Abstract
The role of cyclin B1 in the clinical therapeutic sensitivity of human esophageal squamous cell carcinoma (ESCC) remains to be defined. In this study, we found that elevated cyclin B1 expression attenuated the apoptosis induced by cisplatin or paclitaxel, while knockdown of cyclin B1 enhanced cisplatin or paclitaxel sensitivity in ESCC cells. Cyclin B1-mediated apoptosis may rely on the Bcl-2-dependent mitochondria-regulated intrinsic death pathway, and the antagonizing effect of cyclin B1 on chemotherapeutic agent-induced apoptosis was through PTEN/Akt pathway. Therefore, cyclin B1 might be a therapeutic target for the development of specific and efficient approaches in the treatment of ESCC.
Keywords: :
Introduction
The worldwide incidence of esophageal cancer has steadily increased over the past 20 y.Citation1,Citation2 Worldwide, esophageal cancer is the sixth leading cause of cancer death, and in China, it is the fourth leading cause of death.Citation3 Chemotherapy plays a major role in palliative therapy and is currently the primary mode of treatment for recurrent or metastatic esophageal cancer. Most of the chemotherapeutic agents, including cisplatin and paclitaxel, have been evaluated previously in esophageal cancer.Citation4 However, these agents have disadvantages because they have a toxic effect on normal tissue and some patients exhibit drug resistance. Thus, it is necessary to better understand the molecular basis of carcinogenesis and to elucidate the signal transduction pathways regulating cell apoptosis, which will provide the impetus for the development of novel molecularly targeted anticancer therapy. It has been illuminated that many proteins can regulate apoptosis in esophageal cancer, including TRAIL,Citation5,Citation6 CDKs,Citation7 caspases,Citation5,Citation6,Citation8 p53,Citation9-Citation11 survivin,Citation10 N-cadherin,Citation12 Aurora-ACitation13 and so on. Within these contexts, therapeutic strategies, which aim to direct induction of cell apoptosis by regulating these proteins, have attracted a great deal of attention. However, none of these inhibitors have been very effective in treating esophageal cancer.
Cyclin B1 is a key component of the cell cycle machinery, which control the G2 to M transition.Citation14,Citation15 Cyclin B1 plays an important role in tumor development and progression. Overexpression of cyclin B1 is observed in various cancers, including esophageal squamous cell carcinoma (ESCC), laryngeal squamous cell carcinoma and colorectal carcinoma.Citation3,Citation16,Citation17 Previously, we have found that the overexpression of cyclin B1 in human ESCC cells can promote invasive growth and metastasis. In addition, cyclin B1 plays an important role in apoptosis following chemotherapeutic drug treatment in many different types of tumor cells. However, the mechanisms by which cyclin B1 affects chemotherapeutic-induced apoptosis are not clearly understood.
The phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is one of the most well-studied tumor-suppressor genes. PTEN mutations have been found in a broad variety of human cancers.Citation18-Citation20 PTEN antagonizes phosphoinositol-3-kinase (PI3K) signaling by dephosphorylating phosphatidylinositol-3,4,5-triphosphate (PtdIns(3,4,5)P3) into inactive PtdIns(4,5)P2 and, thus, inactivating downstream effectors, notably Akt. Akt (also known as protein kinase B) is a serine/threonine protein kinase.Citation21 Growth factors or cytokines via the product of PI3K, PtdIns(3,4,5)P3 and the phosphoinositide-dependent kinase 1 and 2 (PDK1/2) can activate Akt by phosphorylation at threonine 308 and serine 473 residues.Citation22,Citation23 The active form of Akt (phosphorylation of Akt) improves cell proliferation and survival by regulating downstream molecules that mediate cell cycles and apoptosis.Citation24 PTEM/Akt pathway plays a critical role in controlling the balance between pro-apoptotic and anti-apoptotic pathways.
We attempted to determine whether altering cyclin B1 in ESCC cells has an effect on chemotherapeutic-induced (cisplatin and paclitaxel) apoptosis. In this report, we demonstrate that cyclin B1 plays an antagonizing role to chemotherapeutic-induced apoptosis in ESCC cells, and this may be due to the Bcl-2-dependent mitochondria-regulated intrinsic death-signaling pathway. We also demonstrated that cyclin B1 antagonized chemotherapeutic-induced apoptosis in human ESCC cells through PTEN/Akt signaling pathway.
Results
Higher expression of cyclin B1 in ESCC cells decreases cisplatin- and paclitaxel-induced apoptosis
ESCC KYSE 150 cells, which had relatively low endogenous cyclin B1 protein, were established clone cells overexpressing cyclin B1 in our laboratory. These clones (High-CycB1–1, High-CycB1–2) and the control cell lines (pcDNA3.1) were previously described and used in our prior publicationsCitation3 (). To evaluate the effect of cyclin B1 on chemotherapeutic-induced apoptosis, the clone cells were treated with cisplatin or paclitaxel, and apoptosis was assessed. MTS analysis showed a greater survival rate in the KYSE150/High-CycB1–1 and KYSE150/High-CycB1–2 cells compared with the KYSE150/pcDNA3.1 cells (). Consistently, after cells were stained with FITC labeled annexin V and Propidium iodide (PI), flow cytometry analysis indicated that after cisplatin or paclitaxel treatment, the apoptotic cells (early apoptotic + late apoptotic) in the KYSE150/High-CycB1–1 and KYSE150/High-CycB1–2 cells were substantially diminished compared with the KYSE150/pcDNA3.1 cells (). As shown in , the apoptotic fraction in the population in untreated KYSE150/pcDNA3.1 cells were 13.3% (5.7% + 7.6%), whereas the KYSE150/High-CycB1–1 and KYSE150/High-CycB1–2 cells were 3.2% (1.2% + 2.0%) and 7.7% (1.7% + 6.0%), respectively. However, after exposure to cisplatin, the apoptotic fraction in the population of KYSE150/pcDNA3.1 cells increased markedly to 41.7% (12.9% + 28.8%), whereas the apoptotic fraction in the population were 16.5% (11.0% + 5.5%) and 18.4% (11.7% + 6.7%) in the KYSE150/High-CycB1–1 and KYSE150/High-CycB1–2 cells, respectively. Similarly, we treated cells with paclitaxel and found the apoptotic fraction in the population of the KYSE150/pcDNA3.1 cells were 33.7% (1.5% + 32.2%), whereas the KYSE150/High-CycB1–1 and KYSE150/High-CycB1–2 cells were 19.5% (2.5% + 17.0%) and 15.7% (9.6% + 6.1%), respectively. These results showed that there were more apoptotic cells in the pcDNA3.1 cells than the High-CycB1 1–2 cells when the cells were exposed to cisplatin or paclitaxel. We further examined the nuclear morphology of ESCC cell lines treated with cisplatin or paclitaxel. After treatment, these cells were stained with DAPI (). The KYSE150/High-CycB1–1 and KYSE150/High-CycB1–2 cells exhibited less typical apoptotic bodies in the nuclear morphology compared with the KYSE150/pcDNA3.1 cells. In agreement with the above observations, western blot analysis indicated significantly less PARP cleavages in the KYSE150/High-CycB1 cells compared with the KYSE150/pcDNA3.1 cells after treatment with cisplatin or paclitaxel (). These results suggest that a high level of cyclin B1 protein reduces cisplatin- and paclitaxel-induced apoptosis in ESCC cells.
