Abstract
Mucin 1 (MUC1) is a heterodimeric glycoprotein that is aberrantly overexpressed in most human breast cancers. The oncogenic MUC1-C subunit promotes survival and blocks the apoptotic response to genotoxic anticancer agents. In the present studies, human MCF-7 and ZR-75-1 breast cancer cells were treated with the MUC1-C inhibitor, GO-203, a cell-penetrating peptide that blocks MUC1-C homodimerization and thereby its oncogenic function. Treatment with GO-203 was found to promote the apoptotic response of MCF-7 and ZR-75-1 cells to the therapeutic drugs taxol and doxorubicin (DOX). This effect was (1) attenuated by a pan-caspase inhibitor, and (2) mediated, at least in part, by activation of the effector caspase-7 and cleavage of the downstream substrate PARP. Further analysis of the interaction between GO-203 and taxol using isobolograms, which evaluate the nature of the interaction of two drugs, demonstrated that the combination is highly synergistic. These results were supported by combination index (CI) analysis with values of less than 1. GO-203 was also highly synergistic with DOX in studies of both MCF-7 and ZR-75-1 breast cancer cells. These findings indicate that blocking MUC1-C function could be effective in combination with taxol and DOX for the treatment of breast cancer.
Keywords: :
Introduction
Mucin 1 (MUC1) is a heterodimeric transmembrane protein that is expressed at the apical borders of normal secretory epithelial cells.Citation1 MUC1 consists of an N-terminal ectodomain (MUC1-N) in a cell surface complex with the C-terminal transmembrane subunit (MUC1-C).Citation1 The MUC1 heterodimer functions in protecting the epithelial layer from stress imposed by the external environment.Citation1 In contrast to normal epithelial cells, the MUC1 dimeric complex is aberrantly overexpressed in human breast and other cancers.Citation1,Citation2 These findings have supported the contention that the physiologic function of MUC1 has been appropriated and subverted by carcinomas to protect them against stress-induced death. In this context, overexpression of the MUC1-C subunit is sufficient to induce transformation and to block stress-induced apoptotic responses.Citation1,Citation2
The MUC1-C subunit consists of a 58-amino acid extracellular domain, a transmembrane domain and a 72-amino acid cytoplasmic domain.Citation1,Citation2 The MUC1-C extracellular domain associates with galectin-3 and thereby forms complexes with growth factor receptors at the cell surface.Citation3 In addition, the MUC1-C cytoplasmic domain interacts with effectors, such as phosphoinositide 3-kinase (PI3K), NF-B and p53, that have been linked to the regulation of cell growth and death.Citation4-Citation6 The overexpression of MUC1 in carcinoma cells is associated with the accumulation of MUC1-C homodimers in the cytoplasm and the targeting of this subunit to the nucleusCitation7-Citation9 and mitochondria.Citation10,Citation11 The formation of MUC1-C homodimers is conferred by a CQC motif in the cytoplasmic domain and is necessary for its oncogenic function.Citation1,Citation2 Accordingly, cell-penetrating peptides and small molecules have been developed to block the MUC1-C CQC motif and thereby the formation of homodimers.Citation12-Citation14 The first generation L-amino acid peptide inhibitor, GO-201, was derived from the NH2-terminal region of the MUC1-C cytoplasmic domain that contains the CQC motif and was linked to a poly d-arginine transduction domain.Citation12 GO-201 was subsequently converted to a second-generation inhibitor, GO-203, which is shorter and contains all D-amino acids.Citation14,Citation15 Importantly, treatment of breast and other types of MUC1-expressing carcinoma cells with these peptide MUC1-C inhibitors is associated with (1) disruption of MUC1-C homodimers, (2) inhibition of growth and (3) induction of death, which has predominantly been necrotic.Citation12,Citation14-Citation16 Based on these findings, the MUC1-C inhibitor, GO-203, has entered Phase I evaluation for patients with refractory breast and other carcinomas.
