer Therapy. Totowa, NJ: Humana Press, Inc., 1999:261-80.
- Shih C, Thornton DE. Preclinical Pharmacology Studies and the Clinical Development of a Novel Multitargeted Antifolate, MTA (LY231514). In: Jackman AL, ed. Anticancer Drug Development Guide: Antifolate Drugs in Cancer Therapy. Totowa, NJ: Humana Press, Inc., 1999:183-201.
- Trent DF, Seither RL, Goldman ID. Rate and extent of interconversion of tetrahydrofolate cofactors to dihydrofolate after cessation of dihydrofolate reductase activity in stationary versus log phase L1210 leukemia cells. J Biol Chem 1991; 266:5445 - 9; PMID: 1825999
- Allegra CJ, Fine RL, Drake JC, Chabner BA. The effect of methotrexate on intracellular folate pools in human MCF-7 breast cancer cells. Evidence for direct inhibition of purine synthesis. J Biol Chem 1986; 261:6478 - 85; PMID: 3700401
- Matherly LH, Seither RL, Goldman ID. Metabolism of the diaminoantifolates: biosynthesis and pharmacology of the 7-hydroxyl and polyglutamyl metabolites of methotrexate and related antifolates. Pharmacol Ther 1987; 35:27 - 56; http://dx.doi.org/10.1016/0163-7258(87)90104-5; PMID: 2447596
- Trent DF, Seither RL, Goldman ID. Compartmentation of intracellular folates. Failure to interconvert tetrahydrofolate cofactors to dihydrofolate in mitochondria of L1210 leukemia cells treated with trimetrexate. Biochem Pharmacol 1991; 42:1015 - 9; http://dx.doi.org/10.1016/0006-2952(91)90283-B; PMID: 1831361
- Matherly LH, Muench SP. Evidence for a localized conversion of endogenous tetrahydrofolate cofactors to dihydrofolate as an important element in antifolate action in murine leukemia cells. Biochem Pharmacol 1990; 39:2005 - 14; http://dx.doi.org/10.1016/0006-2952(90)90622-R; PMID: 2141258
- Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr 2010; 30:57 - 81; http://dx.doi.org/10.1146/annurev.nutr.012809.104810; PMID: 20645850
- Matherly LH, Voss MK, Anderson LA, Fry DW, Goldman ID. Enhanced polyglutamylation of aminopterin relative to methotrexate in the Ehrlich ascites tumor cell in vitro. Cancer Res 1985; 45:1073 - 8; PMID: 2578870
- Menter A, Thrash B, Cherian C, Matherly LH, Wang L, Gangjee A, et al. Intestinal transport of aminopterin enantiomers in dogs and humans with psoriasis is stereoselective: evidence for a mechanism involving the proton-coupled folate transporter. J Pharmacol Exp Ther 2012; 342:696 - 708; http://dx.doi.org/10.1124/jpet.112.195479; PMID: 22653877
- Cole PD, Drachtman RA, Masterson M, Smith AK, Glod J, Zebala JA, et al. Phase 2B trial of aminopterin in multiagent therapy for children with newly diagnosed acute lymphoblastic leukemia. Cancer Chemother Pharmacol 2008; 62:65 - 75; http://dx.doi.org/10.1007/s00280-007-0576-7; PMID: 17768625
- Sirotnak FM, DeGraw JI, Schmid FA, Goutas LJ, Moccio DM. New folate analogs of the 10-deaza-aminopterin series. Further evidence for markedly increased antitumor efficacy compared with methotrexate in ascitic and solid murine tumor models. Cancer Chemother Pharmacol 1984; 12:26 - 30; PMID: 6690070
- Schmid FA, Sirotnak FM, Otter GM, DeGraw JI. New folate analogs of the 10-deaza-aminopterin series: markedly increased antitumor activity of the 10-ethyl analog compared to the parent compound and methotrexate against some human tumor xenografts in nude mice. Cancer Treat Rep 1985; 69:551 - 3; PMID: 4005878
- Sirotnak FM, Otter GM, Schmid FA. Markedly improved efficacy of edatrexate compared to methotrexate in a high-dose regimen with leucovorin rescue against metastatic murine solid tumors. Cancer Res 1993; 53:587 - 91; PMID: 8425192
- Sirotnak FM, DeGraw JI, Colwell WT, Piper JR. A new analogue of 10-deazaaminopterin with markedly enhanced curative effects against human tumor xenografts in mice. Cancer Chemother Pharmacol 1998; 42:313 - 8; http://dx.doi.org/10.1007/s002800050823; PMID: 9744777
- Krug LM, Azzoli CG, Kris MG, Miller VA, Khokhar NZ, Tong W, et al. 10-propargyl-10-deazaaminopterin: an antifolate with activity in patients with previously treated non-small cell lung cancer. Clin Cancer Res 2003; 9:2072 - 8; PMID: 12796370