Figure 1A–C. Overexpression of cyclin B1 contributes to KYSE150 cells resistance to cisplatin or paclitaxel. (A) Western blot analysis of the protein levels of cyclin B1 and actin in KYSE150/pcDNA3.1 and High-CycB1 1–2 cells. (B) Effects of cyclin B1 overexpression on the viability in the KYSE150/pcDNA3.1, High-CycB1–1 and High-CycB1–2 cell lines after treatment with cisplatin, paclitaxel or a control reagent by MTS assay. (C)(a) KYSE150/pcDNA3.1, High-CycB1–1 and High-CycB1–2 cells were not treated or treated with cisplatin and paclitaxel for 24 h, and the cells stained with annexin V and propidium iodide (PI) were analyzed by flow cytometry. The lower left quadrant contains the vital (annexin V-/PI-) population, the upper left quadrant contains the damaged (annexin V-/PI+) population, the upper right quadrant contains the late apoptotic (annexin V+/PI+) cells and the lower right quadrant contains the early apoptotic (annexin V+/PI-) cells. (C)(b) The bar chart shows the percentage of apoptotic cells (early apoptotic + late apoptotic).
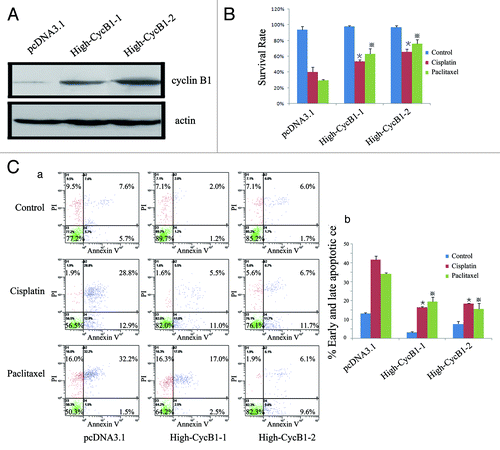
Figure 1D and E. (D)(a) KYSE150/pcDNA3.1, High-CycB1–1 and High-CycB1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h and apoptotic cells were analyzed by DAPI stain (200 × ). (D)(b) The bar chart shows the percentage of apoptotic cells. (E)(a) Overexpression of cyclin B1 inhibits PARP cleavages induced by cisplatin and paclitaxel. KYSE150/pcDNA3.1, High-CycB1–1, High-CycB1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h. PARP were detected by western blot analysis. Actin was used as an equal loading control. (E)(b) The bar chart shows the percentage of PARP-cleaved [PARP-cleaved / (PARP-cleaved + full-length PARP)]. All experiments were performed at least three times with consistent and repeatable results. Each value is expressed as mean ± SD (n = 3). * and ※ p < 0.05 as compared with the control.
![Figure 1D and E. (D)(a) KYSE150/pcDNA3.1, High-CycB1–1 and High-CycB1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h and apoptotic cells were analyzed by DAPI stain (200 × ). (D)(b) The bar chart shows the percentage of apoptotic cells. (E)(a) Overexpression of cyclin B1 inhibits PARP cleavages induced by cisplatin and paclitaxel. KYSE150/pcDNA3.1, High-CycB1–1, High-CycB1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h. PARP were detected by western blot analysis. Actin was used as an equal loading control. (E)(b) The bar chart shows the percentage of PARP-cleaved [PARP-cleaved / (PARP-cleaved + full-length PARP)]. All experiments were performed at least three times with consistent and repeatable results. Each value is expressed as mean ± SD (n = 3). * and ※ p < 0.05 as compared with the control.](/cms/asset/9d75a22d-a956-4d6d-886a-3063e0c3eb18/kcbt_a_10922627_f0002.gif)
Knockdown of endogenous cyclin B1 protein with siRNA increases cisplatin- and paclitaxel-induced apoptosis
To further determine whether inhibition of cyclin B1 in the high-endogenous cyclin B1 EC9706 cells would affect cisplatin- or paclitaxel-induced apoptosis in ESCC cells, we stably transfected with siRNA specific for cyclin B1 in EC9706 cells and established clone cells (EC9706/CycB1-siRNA-1, CycB1-siRNA-2 and control-siRNA)Citation3 (). In the survival rate analysis, CycB1 siRNA 1–2 cells showed a lower survival rate compared with control siRNA cells () following treatment with cisplatin or paclitaxel. In the flow cytometric analysis, we found that when we compared the control group to the cisplatin group, the apoptotic cells (early apoptotic + late apoptotic) sharply increased from 11.4% (7.5% + 3.9%) to 29.5% (16.5% + 13.0%) in the EC9706 CycB1-siRNA-1 cells, increased from 11.4% (7.6% + 3.8%) to 23.2% (11.4% + 11.8%) in the CycB1-siRNA-2 cells and increased from 5.1% (2.8% + 2.3%) to 16.0% (7.7% + 8.3%) in the control-siRNA cells (). In the paclitaxel group, the apoptotic cells were 40.8% (4.5% + 36.3%) in the CycB1-siRNA-1 cells and 33.7% (17.5% + 16.2%) in the CycB1-siRNA-2 cells, whereas the apoptotic cells were 21.6% (14.5% + 7.1%) in the control-siRNA cells. The result showed that the CycB1-siRNA 1–2 cells were more sensitive to cisplatin and paclitaxel than the control-siRNA cells. We also performed DAPI analysis. The results showed that there were more typical apoptotic bodies in the EC9706 CycB1 siRNA 1–2 cells compared with the control siRNA cells after the cells were treated with cisplatin or paclitaxel (). Furthermore, western blot analysis revealed greater PARP cleavages in the EC9706 CycB1 siRNA 1–2 cells compared with the control-siRNA cells treated with cisplatin or pacletaxel (). These results provide further evidence that reduced cyclin B1 protein increases cisplatin- and paclitaxel-induced apoptosis in ESCC cells.