The overexpression of MUC1-C in breast cancer cells is associated with resistance to death in the response to treatment with diverse cytotoxic chemotherapeutic agents.Citation4,Citation5,Citation10,Citation17 Taxol and doxorubicin (DOX) are two cytotoxic agents that are commonly used in the treatment of breast cancer. The present studies demonstrate that targeting the MUC1-C subunit in breast cancer cells is highly synergistic with both taxol and DOX. These results provide an experimental framework for the combination of MUC1-C inhibitors with cytotoxic anticancer agents in the clinic.
Results
Combining the MUC1-C inhibitor GO-203 with taxol and doxorubicin (DOX) in the treatment of MCF-7 breast cancer cells
GO-203 is a D-amino acid cell-penetrating peptide inhibitor of MUC1-C dimerization that contains a poly-Arg transduction domain linked to the CQCRRKN amino acid sequence derived from the MUC1-C cytoplasmic domain (). Treatment of breast and other carcinoma cells with GO-203 is associated with disruption of MUC1-C homodimers and cell death.Citation14,Citation15 Consistent with previous studies of MUC1-C inhibitors,Citation12 analysis of MCF-7 breast cancer cells after treatment with the IC50 value of GO-203 (; 5.6 μM) for 72 h demonstrated an accumulation of cells in G1 and S phases (). GO-203 treatment was also associated with a modest 9% population of apoptotic cells in sub-G1 phase (). By comparison, treatment with taxol at the IC50 (; 28 nM) resulted in the arrest of cells in G2/M phase and 11% in the sub-G1 fraction (). By contrast to either agent alone, the combination of GO-203 and taxol was associated with a marked increase of 37% in the sub-G1 population, consistent with an enhanced induction of apoptosis (). Treatment of MCF-7 cells with GO-203 alone and DOX alone (; IC50 = 0.33 μM) was each associated with < 10% of cells in the sub-G1 fraction (). However, the combination of GO-203 and DOX resulted in over 50% of apoptotic cells (). These results indicate that the apoptotic response to the combination of the MUC1-C inhibitor with taxol or DOX is more than additive compared with that obtained with these agents alone.
Figure 1. Combining GO-203 with taxol and DOX increases the induction of MCF-7 cell apoptosis. (A) Schema of the MUC1-C subunit with the sequence of the 72-amino acid cytoplasmic domain. GO-203 is a D-amino acid peptide that includes a poly-Arg transduction domain and the CQCRRKN sequence (shaded) derived from the MUC1-C cytoplasmic domain. (B) MCF-7 cells were left untreated (control) and treated with 5.6 μM GO-203 (), 28 nM taxol () or the combination of both agents for 72 h. GO-203 was added every 24 h. (C) MCF-7 cells were left untreated (control) and treated with 5.6 M GO-203, 0.33 μM DOX () or the combination of both agents for 72 h. GO-203 was added every 24 h. The cells were fixed and analyzed for cell cycle distribution by flow cytometry. The percentage of sub-G1 cells is included in the panels.
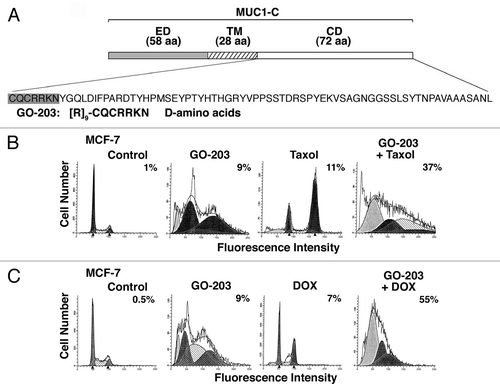
Table 1. IC50 values of GO-203, taxol and DOX
Activation of caspase-7 in response to treatment of MCF-7 cells with GO-203 in combination with taxol and DOX
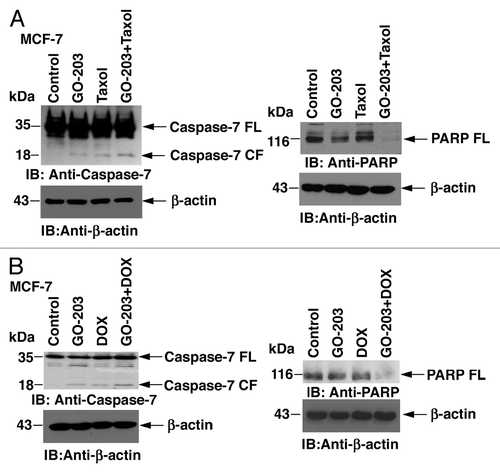
MCF-7 cells are null for the effector caspase-3; consequently, we studied activation of caspase-7. Treatment with GO-203 alone was associated with a modest increase in the cleaved form of caspase-7 (, left). Similar results were obtained in response to treatment with taxol alone (, left). Moreover, the combination of GO-203 and taxol was associated with a greater increase in cleaved caspase-7 as compared with either agent alone (, left). Consistent with these results, cleavage of the effector caspase target, PARP, was more pronounced with the GO-203/taxol combination than that found with each agent alone (, right). Similar results were obtained when the MCF-7 cells were treated with GO-203 and DOX (). Cleavage of both caspase-7 and PARP was greater with the GO-203/DOX combination than with GO-203 or DOX alone (, left and right). These results indicate that the increased apoptotic response to the combination of GO-203 with taxol and DOX is mediated, at least in part, to activation of caspase-7.