- O’Connor OA, Horwitz S, Hamlin P, Portlock C, Moskowitz CH, Sarasohn D, et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol 2009; 27:4357 - 64; http://dx.doi.org/10.1200/JCO.2008.20.8470; PMID: 19652067
- Jackman AL, Calvert AH. Folate-based thymidylate synthase inhibitors as anticancer drugs. Ann Oncol 1995; 6:871 - 81; PMID: 8624289
- Jackman AL, Taylor GA, Gibson W, Kimbell R, Brown M, Calvert AH, et al. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: a new agent for clinical study. Cancer Res 1991; 51:5579 - 86; PMID: 1913676
- Surmont VF, van Meerbeeck JP. Raltitrexed in mesothelioma. Expert Rev Anticancer Ther 2011; 11:1481 - 90; http://dx.doi.org/10.1586/era.11.136; PMID: 21999120
- Theti DS, Bavetsias V, Skelton LA, Titley J, Gibbs D, Jansen G, et al. Selective delivery of CB300638, a cyclopenta[g]quinazoline-based thymidylate synthase inhibitor into human tumor cell lines overexpressing the alpha-isoform of the folate receptor. Cancer Res 2003; 63:3612 - 8; PMID: 12839949
- Jackson RC, Harkrader RJ. The contributions of de-novo and salvage pathways of nucleotide biosynthesis in normal and malignant cells. In: Tattersall MHN, Fox RM, eds. Nucleosides and Cancer Treatment. Sydney: Academic Press, 1981:18-31.
- Howell SB, Mansfield SJ, Taetle R. Thymidine and hypoxanthine requirements of normal and malignant human cells for protection against methotrexate cytotoxicity. Cancer Res 1981; 41:945 - 50; PMID: 6257387
- Moran RG, Baldwin SW, Taylor EC, Shih C. The 6S- and 6R-diastereomers of 5, 10-dideaza-5, 6, 7, 8-tetrahydrofolate are equiactive inhibitors of de novo purine synthesis. J Biol Chem 1989; 264:21047 - 51; PMID: 2592365
- Taylor EC, Harrington PJ, Fletcher SR, Beardsley GP, Moran RG. Synthesis of the antileukemic agents 5,10-dideazaaminopterin and 5,10-dideaza-5,6,7,8-tetrahydroaminopterin. J Med Chem 1985; 28:914 - 21; http://dx.doi.org/10.1021/jm00145a012; PMID: 4009615
- Jansen G. Receptor- and carrier- mediated transport system for folates and antifolates. In: Jackman AL, ed. Anticancer Drug Development Guide: Antifolate Drugs in Cancer Therapy. Totowa, NJ: Humana Press Inc., 1999:293-321.
- Beardsley GP, Moroson BA, Taylor EC, Moran RG. A new folate antimetabolite, 5,10-dideaza-5,6,7,8-tetrahydrofolate is a potent inhibitor of de novo purine synthesis. J Biol Chem 1989; 264:328 - 33; PMID: 2909524
- Matherly LH, Angeles SM, McGuire JJ. Determinants of the disparate antitumor activities of (6R)-5,10-dideaza-5,6,7,8-tetrahydrofolate and methotrexate toward human lymphoblastic leukemia cells, characterized by severely impaired antifolate membrane transport. Biochem Pharmacol 1993; 46:2185 - 95; http://dx.doi.org/10.1016/0006-2952(93)90608-Y; PMID: 7506026
- Ray MS, Muggia FM, Leichman CG, Grunberg SM, Nelson RL, Dyke RW, et al. Phase I study of (6R)-5,10-dideazatetrahydrofolate: a folate antimetabolite inhibitory to de novo purine synthesis. J Natl Cancer Inst 1993; 85:1154 - 9; http://dx.doi.org/10.1093/jnci/85.14.1154; PMID: 8320744
- Roberts JD, Poplin EA, Tombes MB, Kyle B, Spicer DV, Grant S, et al. Weekly lometrexol with daily oral folic acid is appropriate for phase II evaluation. Cancer Chemother Pharmacol 2000; 45:103 - 10; http://dx.doi.org/10.1007/s002800050017; PMID: 10663624
- Boritzki TJ, Barlett CA, Zhang C, Howland EF. AG2034: a novel inhibitor of glycinamide ribonucleotide formyltransferase. Invest New Drugs 1996; 14:295 - 303; http://dx.doi.org/10.1007/BF00194533; PMID: 8958185
- Bissett D, McLeod HL, Sheedy B, Collier M, Pithavala Y, Paradiso L, et al. Phase I dose-escalation and pharmacokinetic study of a novel folate analogue AG2034. Br J Cancer 2001; 84:308 - 12; http://dx.doi.org/10.1054/bjoc.2000.1601; PMID: 11161393
- Budman DR, Johnson R, Barile B, Bowsher RR, Vinciguerra V, Allen SL, et al. Phase I and pharmacokinetic study of LY309887: a specific inhibitor of purine biosynthesis. Cancer Chemother Pharmacol 2001; 47:525 - 31; http://dx.doi.org/10.1007/s002800000272; PMID: 11459206
- Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, et al. A dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5- yl)ethyl]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthase. J Med Chem 1992; 35:4450 - 4; http://dx.doi.org/10.1021/jm00101a023; PMID: 1447744
- Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res 1997; 57:1116 - 23; PMID: 9067281
- Zhao R, Gao F, Goldman ID. Marked suppression of the activity of some, but not all, antifolate compounds by augmentation of folate cofactor pools within tumor cells. Biochem Pharmacol 2001; 61:857 - 65; http://dx.doi.org/10.1016/S0006-2952(01)00532-9; PMID: 11274972
- Zhao R, Chattopadhyay S, Hanscom M, Goldman ID. Antifolate resistance in a HeLa cell line associated with impaired transport independent of the reduced folate carrier. Clin Cancer Res 2004; 10:8735 - 42; http://dx.doi.org/10.1158/1078-0432.CCR-04-0932; PMID: 15623659
- Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumors: contribution to the preservation of methotrexate pharmacologic activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res 2004; 10:718 - 27; http://dx.doi.org/10.1158/1078-0432.CCR-1066-03; PMID: 14760095
- Zhao R, Hanscom M, Chattopadhyay S, Goldman ID. Selective preservation of pemetrexed pharmacological activity in HeLa cells lacking the reduced folate carrier: association with the presence of a secondary transport pathway. Cancer Res 2004; 64:3313 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-03-3953; PMID: 15126375
- Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res 2009; 69:5467 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-08-4979; PMID: 19549896
- Rothbart SB, Racanelli AC, Moran RG. Pemetrexed indirectly activates the metabolic kinase AMPK in human carcinomas. Cancer Res 2010; 70:10299 - 309; http://dx.doi.org/10.1158/0008-5472.CAN-10-1873; PMID: 21159649
- Bareford MD, Park MA, Yacoub A, Hamed HA, Tang Y, Cruickshanks N, et al. Sorafenib enhances pemetrexed cytotoxicity through an autophagy-dependent mechanism in cancer cells. Cancer Res 2011; 71:4955 - 67; http://dx.doi.org/10.1158/0008-5472.CAN-11-0898; PMID: 21622715
- Cohen MH, Cortazar P, Justice R, Pazdur R. Approval summary: pemetrexed maintenance therapy of advanced/metastatic nonsquamous, non-small cell lung cancer (NSCLC). Oncologist 2010; 15:1352 - 8; http://dx.doi.org/10.1634/theoncologist.2010-0224; PMID: 21148615
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011; 27:441 - 64; http://dx.doi.org/10.1146/annurev-cellbio-092910-154237; PMID: 21985671
- Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol 1993; 265:C1015 - 29; PMID: 8238296
- McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am J Physiol Cell Physiol 2000; 278:C676 - 88; PMID: 10751317
- Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol 2010; 2010:427694; http://dx.doi.org/10.1155/2010/427694; PMID: 20454640
- Chiche J, Le Fur Y, Vilmen C, Frassineti F, Daniel L, Halestrap AP, et al. In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int J Cancer 2012; 130:1511 - 20; http://dx.doi.org/10.1002/ijc.26125; PMID: 21484790
- Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009; 69:358 - 68; http://dx.doi.org/10.1158/0008-5472.CAN-08-2470; PMID: 19118021
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 2009; 212:1672 - 83; http://dx.doi.org/10.1242/jeb.029454; PMID: 19448077
- Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol 2009; 212:1697 - 706; http://dx.doi.org/10.1242/jeb.028563; PMID: 19448079
- Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging 2002; 16:430 - 50; http://dx.doi.org/10.1002/jmri.10181; PMID: 12353258
- Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, Zandt R, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008; 453:940 - 3; http://dx.doi.org/10.1038/nature07017; PMID: 18509335
- Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011; 11:671 - 7; http://dx.doi.org/10.1038/nrc3110; PMID: 21833026