Figure 2A–C. Suppression of cyclin B1 contributes to sensitization of EC9706 cells to cisplatin or paclitaxel. (A) Western blot analysis of the protein levels of cyclin B1 and actin in EC9706 control-siRNA and CycB1 siRNA 1–2 cells; (B) Effects of cyclin B1 suppression on the viability in the EC9706 control-siRNA and CycB1 siRNA 1–2 cells lines after treatment with cisplatin, paclitaxel or a control reagent by MTS assay. (C)(a) EC9706 control-siRNA and CycB1 siRNA 1–2 cells were not treated or treated with cisplatin and paclitaxel for 24 h and the cells stained with annexin V and propidium iodide (PI) were analyzed by flow cytometry. The lower left quadrant contains the vital (annexin V-/PI-) population, the upper left quadrant contains the damaged (annexin V-/PI+) population, the upper right quadrant contains the late apoptotic (annexin V+/PI+) cells and the lower right quadrant contains the early apoptotic (annexin V+/PI-) cells. (C)(b) The bar chart shows the percentage of apoptotic cells (early apoptotic + late apoptotic).
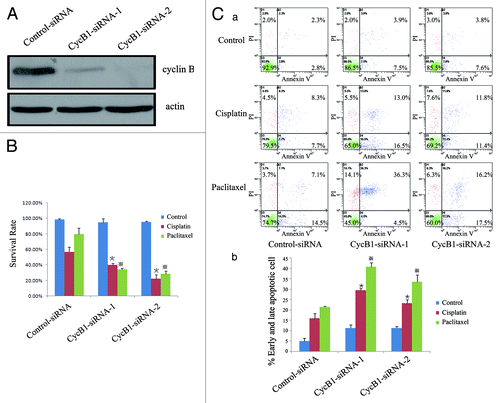
Figure 2D and E. (D)(a) EC9706 control-siRNA and CycB1 siRNA 1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h, and apoptotic cells were analyzed by DAPI stain (200 × ). (D)(b) The bar chart shows the percentage of apoptotic cells. (Ea) Suppression of cyclin B1 increases PARP cleavages induced by cisplatin or paclitaxel. EC9706 control-siRNA and CycB1 siRNA 1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h. PARP were detected by western blot analysis. Actin was used as an equal loading control. (E)(b) The bar chart shows the percentage of PARP-cleaved [PARP-cleaved / (PARP-cleaved + full-length PARP)]. All experiments were performed at least three times with consistent and repeatable results. Each value is expressed as mean ± SD (n = 3). *and ※ p < 0.05 as compared with the control.
![Figure 2D and E. (D)(a) EC9706 control-siRNA and CycB1 siRNA 1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h, and apoptotic cells were analyzed by DAPI stain (200 × ). (D)(b) The bar chart shows the percentage of apoptotic cells. (Ea) Suppression of cyclin B1 increases PARP cleavages induced by cisplatin or paclitaxel. EC9706 control-siRNA and CycB1 siRNA 1–2 cells were treated with cisplatin, paclitaxel or a control reagent for 24 h. PARP were detected by western blot analysis. Actin was used as an equal loading control. (E)(b) The bar chart shows the percentage of PARP-cleaved [PARP-cleaved / (PARP-cleaved + full-length PARP)]. All experiments were performed at least three times with consistent and repeatable results. Each value is expressed as mean ± SD (n = 3). *and ※ p < 0.05 as compared with the control.](/cms/asset/afa5518a-bd0c-4931-ac7b-63243b316264/kcbt_a_10922627_f0004.gif)
To confirm our results in cells with transient transfection of cyclin B1 plasmid or cyclin B1 siRNA, we transiently transfected pcDNA3.1 cyclin B1 plasmid and control plasmid in KYSE 150 cells, while cyclin B1 siRNA and control siRNA were transiently transfected in EC9706 cells (Fig. S1A and a). Consistently with stable clone cells, after cells were stained with FITC labeled annexin V and PI, flow cytometry analysis displayed that after cisplatin or paclitaxel treatment, there were more apoptotic cells in KYSE 150 control cells than KYSE 150-Cyc B1 cells, while there were also more apoptotic cells in EC 9706 Cyc B1-siRNA cells than control-siRNA cells (Fig. S1A and b–c). The similar trend were also detected in three parental ESCC cells (KYSE450, KYSE 510 and COLO 680N) harboring different cyclin B1 (Fig. S1B). All the results confirm that cyclin B1 antagonizes chemotherapeutic-induced apoptosis in ESCC cells.
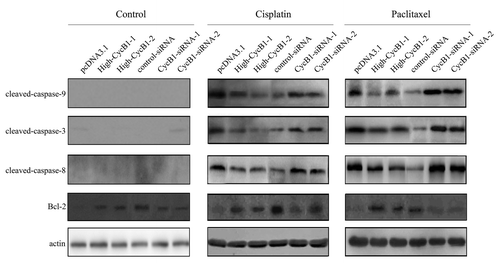
Involvement of caspase-9, caspase-3, caspase-8 and Bcl-2 in cisplatin- or paclitaxel-induced apoptosis in ESCC cells
The mitochondrial release of cytochrome c, which is required for the activation of caspase-9 and caspase-3, is a critical event for chemotherapy-mediated apoptosis.Citation8,Citation25 To understand the death-signaling pathway underlying cisplatin- and paclitaxel-mediated apoptosis in ESCC cells, we investigated the activation of caspase-9 and caspase-3 after ESCC cells were treated with cisplatin or paclitaxel. As shown in , more cleaved caspase-9 was detected in the KYSE150/pcDNA3.1 cells compared with the KYSE150/High-CycB1 1–2 cells, whereas there was also more cleaved caspase-9 in the EC9706 CycB1 siRNA 1–2 cells compared with the EC9706 control-siRNA cells. A high level of cleaved caspase-3 was also detected in the KYSE150/pcDNA3.1 and EC9706 CycB1 siRNA 1–2 cells along with caspase-9 activation (). To test whether caspase-8 was also involved in the apoptotic process induced by cisplatin or paclitaxel in ESCC cells, we examined the expression of caspase-8 activation after the cells were exposed to cisplatin or paclitaxel and found that more activated caspase-8 was found in the KYSE150/pcDNA3.1 cells compared with the KYSE150/High-CycB1 1–2 cells, whereas there was more cleaved caspase-8 in the EC9706 CycB1 siRNA 1–2 cells compared with the EC9706 control-siRNA cells (). These results suggest that apoptosis in ESCC activated by cisplatin or paclitaxel is associated with both the activation of caspase-9 and caspase-8. Bcl-2 is an important protein during the execution of apoptosis; it plays a role in protecting against apoptosis and providing resistance to cell death stimuli, including the classic chemotherapeutic drugs.Citation26 We examined the relationship between cyclin B1 and Bcl-2 on cisplatin- or paclitaxel-induced apoptosis. Western blot results showed that KYSE150/High-CycB1–1 and High-CycB1–2 cells expressed more Bcl-2 protein compared with the KYSE150/pcDNA3.1 cells, and there was more Bcl-2 protein in the EC9706 control-siRNA cells than in the EC9706 CycB1 siRNA 1–2 cells (), suggesting that Bcl-2 was also involved in the regulation of cyclin B1-mediated apoptosis.