Figure 2. Activation of effector caspase-7 in MCF-7 cells treated with GO-203, taxol and DOX. (A) MCF-7 cells were treated with 5.6 μM GO-203, 28 nM taxol or the combination of both agents for 48 h. GO-203 was added every 24 h. B. MCF-7 cells were treated with 5.6 μM GO-203, 0.33 μM DOX, or the combination of both agents for 48 h. GO-203 was added every 24 h. Cytosolic lysates were immunoblotted with the indicated antibodies (left). Total cell lysates were immunoblotted with anti-PARP and anti-actin (right). FL, full-length; CF, cleaved fragment.
Effects of combining GO-203 with taxol and DOX in the treatment of ZR-75-1 breast cancer cells
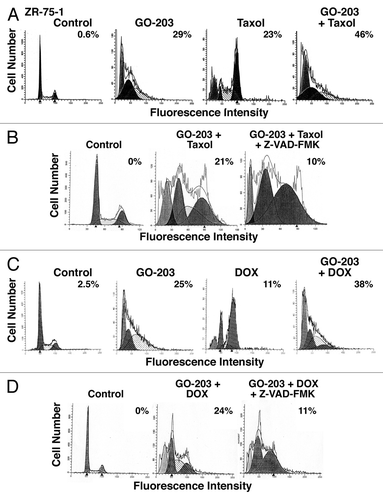
To extend the results obtained with MCF-7 cells, we similarly analyzed the effects of GO-203 and taxol on ZR-75-1 cells. Treatment with GO-203 alone for 72 h (; IC50 = 2.9 μM) was associated with an arrest of ZR-75-1 cells in G1 phase and 29% in the sub-G1 population (). Taxol treatment for 72 h (; IC50 = 85 nM) resulted in a predominant arrest of cells in G2/M phase and 23% in the sub-G1 fraction (). In addition, treatment with the GO-203/taxol combination for 72 h increased the apoptotic response to 46% (). To extend this analysis, we found that treatment of ZR-75-1 cells with GO-203/taxol for 48 h in the presence of the pan-caspase inhibitor Z-VAD-FMK attenuated the apoptotic response (). Treatment of ZR-75-1 cells with GO-203 alone and DOX alone (; IC50 = 0.53 μM) was associated with 25% and 11% of cells in the sub-G1 population, respectively (). The GO-203/DOX combination further resulted in more than an additive increase in the apoptotic response (). Moreover, this response to the GO-203/DOX combination was attenuated by the addition of Z-VAD-FMK ().
Figure 3. GO-203 increases the apoptotic response of ZR-75-1 cells to taxol and DOX. (A) ZR-75-1 cells were left untreated (control) and treated with 2.9 μM GO-203 (), 85 nM taxol () or the combination of both agents for 72 h. GO-203 was added every 24 h. (B) ZR-75-1 cells were left untreated (control) and treated with the combination of 2.9 μM GO-203 and 85 nM taxol in the absence and presence of 4 μM Z-VAD-FMK for 48 h. GO-203 was added every 24 h. (C) ZR-75-1 cells were left untreated (control) and treated with 2.9 μM GO-203, 0.53 μM DOX () or the combination of both agents for 72 h. GO-203 was added every 24 h. (D) ZR-75-1 cells were left untreated (control) and treated with the combination of 2.9 M GO-203 and 0.53 μM DOX in the absence and presence of 4 μM Z-VAD-FMK for 48 h. GO-203 was added every 24 h. The cells were fixed and analyzed for cell cycle distribution by flow cytometry. The percentage of sub-G1 cells is included in the panels.