- Anderson CM, Thwaites DT. Hijacking solute carriers for proton-coupled drug transport. Physiology (Bethesda) 2010; 25:364 - 77; http://dx.doi.org/10.1152/physiol.00027.2010; PMID: 21186281
- Anderson CM, Jevons M, Thangaraju M, Edwards N, Conlon NJ, Woods S, et al. Transport of the photodynamic therapy agent 5-aminolevulinic acid by distinct H+-coupled nutrient carriers coexpressed in the small intestine. J Pharmacol Exp Ther 2010; 332:220 - 8; http://dx.doi.org/10.1124/jpet.109.159822; PMID: 19789362
- Nakanishi T, Tamai I, Takaki A, Tsuji A. Cancer cell-targeted drug delivery utilizing oligopeptide transport activity. Int J Cancer 2000; 88:274 - 80; http://dx.doi.org/10.1002/1097-0215(20001015)88:2<274::AID-IJC20>3.0.CO;2-5; PMID: 11004680
- Sun Y, Sun J, Shi S, Jing Y, Yin S, Chen Y, et al. Synthesis, transport and pharmacokinetics of 5′-amino acid ester prodrugs of 1-beta-D-arabinofuranosylcytosine. Mol Pharm 2009; 6:315 - 25; http://dx.doi.org/10.1021/mp800200a; PMID: 19115956
- Anderson CM, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, et al. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: An intestinal nutrient/drug transporter in human and rat. Gastroenterology 2004; 127:1410 - 22; http://dx.doi.org/10.1053/j.gastro.2004.08.017; PMID: 15521011
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, et al. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther 2006; 318:521 - 9; http://dx.doi.org/10.1124/jpet.106.104364; PMID: 16702441
- Wong SC, Proefke SA, Bhushan A, Matherly LH. Isolation of human cDNAs that restore methotrexate sensitivity and reduced folate carrier activity in methotrexate transport-defective Chinese hamster ovary cells. J Biol Chem 1995; 270:17468 - 75; http://dx.doi.org/10.1074/jbc.270.29.17468; PMID: 7615551
- Shih C, Barnett CJ, Grindey GB, Pearce HL, Engelhardt JA, Todd GC, et al. Structural Features That Determine the Biological Activity of Pyrrolo[2,3-d]pyrimidine Based Antifolates. The 10th International Symposium, Chemistry and Biology of Pteridines and Folates. Orange Beach, AL, 1993:Abstr F15.
- Gangjee A, Zeng Y, McGuire JJ, Mehraein F, Kisliuk RL. Synthesis of classical, three-carbon-bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates. J Med Chem 2004; 47:6893 - 901; http://dx.doi.org/10.1021/jm040123k; PMID: 15615538
- Gangjee A, Zeng Y, McGuire JJ, Kisliuk RL. Synthesis of classical, four-carbon bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates. J Med Chem 2005; 48:5329 - 36; http://dx.doi.org/10.1021/jm058213s; PMID: 16078850
- Gates SB, Worzalla JF, Shih C, Grindey GB, Mendelsohn LG. Dietary folate and folylpolyglutamate synthetase activity in normal and neoplastic murine tissues and human tumor xenografts. Biochem Pharmacol 1996; 52:1477 - 9; http://dx.doi.org/10.1016/S0006-2952(96)00554-0; PMID: 8937460
- Ifergan I, Jansen G, Assaraf YG. Cytoplasmic confinement of breast cancer resistance protein (BCRP/ABCG2) as a novel mechanism of adaptation to short-term folate deprivation. Mol Pharmacol 2005; 67:1349 - 59; http://dx.doi.org/10.1124/mol.104.008250; PMID: 15657365
- Bronder JL, Moran RG. Antifolates targeting purine synthesis allow entry of tumor cells into S phase regardless of p53 function. Cancer Res 2002; 62:5236 - 41; PMID: 12234990
- Bronder JL, Moran RG. A defect in the p53 response pathway induced by de novo purine synthesis inhibition. J Biol Chem 2003; 278:48861 - 71; http://dx.doi.org/10.1074/jbc.M304844200; PMID: 14517211
- Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res 2003; 9:2108 - 13; PMID: 12796375
- Hori H, Tran P, Carrera CJ, Hori Y, Rosenbach MD, Carson DA, et al. Methylthioadenosine phosphorylase cDNA transfection alters sensitivity to depletion of purine and methionine in A549 lung cancer cells. Cancer Res 1996; 56:5653 - 8; PMID: 8971171
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 2008; 320:103 - 6; http://dx.doi.org/10.1126/science.1152241; PMID: 18388293