Figure 3. Western blot analysis of the protein levels of cleaved-caspase-9, cleaved-caspase-3, cleaved-caspase-8, Bcl-2 and actin in KYSE150/pcDNA3.1, High-CycB1 1–2, EC9706 control-siRNA and CycB1 siRNA 1–2 cells after treatment with cisplatin, paclitaxel or a control reagent for 24 h. All experiments were performed at least three times with consistent and repeatable results.
Cyclin B1 mediates cisplatin- or paclitaxel-induced apoptosis in ESCC cells through PTEN/Akt pathway
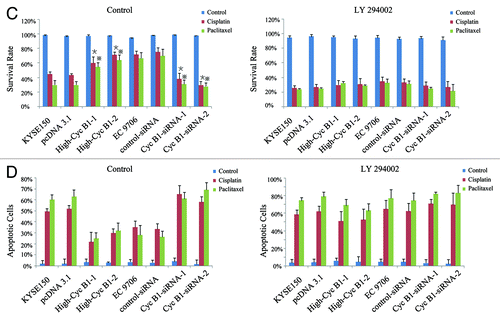
PTEN, which is a major negative regulator of the PI3K/AKT signaling pathway,Citation27,Citation28 acts as a negative regulator of apoptosis in many cancer cells.Citation29,Citation30 To further investigate the role of PTEN in the process of cyclin B1-mediated apoptosis, we analyzed the expression of PTEN in our models and added the parental cells. After treatment with cisplatin or paclitaxel, KYSE150/High-CycB1–1 and High-CycB1–2 cells expressed less PTEN protein compared with the KYSE150 and pcDNA3.1 cells, and there was also less PTEN protein in the EC9706 and control-siRNA cells than in the EC9706 CycB1 siRNA 1–2 cells (). Therefore, our results suggest that cyclin B1 induced its antitumor chemotherapeutics effect by regulating the expression of PTEN. P53 also plays an important role in apoptosis in many cancer cells, and PTEN can increase the cellular content and transactivation of p53.Citation31 However, there were no changes in the level of p53 protein in our models. Akt is a downstream factor of PTEN, which plays a critical role in mediating growth promotion and cell survival signaling.Citation24,Citation32 We found that overexpression or suppression of cyclin B1 could not directly affect the expression of total AKT and phosphorylated form of Akt (p-Akt). However, after cisplatin or paclitaxel treatment, KYSE150/High-CycB1–1 and High-CycB1–2 cells expressed more p-Akt compared with the KYSE150 and pcDNA3.1 cells, and there was also more p-Akt in the EC9706 and control-siRNA cells than in the EC9706 CycB1 siRNA 1–2 cells, while the total Akt protein did not exhibit a difference (). To identify whether cyclin B1-mediated apoptosis depends on PTEN/Akt signaling pathways, we preincubated cells with LY 294002 (50 μmol/L) for 2 h before cisplatin or paclitaxel treatment, and LY 294002 effectively inhibited the activity of p-AKT, while did not affect the expression of AKT. As showed in , LY 294002 did not affect survival rate when cells were treated with control agent. However, after cisplatin or paclitaxel treatment, cells pretreated with LY 294002 exhibited lower survival rate compared with no LY 294002 pretreated in High-CycB1 1–2 cells by MTS assay, the survival rates were not significantly different among High-CycB1–1, High-CycB1–2 KYSE 150 and pcDNA3.1 cells undergoing LY 294002 treatment. In EC9706 clone cells, LY 294002 also decreased the survival rate compared with no LY 294002 pretreated in EC9706 and control-siRNA cells and the survival rates were also not significant difference among EC9706, control-siRNA, CycB1 siRNA 1 and CycB1 siRNA 2 cells (). When cells pretreated with LY 294002 were stained with DAPI, the percent of apoptotic cells were also not significantly different between both in KYSE150 clone cells and EC9706 clone cells (). All these results suggest that LY 294002 blocked the antagonizing effect of cyclin B1 in chemotherapeutic-induced apoptosis in human ESCC cells. It is demonstrated that cyclin B1 mediates cisplatin- or paclitaxel-induced apoptosis in ESCC cells through PTEN/Akt pathway.
Figure 4 and B. PTEN-PI3K/Akt pathway involves in cyclin B1-mediated chemotherapeutic-induced apoptosis. (A) Western blot analysis of the protein levels of PTEN, p53, Akt, p-Akt and actin in KYSE150, pcDNA3.1, High-CycB1 1–2, EC9706, control-siRNA and CycB1 siRNA 1–2 cells after treatment with cisplatin, paclitaxel or a control reagent for 24 h. (B) Inhibitor of PI3K/Akt (LY 294002) reduces the phosphorylation of Akt (p-Akt). KYSE150, pcDNA3.1, High-CycB1 1–2, EC9706, control-siRNA and CycB1 siRNA 1–2 cells were incubated with LY 294002 (50 μmol/L) for 2 h. Cell lysates were subjected to western blotting with indicated antibodies. Protein expression levels were normalized with actin.
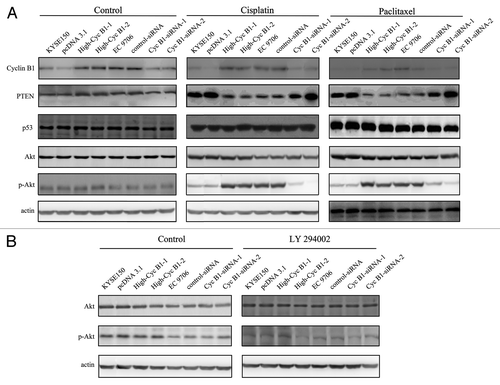
Figure 4 and D. (C) Effects of LY 294002 on cyclin B1-mediated chemotherapeutic-induced apoptosis in KYSE150, pcDNA3.1, High-CycB1 1–2, EC9706, control-siRNA and CycB1 siRNA 1–2 cells by MTS assay. Cells were preincubated with LY 294002 (50 μmol/L) for 2 h, then cisplatin or paclitaxel was added and incubated for 24 h. Twenty μl of MTS reagent was added to each well, followed by a 1–4 h incubation at 37°C and 5% CO2. The OD was read at 490 nm with a microplate reader. (D) Effects of LY 294002 on cyclin B1-mediated chemotherapeutic-induced apoptosis in KYSE150, pcDNA3.1, High-CycB1 1–2, EC9706, control-siRNA and CycB1 siRNA 1–2 cells by DAPI stain. Cells were preincubated with LY 294002 (50 μmol/L) for 2 h, then cisplatin or paclitaxel was added and incubated for 24 h and apoptosis was detected by DAPI stain. All experiments were performed at least three times with consistent and repeatable results. Each value is expressed as mean ± SD (n = 3). *and ※ p < 0.05 as compared with the control.