In association with these results, activation of caspase-3 was moderately increased in the response of ZR-75-1 cells to the combination as compared with GO-203 or taxol alone (, left). In addition, cleavage of caspase-7 was more pronounced with the GO-203/taxol combination as compared with that obtained with these agents alone (, left). Moreover, cleavage of PARP was greater with the GO-203/taxol combination (, right). In studies with DOX treatment, cleavage of caspase-3 was observed in the GO-203-treated ZR-75-1 cells, but not as shown previously in the response to DOX,Citation18 and was found at a similar levels in the response to the GO-203/DOX combination (, left). Nonetheless, activation of caspase-7 and cleavage of PARP were both increased with the GO-203/DOX combination compared with treatment with either agent alone (, right and left). These results indicate that combining GO-203 with taxol and DOX is more effective in inducing caspase activation and apoptosis in breast cancer cells.
Figure 4. Activation of caspase-3 and caspase-7 in ZR-75-1 cells treated with GO-203 in combination with taxol and DOX. (A) ZR-75-1 cells were treated with 2.9 μM GO-203, 85 nM taxol or the combination of both agents for 48 h. GO-203 was added every 24 h. (B) ZR-75-1 cells were treated with 2.9 μM GO-203, 0.53 μM DOX or the combination of both agents for 48 h. GO-203 was added every 24 h. Cytosolic lysates were immunoblotted with the indicated antibodies (left). Total-cell lysates were immunoblotted with anti-PARP and anti-actin (right). FL, full-length; CF, cleaved fragment.

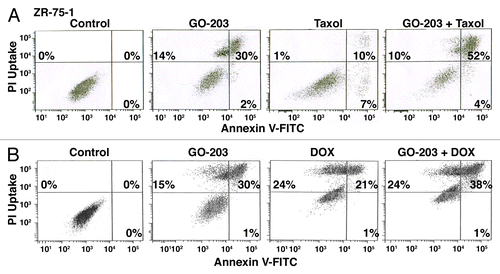
Previous studies assessing the effects of treating MUC1+ carcinoma cells with MUC1-C inhibitors have largely focused on the induction of necrotic cell death.Citation12,Citation15,Citation16 To further define the response of ZR-75-1 cells to GO-203 in combination with taxol or DOX, we analyzed staining with propidium iodide (PI) and annexin V to assess both apoptosis and necrosis. Treatment of ZR-75-1 cells with GO-203 alone was associated with induction of necrotic (upper left quadrant), apoptotic (lower right quadrant) and late apoptotic/necrotic (upper right quadrant) cells (). Treatment with taxol alone was associated with the induction of predominantly apoptotic cells (). Moreover, the GO-203/taxol combination resulted in death that was predominantly late apoptotic/necrotic (). Similar results were obtained when ZR-75-1 cells were treated with GO-203/DOX (), indicating that combining GO-203 with these cytotoxic agents can potentiate both apoptosis and necrosis.
Figure 5. Combining GO-203 with taxol or DOX induces late apoptosis/necrosis. (A) ZR-75-1 cells were treated with 2.9 μM GO-203, 85 nM taxol or the combination of both agents for 48 h. GO-203 was added every 24 h. (B) ZR-75-1 cells were treated with 2.9 μM GO-203, 0.53 μM DOX or the combination of both agents for 48 h. GO-203 was added every 24 h. The cells were then incubated with PI/annexin V and analyzed by flow cytometry. The percentage of necrotic (upper left quadrant), apoptotic (lower right quadrant) and late apoptotic/necrotic (upper right quadrant) cells is indicated in the panels.
Evaluation of combining GO-203 with taxol and DOX by isobologram and combination index analyses
The studies reported above support a potential additive or synergistic interaction for GO-203 in combination with taxol and DOX. To more precisely define the level of that interaction, we used the Chou-Talalay method for drug combinations that is based on the median-effect equation.Citation19,Citation20 Studies were first performed to define the effects of GO-203 in combination with taxol in the treatment of MCF-7 cells. Using the half-maximal inhibitory concentrations (), GO-203 and taxol were tested alone for effects on MCF-7 cell growth at 1/8X, 1/4X, 1/2X 1X, 2X and 4X the IC50 and at equipotent concentrations at the same ratios in combination (, left). Isobologram analysis at the ED50, ED75 and ED90 values confirmed synergy of the GO-203/taxol combination (, right) with CIs < 1 (). The MCF-7 cells were also treated with GO-203 alone, DOX alone and the GO-203/DOX combination (, left). Synergy of the combination was confirmed by isobologram analysis (, right, ).
Figure 6. Synergistic interaction of GO-203 in combination with taxol and DOX in the treatment of MCF-7 cells. (A) MCF-7 cells were exposed to fixed IC50 ratios GO-203 alone, taxol alone and the GO-203/taxol combination. The dose-effect curves for GO-203 alone (+), taxol alone (8) and GO-203/taxol (X) are shown in the left panel. The multiple effect-level isobologram analysis is shown in the right panel for the ED90 (solid circles), ED75 (solid squares) and ED50 (solid triangles). The CI values are listed in . (B) MCF-7 cells were treated with fixed IC50 ratios of GO-203 alone, DOX alone and the GO-203/DOX combination. The dose-effect curves for GO-203 alone (+), DOX alone (8) and GO-203/DOX (X) are shown in the left panel. The multiple effect-level isobologram analysis is shown in the right panel for the ED90 (solid circles), ED75 (solid squares) and ED50 (solid triangles). The CI values are listed in .
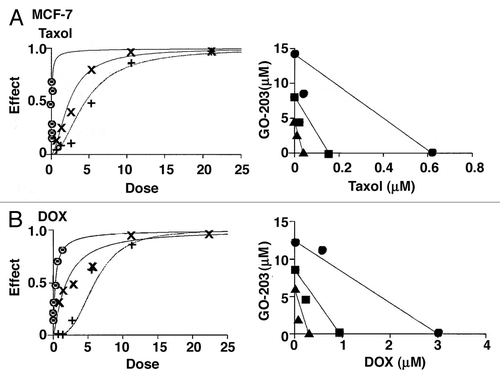
Table 2. Combination indices
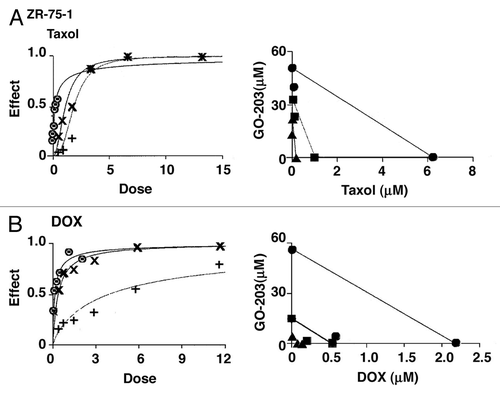
Similar studies were performed in ZR-75-1 cells using GO-203 alone, taxol alone and the combination (, left). A synergistic interaction was confirmed by isobologram analysis (, right) and demonstration of CIs that ranged from 0.73–0.80 (). Moreover, the GO-203/DOX combination in the treatment of ZR-75-1 cells was found to be highly synergistic with CI values of 0.34–0.59 (, left and right and ). These results support a synergistic interaction by combining GO-203 with taxol and DOX in the treatment of MCF-7 and ZR-75-1 cells.