Discussion
It has been reported that overexpression of cyclin B1 promotes proliferation in human ESCC cells. Thus, suppression of cyclin B1 might be a unique and effective strategy in the development of targeted ESCC therapy.Citation3 However, the effect of cyclin B1 on chemotherapeutic-induced apoptosis in ESCC cells remains unknown. In the present study, we find that elevated cyclin B1 expression levels greatly attenuate the apoptosis induced by cisplatin or paclitaxel in ESCC cells. In contrast, suppression of cyclin B1 protein expression sensitizes ESCC cells to cisplatin- and paclitaxel-induced apoptosis. Otherwise, caspase-9, caspase-3, caspase-8 and Bcl-2 are involved in the process of apoptosis. We also observe that the antagonizing effect of cyclin B1 on therapeutic agent-induced apoptosis is through PTEN/Akt pathway. Overall, these observations suggest that suppressing the expression of cyclin B1 or combining with inhibitor of Akt could become a powerful therapeutic strategy in the treatment of ESCC.
Cyclin B1 is an important regulator in both the cell cycle process and the control of apoptosis. Reduced expression of cyclin B1 protein by siRNA in HeLa cells increased sensitivity to paclitaxel-induced apoptosis.Citation16,Citation33,Citation34 However, Borgne et al. showed that reduction of cyclin B1 protein by siRNA or by chemical inhibition decreased the number of apoptotic cells in HT 29 cells,Citation7 and there was a positive correlation between cyclin B1 and chemotherapy-induced apoptosis in prostate cancer cells.Citation35 All of these findings show that cyclin B1 protein plays different roles in various types of cells in the process of apoptosis. To elucidate the effect of cyclin B1 on cisplatin- or paclitaxel-induced apoptosis in ESCC cells, we established clones that overexpress cyclin B1 in ESCC KYSE150 cell lines.Citation3 In this model, we obtained evidence that ESCC cells were resistant to apoptosis when cyclin B1 protein was overexpressed. Additionally, we stably transfected cyclin B1 siRNA into ESCC EC9706 cells and obtained isogenic cell lines with endogenous cyclin B1 knockdown.Citation3 Using this model, we found that decreased cyclin B1 in ESCC cells made the cells more sensitive to cisplatin- or paclitaxel-induced apoptosis. These findings demonstrate that cyclin B1 is a critical regulator of apoptosis induced by cisplatin and paclitaxel and there is a negative correlation between cyclin B1 protein levels and cisplatin- and paclitaxel-induced apoptosis in ESCC cells.
Death ligand-mediated induction of apoptosis can be classified into type I (extrinsic pathway) or type II (intrinsic pathway) depending on the involvement of the mitochondria in the process of apoptosis.Citation5,Citation6 In type I cells, active caspase-8 cleaves caspase-3, which leads to cell death induction. In type II cells, activation of the mitochondrial upstream proteins is required for the induction of apoptosis following a death receptor stimulus.Citation5,Citation36 The bridging element between the two types of apoptosis is the caspase-8-mediated cleavage of Bid.Citation5,Citation36 Caspase-8 cleaves Bid into tBid, which initiates the mitochondrial apoptosis pathway leading to release of cytochrome c and SMAC/DIABLO. Next, cytochrome c, together with Apaf-1 forms the apoptosome, an activation platform for caspase-9. SMAC/DIABLO blocks the inhibitory function of XIAP, thereby allowing caspase-9 and -3 activation and consequently the induction of apoptosis.Citation5 To investigate the mechanism of the death signaling pathway involved in the process of cyclin B1-mediated apoptosis, we assayed the activation of caspase-9, caspase-3 and caspase-8 when cells were exposed to cisplatin or paclitaxel. We directly observed that the activation of caspase-9, caspase-3 and caspase-8 was elevated in KYSE150/pcDNA3.1 and EC9706 CycB1 siRNA 1–2 cells compared with their control cells after exposure to cisplatin or paclitaxel. We further illustrated the types of cyclin B1-mediated apoptosis. Type I cells are not sensitive to Bcl-2, whereas in type II cells, apoptosis can be abrogated by Bcl-2.Citation6 It is known that Bcl-2 exerts a protective effect against apoptosis and provides resistance to cell-death stimuli, including classic chemotherapeutic drugs.Citation26 We analyzed the expression of Bcl-2 by western blot in our two models. The results showed that the expression of Bcl-2 protein was lower in KYSE150/pcDNA3.1 and EC9706 CycB1 siRNA 1–2 cells compared with KYSE150/High-CycB1 1–2 and EC9706 control-siRNA cells after exposing the cells to cisplatin or paclitaxel. These results suggested that Bcl-2 was involved in the process of cyclin B1-mediated apoptosis in ESCC cells and served as a negative regulator during the process. The mechanism of cyclin B1-mediated apoptosis may rely on the Bcl-2-dependent mitochondria-regulated intrinsic death-signaling pathway. It is possible to deduce that the elevated caspase-8 activity in ESCC cells exposed to cisplatin or paclitaxel was probably not derived from the extrinsic pathway. Tsai et al. have reported that activation of cytotoxic procaspase-8 can alternatively occur by activated caspase-3-mediated or caspase-9-mediated proteolytic cleavage via the intrinsic death signaling subsequent to death receptor activation.Citation6,Citation37
Paclitaxel is a highly effective drug in treating tumors because of its ability to bind tubulin and disturb microtubule dynamics,Citation38,Citation39 which generally results in an impairment of the G2/M transition during mitosis and leads to cell death by apoptosis.Citation40,Citation41 Cisplatin, one of the most widely used anticancer drugs, is believed to induce tumor cell death as a result of the formation of cisplatin-DNA adducts, which inhibit DNA replication and transcription.Citation42 The above reports indicate that the mechanisms of paclitaxel- and cisplatin-induced apoptosis are different. However, our studies found that cyclin B1 protein was able to antagonize apoptosis induced by both paclitaxel and cisplatin and that the suppression of endogenous cyclin B1 sensitized ESCC cells to apoptosis after the cells were treated with paclitaxel or cisplatin. These findings suggest that cyclin B1 may be a common regulatory factor during the process of apoptosis when cells are exposed to paclitaxel or cisplatin.