Figure 7. GO-203 is synergistic in combination with taxol and DOX in the ZR-75-1 cell model. (A) ZR-75-1 cells were exposed to fixed IC50 ratios of GO-203 alone, taxol alone and the GO-203/taxol combination. The dose-effect curves for GO-203 alone (+), taxol alone (8) and GO-203/taxol (X) are shown in the left panel. The multiple effect-level isobologram analysis is shown in the right panel for the ED90 (solid circles), ED75 (solid squares) and ED50 (solid triangles). The CI values are listed in . (B) ZR-75-1 cells were treated with fixed IC50 ratios of GO-203 alone, DOX alone and the GO-203/DOX combination. The dose-effect curves for GO-203 alone (+), DOX alone (8) and GO-203/DOX (X) are shown in the left panel. The multiple effect-level isobologram analysis is shown in the right panel for the ED90 (solid circles), ED75 (solid squares) and ED50 (solid triangles). The CI values are listed in .
Discussion
MUC1-C inhibition is effective in enhancing the cell death response to taxol and DOX
The overexpression of MUC1, as found in human breast cancer cells, is sufficient to block the apoptotic response to oxidative stress,Citation21 hypoxiaCitation22 and glucose deprivation.Citation23 Overexpression of MUC1 also blocks the induction of apoptosis in the response of cells to treatment with genotoxic drugs.Citation4,Citation5,Citation10,Citation17 These findings invoked the possibility that MUC1 inhibitors could be effective in combination with certain anticancer agents. Cell-penetrating peptides and small molecules have been identified that block dimerization and thereby function of the pro-survival MUC1-C subunit.Citation12-Citation14 These MUC1-C inhibitors are effective in attenuating growth and inducing predominantly necrotic death of breast cancer cells.Citation12,Citation13 However, to date, MUC1-C inhibitors have not been studied for their potential to interact in a positive way with approved anticancer drugs. To examine this possibility, we selected taxol and DOX, both of which are widely used in the treatment of breast cancer. Taxol stabilizes microtubules and thereby disrupts microtubule-dependent structures that are required for mitosis. Treatment of breast cancer cells with the MUC1-C inhibitor, GO-203 and taxol was associated with an apoptotic response, as determined by cells with sub-G1 DNA content, which was greater than that achieved with either agent alone. Similar results were obtained with DOX, an anthracycline that intercalates in DNA and induces strand breaks. In concert with these results, we found that (1) activation of the effector caspase-7 was increased to a greater extent with the GO-203/taxol and GO-203/DOX combinations than that observed with these agents alone, and (2) the apoptotic response is attenuated by a pan-caspase inhibitor. In concert with these results and the effects of GO-203 alone on necrotic cell death,Citation12,Citation15,Citation16 PI/annexin V staining demonstrated that combining GO-203 with taxol and DOX increases a late apoptotic/necrotic response. These findings indicate that GO-203 may potentiate cell death by both apoptotic and necrotic mechanisms. GO-203 also induces death of lung and prostate cancer cellsCitation15,Citation16; thus, other studies will be needed to determine whether inhibiting MUC1-C in these cell types is effective in combination with anticancer agents.
Why would MUC1 play a role in blocking the death response to genotoxic anticancer agents?
The MUC1 heterodimer functions in physically protecting the single-cell epithelial layer from various forms of stress.Citation1 Additionally, the MUC1-C subunit localizes to the nucleus of breast cancer cells, where it interacts with transcription factors, such as TCF7L2, and promotes growth and survival.Citation24,Citation25 MUC1-C also localizes to the mitochondrial outer membrane, where it blocks loss of the mitochondrial transmembrane potential and activation of the intrinsic apoptotic pathway.Citation10 The mechanistic basis for such an anti-apoptotic effect resides, at least in part, in direct binding of MUC1-C to the BH3 domain of the pro-apoptotic BAX protein.Citation26 The interaction between MUC1-C and BAX is induced in the response of breast cancer cells to genotoxic agents, such as DOX, and blocks BAX dimerization and BAX-mediated release of mitochondrial cytochrome c.Citation26 Notably, binding of MUC1-C to BAX is disrupted by GO-203 in vitro and in breast cancer cells.Citation26 Thus, treatment with GO-203 alone can promote an apoptotic response and conceivably potentiate that induced by genotoxic agents.Citation26 Indeed, the present results show that inhibition of MUC1-C is highly synergistic with taxol and DOX. These observations are supported by the demonstration that the treatment with the GO-203/taxol combination is associated with CIs of less than 1. Similar effects were found with the GO-203/DOX combination, indicating that targeting the MUC1-C subunit may enhance the therapeutic index of taxol or DOX. Such findings may also be applicable to other classes of anticancer agents. In that sense, MUC1-C protects against the apoptotic response to the (1) alkylating agent, cisplatin, (2) topoisomerase inhibitor, etoposide, (3) anti-metabolite, cytosine arabinoside and (4) microtubule-disrupting agent, vinblastine.Citation4,Citation5,Citation10,Citation17 Thus, blocking MUC1-C function may as well be additive or synergistic with these agents by potentiating the apoptotic or necrotic death response. GO-203 is presently under Phase I study in patients with refractory solid tumors. With establishment of the maximum tolerated GO-203 dose, the present findings provide support for evaluating GO-203 in combination with taxol and DOX for the treatment of patients with breast cancer.