The underlying mechanisms of cyclin B1-mediated apoptosis might include several aspects in ESCC cells. Thus, we further elucidated the underlying factors contributing to cyclin B1-mediated apoptosis. The tumor suppressor PTEN controls a variety of cellular functions, including cell proliferation and survival. It has been demonstrated that the knockdown of PTEN can stimulate cell proliferation and reduce apoptosis in many cancers.Citation43,Citation44 To detect if PTEN involves in cisplatin- or paclitaxel-induced apoptosis in our models, we examined PTEN by western blot in the KYSE150 and EC9706 cell lines treated with cisplatin or paclitaxel. We found that after cisplatin or paclitaxel treatment, PTEN in KYSE150/High-CycB1 1–2 cells were significantly lowered compared with KYSE150/pcDNA3.1 cells, and that PTEN in EC9706 control-siRNA cells were also significantly lowered compared with EC9706 CycB1 siRNA 1–2 cells. Otherwise, PTEN can increase the cellular content and transactivation of p53.Citation31 However, there were no changes at the level of p53 protein in our model. These results suggest that the antagonizing effect of overexpression cyclin B1 on cisplatin- or paclitaxel-induced apoptosis is related to lower PTEN, which is through p53-independent mechanism. The major substrate of PTEN is PtdIns(3,4,5)P3, and PTEN can dephosphorylate PtdIns(3,4,5)P3 into PtdIns(4,5)P2, which blocks Akt activation. Therefore, PTEN is a negative regulator of Akt and negatively regulating Akt-triggered signaling. The Akt pathway is an important anti-apoptotic signaling pathway regulating cell growth and survival.Citation24,Citation32 Phosphorylation of Akt can block the activity of many proapoptotic proteins and negatively regulate Bcl-2 to block cytochrome C release from the mitochondria.Citation22,Citation45 Akt is observed in a variety of cancers with a high level,Citation46 while PTEN is mutated in advanced tumors.Citation47 Therefore, we hypothesized that cyclin B1 may stimulate Akt signaling pathway to protect ESCC cells against chemotherapeutic drugs. Our further investigation exhibits that phosphorylation of Akt was involved in the process of cyclin B1-mediated chemotherapeutic-induced apoptosis. Cells with more cyclin B1 were found more p-Akt than the cells produced less cyclin B1 after cisplatin or paclitaxel treatment. To further confirm the role of Akt signaling pathway in our models, cells were pretreated with PI3K/Akt inhibitor LY 294002. We determinate that the resistant effect of cyclin B1 in chemotherapeutic-induced apoptosis could be blocked by LY 294002. All these results suggest that cyclin B1 antagonized chemotherapeutic-induced apoptosis in human ESCC cells via PTEN/Akt signaling pathway.
In summary, our study identifies the important role of cyclin B1 in the chemotherapeutic-induced apoptosis, and elucidates the molecular mechanisms underlying cyclin B1 antagonizing effect in chemotherapeutic-induced apoptosis in ESCC cells. In addition to elucidating better understanding of the molecular and biological mechanisms of apoptotic progression in ESCC cells, these findings may provide significant clinical application, which suppression of cyclin B1 or combining with inhibitor of Akt in ESCC could be an attractive strategy for cisplatin and paclitaxel therapy.
Material and Methods
Cells and reagents
The KYSE150, EC9706, KYSE 450, KYSE 510 and COLO 680N human ESCC cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2. The EC9706 cell line was generously provided by Dr. Mingrong Wang of the Cancer Institute (Hospital), Chinese Academy of Medical Sciences.Citation48 The KYSE150 cell line was generously provided by Dr. Shemada of Kyoto University.Citation49 KYSE 450, KYSE 510 and COLO 680N cells were stored in our laboratory. Cyclin B1 overexpression stable cell lines (High-CycB1–1, High-CycB1–2), the control cell line (pcDNA3.1) in KYSE150 cells and the isogenic cell lines with endogenous cyclin B1 knockdown in ESCC EC9706 cells (CycB1-siRNA-1, CycB1-siRNA-2 and control-siRNA) have been established in our laboratory. All clones were previously described and used in our prior publications.Citation3 Cisplatin and paclitaxel were purchased from the pharmacy of the clinical center (Cancer Institute and Hospital, Chinese Academy of Medical Sciences). PI3K/Akt inhibitor LY 294002 was purchased from Sigma Inc. Cells were seeded the day prior to treatment at a density of about 5 × 105 cells/ ml and treated with cisplatin and paclitaxel. Preliminary experiments were done to assess the optimum concentration (ED50) of cisplatin (32.0 μmol/L) and paclitaxel (32.0 μmol/L) for ESCC cells. ESCC cells were grown in 10% FCS/RPMI 1640 and exposed to cisplatin or paclitaxel for 24 h continuously. Cells were harvested for western blot analysis, DAPI apoptosis analysis, flow cytometric analysis and survival analysis.
Western blotting
Cells were collected and centrifuged for harvest. Cells were lysed on ice for 40 min in Ripa buffer (10 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X, 0.1% Na-Deoxycholate, 0.1% SDS and 5 mM EDTA) containing Complete Protease Inhibitor Cocktail (Sigma). Lysates were cleared by centrifugation at 12,000 rcf for 20 min at 4°C. A BCA protein assay was performed to determine total protein concentration, and 20 μg of total protein was loaded into each well of a 15% SDS–PAGE. Gels were transferred onto PVDF membranes (Bio-Rad), blocked with 5% milk-PBS and incubated overnight at 4°C with PARP Abs, Bcl-2 Abs, cyclin B1 Abs, p53 Abs, cleaved caspase-3 Abs, cleaved caspase-9 Abs, cleaved caspase-8 Abs, protein kinase B (Akt) Abs, pAkt (Serine 473) Abs (Cell Signaling) and β-actin Abs (Sigma). The membranes were washed and incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies at 1:3,000 in 5% milk and then washed and detected by ECL (Applygen Technologies Inc.) and exposure to X-ray film.
Plasmids and small-interfering RNA transfection
The siRNA sequences that we used were listed as follows: cyclin B1, 5′-ATGAGAGCCATCCTAATTG-3′, and control siRNA, 5′-UUCUCCGAACGUGUCACGU-3′. Cells were transfected with pcDNA3.1 cyclin B1 plasmids or cyclin B1 siRNA using Lipofectamine2000 (Invitrogen Corporation) according to the manufacturer’s instruction. Fresh medium was added at 6 h after transfection.
Survival rate analysis
Cell survival rate was assessed by adding 1 × 104 cells (in 100 μl) to three wells of a 96-well plate. After cells were treated with or without cisplatin and paclitaxel for 24 h, 20 μl of 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent (Promega) was added to each well, followed by a 1–4 h incubation at 37°C and 5% CO2. The optical densities (OD) were read at 490 nm with a microplate reader. The experiment was conducted in triplicate and repeated three times. Survival rate was calculated as following: (OD sample/ OD control) × 100%.
Flow cytometric analysis
The vital, apoptotic and damaged cells were separated by flow cytometry. The quantitative determination of the percentage of cells undergoing apoptosis was performed using an annexin V-FITC apoptosis detection kit (Cliniscience S.A.) according to the manufacturer’s instructions. In brief, 24 h after treatment with cisplatin or paclitaxel, 2 × 105 cells were labeled fluorescently for detection of apoptotic and necrotic cells by adding 195 μl of annexin V binding buffer and 5 μl of annexin V-FITC to each sample. Samples were mixed gently and incubated at room temperature in the dark for 3 min, then, 10 μl of propidium iodide (PI; Sigma) were added to each sample and incubated at room temperature for 10 min. Before cytometric analysis, the cell suspension was supplemented with 300 μl of annexin V-binding buffer. A minimum of 10,000 cells within the gated region were acquired and analyzed with CellQuest software.
DAPI apoptosis assay
For the DAPI staining apoptosis assay, cells were resuspended in 0.6-mL 4% paraformaldehyde/ PBS for 15 min, washed with PBS and resuspended in 0.5 ml of DAPI (1 μg/ml) /PBS for 10 min. Cells were washed with PBS, 10 μl of concentrated cells were added on a microscope slide and a coverslip was placed on the slide. Cells containing densely stained and fragmented chromatin were identified as end-stage apoptotic using a Nikon fluorescence microscope with a DAPI filter. The number of apoptotic cells in at least 200 total cells was determined from at least four random microscope fields. Changes in apoptosis from cisplatin- or paclitaxel-treated cells were determined as percentage of apoptotic cells in at least five different samples from three independent experiments. Minimal apoptosis was detected in control treated cells (< 0.5%).
Statistics analysis
All experiments were performed and repeated at least three times. Data was analyzed with SPSS 11.5 software.
| Abbreviations: | ||
| CDK1 | = | cyclin-dependent kinase 1 |
| ESCC | = | esophageal squamous cell carcinoma |
| PTEN | = | phosphatase and tensin homologue deleted on chromosome 10 |
| APC | = | anaphase-promoting complex |
Additional material
Download Zip (2.6 MB)Acknowledgments
We thank Dr. Shemada of Kyoto University for providing us with the esophageal carcinoma cell line KYSE150. This work is supported by funding from the 973 National Key Fundamental Research Program of China (2009CB521801) and the National Natural Science Foundation of China (81071633 and 81021061).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349:2241 - 52; http://dx.doi.org/10.1056/NEJMra035010; PMID: 14657432
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55:10 - 30; http://dx.doi.org/10.3322/canjclin.55.1.10; PMID: 15661684
- Song Y, Zhao C, Dong L, Fu M, Xue L, Huang Z, et al. Overexpression of cyclin B1 in human esophageal squamous cell carcinoma cells induces tumor cell invasive growth and metastasis. Carcinogenesis 2008; 29:307 - 15; http://dx.doi.org/10.1093/carcin/bgm269; PMID: 18048386
- Kim JY, Do YR, Park KU, Kim MK, Lee KH, Bae SH, et al. A multi-center phase II study of docetaxel plus cisplatin as first-line therapy in patients with metastatic squamous cell esophageal cancer. Cancer Chemother Pharmacol 2010; 66:31 - 6; http://dx.doi.org/10.1007/s00280-009-1130-6; PMID: 19763571
- Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta 2011; 1813:558 - 63; http://dx.doi.org/10.1016/j.bbamcr.2011.01.026; PMID: 21295084
- Tsai WS, Yeow WS, Chua A, Reddy RM, Nguyen DM, Schrump DS, et al. Enhancement of Apo2L/TRAIL-mediated cytotoxicity in esophageal cancer cells by cisplatin. Mol Cancer Ther 2006; 5:2977 - 90; http://dx.doi.org/10.1158/1535-7163.MCT-05-0514; PMID: 17172403
- Borgne A, Versteege I, Mahé M, Studeny A, Léonce S, Naime I, et al. Analysis of cyclin B1 and CDK activity during apoptosis induced by camptothecin treatment. Oncogene 2006; 25:7361 - 72; http://dx.doi.org/10.1038/sj.onc.1209718; PMID: 16785996
- Jun DY, Kim JS, Park HS, Song WS, Bae YS, Kim YH. Cytotoxicity of diacetoxyscirpenol is associated with apoptosis by activation of caspase-8 and interruption of cell cycle progression by down-regulation of cdk4 and cyclin B1 in human Jurkat T cells. Toxicol Appl Pharmacol 2007; 222:190 - 201; http://dx.doi.org/10.1016/j.taap.2007.04.011; PMID: 17559898
- Tommasi S, Besaratinia A, Wilczynski SP, Pfeifer GP. Loss of Rassf1a enhances p53-mediated tumor predisposition and accelerates progression to aneuploidy. Oncogene 2011; 30:690 - 700; http://dx.doi.org/10.1038/onc.2010.440; PMID: 20890300
- Chang E, Donahue J, Smith A, Hornick J, Rao JN, Wang JY, et al. Loss of p53, rather than beta-catenin overexpression, induces survivin-mediated resistance to apoptosis in an esophageal cancer cell line. J Thorac Cardiovasc Surg 2010; 140:225 - 32; http://dx.doi.org/10.1016/j.jtcvs.2009.11.038; PMID: 20236666
- Lubin DJ, Butler JS, Loh SN. Folding of tetrameric p53: oligomerization and tumorigenic mutations induce misfolding and loss of function. J Mol Biol 2010; 395:705 - 16; http://dx.doi.org/10.1016/j.jmb.2009.11.013; PMID: 19913028
- Li K, He W, Lin N, Wang X, Fan QX. Downregulation of N-cadherin expression inhibits invasiveness, arrests cell cycle and induces cell apoptosis in esophageal squamous cell carcinoma. Cancer Invest 2010; 28:479 - 86; http://dx.doi.org/10.3109/07357900903476745; PMID: 20014942
- Wang X, Dong L, Xie J, Tong T, Zhan Q. Stable knockdown of Aurora-A by vector-based RNA interference in human esophageal squamous cell carcinoma cell line inhibits tumor cell proliferation, invasion and enhances apoptosis. Cancer Biol Ther 2009; 8:1852 - 9; http://dx.doi.org/10.4161/cbt.8.19.9550; PMID: 19770595
- Jin P, Hardy S, Morgan DO. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J Cell Biol 1998; 141:875 - 85; http://dx.doi.org/10.1083/jcb.141.4.875; PMID: 9585407
- Tang L, Zhang Y, Pan H, Luo Q, Zhu XM, Dong MY, et al. Involvement of cyclin B1 in progesterone-mediated cell growth inhibition, G2/M cell cycle arrest, and apoptosis in human endometrial cell. Reprod Biol Endocrinol 2009; 7:144; http://dx.doi.org/10.1186/1477-7827-7-144; PMID: 19968870
- Yuan J, Yan R, Krämer A, Eckerdt F, Roller M, Kaufmann M, et al. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene 2004; 23:5843 - 52; http://dx.doi.org/10.1038/sj.onc.1207757; PMID: 15208674
- Hassan KA, Ang KK, El-Naggar AK, Story MD, Lee JI, Liu D, et al. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res 2002; 62:6414 - 7; PMID: 12438226
- Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res 1997; 57:4736 - 8; PMID: 9354433
- Risinger JI, Hayes K, Maxwell GL, Carney ME, Dodge RK, Barrett JC, et al. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin Cancer Res 1998; 4:3005 - 10; PMID: 9865913
- Marsh DJ, Dahia PL, Caron S, Kum JB, Frayling IM, Tomlinson IP, et al. Germline PTEN mutations in Cowden syndrome-like families. J Med Genet 1998; 35:881 - 5; http://dx.doi.org/10.1136/jmg.35.11.881; PMID: 9832031
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 1991; 254:274 - 7; http://dx.doi.org/10.1126/science.1833819; PMID: 1833819
- Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res 2000; 60:5988 - 94; PMID: 11085518
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 1998; 279:710 - 4; http://dx.doi.org/10.1126/science.279.5351.710; PMID: 9445477
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2:489 - 501; http://dx.doi.org/10.1038/nrc839; PMID: 12094235
- McDonnell MA, Wang D, Khan SM, Vander Heiden MG, Kelekar A. Caspase-9 is activated in a cytochrome c-independent manner early during TNFalpha-induced apoptosis in murine cells. Cell Death Differ 2003; 10:1005 - 15; http://dx.doi.org/10.1038/sj.cdd.4401271; PMID: 12934075
- Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol 1999; 17:2941 - 53; PMID: 10561374
- Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene 2003; 22:8205 - 11; http://dx.doi.org/10.1038/sj.onc.1206878; PMID: 14603261
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 1999; 96:4240 - 5; http://dx.doi.org/10.1073/pnas.96.8.4240; PMID: 10200246
- Chae HD, Broxmeyer HE. SIRT1 Deficiency Downregulates PTEN/JNK/FOXO1 Pathway to Block ROS-Induced Apoptosis in Mouse Embryonic Stem Cells. Stem Cells Dev 2011; 20:1277 - 85; http://dx.doi.org/10.1089/scd.2010.0465; PMID: 21083429
- Li HF, Keeton A, Vitolo M, Maddox C, Rasmussen L, Hobrath J, et al. A high-throughput screen with isogenic PTEN+/+ and PTEN-/- cells identifies CID1340132 as a novel compound that induces apoptosis in PTEN and PIK3CA mutant human cancer cells. J Biomol Screen 2011; 16:383 - 93; http://dx.doi.org/10.1177/1087057110397357; PMID: 21335596
- Yamada K, Kunishima N, Mayanagi K, Ohnishi T, Nishino T, Iwasaki H, et al. Crystal structure of the Holliday junction migration motor protein RuvB from Thermus thermophilus HB8. Proc Natl Acad Sci USA 2001; 98:1442 - 7; http://dx.doi.org/10.1073/pnas.98.4.1442; PMID: 11171970
- Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2002; 94:3127 - 34; http://dx.doi.org/10.1002/cncr.10591; PMID: 12115344
- Xie XH, An HJ, Kang S, Hong S, Choi YP, Kim YT, et al. Loss of Cyclin B1 followed by downregulation of Cyclin A/Cdk2, apoptosis and antiproliferation in Hela cell line. Int J Cancer 2005; 116:520 - 5; http://dx.doi.org/10.1002/ijc.21056; PMID: 15818617
- Yuan J, Krämer A, Matthess Y, Yan R, Spänkuch B, Gätje R, et al. Stable gene silencing of cyclin B1 in tumor cells increases susceptibility to taxol and leads to growth arrest in vivo. Oncogene 2006; 25:1753 - 62; http://dx.doi.org/10.1038/sj.onc.1209202; PMID: 16278675
- Gomez LA, de Las Pozas A, Reiner T, Burnstein K, Perez-Stable C. Increased expression of cyclin B1 sensitizes prostate cancer cells to apoptosis induced by chemotherapy. Mol Cancer Ther 2007; 6:1534 - 43; http://dx.doi.org/10.1158/1535-7163.MCT-06-0727; PMID: 17513602
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94:491 - 501; http://dx.doi.org/10.1016/S0092-8674(00)81590-1; PMID: 9727492
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 1999; 144:281 - 92; http://dx.doi.org/10.1083/jcb.144.2.281; PMID: 9922454
- Kumar N. Taxol-induced polymerization of purified tubulin. Mechanism of action. J Biol Chem 1981; 256:10435 - 41; PMID: 6116707
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979; 277:665 - 7; http://dx.doi.org/10.1038/277665a0; PMID: 423966
- Horwitz SB. Mechanism of action of taxol. Trends Pharmacol Sci 1992; 13:134 - 6; http://dx.doi.org/10.1016/0165-6147(92)90048-B; PMID: 1350385
- Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 1996; 56:816 - 25; PMID: 8631019
- Szymkowski DE, Yarema K, Essigmann JM, Lippard SJ, Wood RD. An intrastrand d(GpG) platinum crosslink in duplex M13 DNA is refractory to repair by human cell extracts. Proc Natl Acad Sci USA 1992; 89:10772 - 6; http://dx.doi.org/10.1073/pnas.89.22.10772; PMID: 1438274
- Akca H, Demiray A, Tokgun O, Yokota J. Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/AKT/NFkB pathway in lung cancer cells. Lung Cancer 2011; 73:302 - 9; http://dx.doi.org/10.1016/j.lungcan.2011.01.012; PMID: 21333374
- Wang T, Lv JH, Zhang XF, Li CJ, Han X, Sun YJ. Tissue inhibitor of metalloproteinase-1 protects MCF-7 breast cancer cells from paclitaxel-induced apoptosis by decreasing the stability of cyclin B1. Int J Cancer 2010; 126:362 - 70; http://dx.doi.org/10.1002/ijc.24753; PMID: 19609944
- Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, et al. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res 1999; 59:2551 - 6; PMID: 10363971
- Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene 2004; 23:5853 - 7; http://dx.doi.org/10.1038/sj.onc.1207721; PMID: 15208673
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275:1943 - 7; http://dx.doi.org/10.1126/science.275.5308.1943; PMID: 9072974
- Han Y, Wei F, Xu X, Cai Y, Chen B, Wang J, et al. [Establishment and comparative genomic hybridization analysis of human esophageal carcinomas cell line EC9706]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2002; 19:455 - 7; PMID: 12476413
- Miyahara T, Ueda K, Akaboshi M, Shimada Y, Imamura M, Utsumi H. Hyperthermic enhancement of cytotoxicity and increased uptake of cis-diamminedichloroplatinum(II) in cultured human esophageal cancer cells. Jpn J Cancer Res 1993; 84:336 - 40; http://dx.doi.org/10.1111/j.1349-7006.1993.tb02875.x; PMID: 8486532