Materials and Methods
Cell culture
Human MCF-7 and ZR-75-1 breast cancer cells were obtained from the American Type Culture Collection (ATCC) and passaged for less than 6 mo with replacement from early passage frozen stocks. No further authentication was performed. MCF-7 cells were grown in DMEM medium supplemented with 10% heated-inactivated FBS (HyClone), 100 units/ml penicillin, 100 g/ml streptomycin. ZR-75-1 cells were cultured in ATCC RPMI 1640 medium containing 10% FBS and antibiotics. Cells were treated with GO-203 (AnaSpec; dissolved in PBS), taxol (Sigma-Aldrich), doxorubicin (DOX) (Sigma-Aldrich) and Z-VAD-FMK (BioVision).
Analysis of cell cycle distribution and death
Cells were fixed with 70% ethanol and incubated in PBS containing RNase (Roche Diagnostics) and propidium iodide (PI; Sigma-Aldrich) as described.Citation12 Cell cycle distribution and sub-G1 DNA content were determined by flow cytometry. Cells were also incubated with PI/annexin V-FITC (Invitrogen) for 15 min and then analyzed by flow cytometry.
Immunoblot analysis
Cell lysates were prepared as described.Citation12 Soluble cytosolic proteins were immunoblotted with anti-caspase-7, anti-caspase-3 (Cell Signaling Technology) and anti–actin (Sigma-Aldrich). Soluble whole-cell proteins were immunoblotted with anti-poly-ADP ribose polymerase (PARP; Cell Signaling Technology) and anti–actin. Reactivity was detected with horseradish peroxidase-conjugated secondary antibodies and chemiluminescence (Perkin-Elmer Life Sciences).
Determination of IC50 and synergism
Cells were seeded on 96-well plates in 100 μl of growth medium at a density of 4,000 cells per well. After 24 h, cells were exposed to drugs in treatment medium. GO-203 was added every 24 h. After incubation for 72 h, cell viability was assessed using the alamar blue viability assay (Invitrogen). The percentage of viable cells was normalized to medium background. Triplicate wells of each treatment were analyzed and each experiment was done three times. The IC50 for each treatment for each cell line was determined by nonlinear regression of the dose-response data using Prism 5.0 for Mac OSX (GraphPad Software). The presence or absence of synergism between GO-203 and taxol or DOX was determined using CalcuSyn software (Biosoft). Briefly, MCF-7 and ZR-75-1 cells were exposed to 1:1 ratios of the respective IC50 values for GO-203 and taxol or DOX at 1/4 x IC50, 1/2 x IC50, IC50, 2 x IC50 and 4 x IC50. The combination index (CI) was calculated to determine the presence of synergism of CI (CI < 1) or antagonism.
| Abbreviations: | ||
| MUC1 | = | mucin 1 |
| MUC1-C | = | MUC1 C-terminal subunit |
| DOX | = | doxorubicin |
| PARP | = | poly-ADP ribose polymerase |
| CI | = | combination index |
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers CA97098 and CA166480. D.K. holds equity in Genus Oncology and is a consultant to the company. The other authors disclosed no potential conflicts of interest.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009; 9:874 - 85; http://dx.doi.org/10.1038/nrc2761; PMID: 19935676
- Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene 2012; In press http://dx.doi.org/10.1038/onc.2012.158; PMID: 22580612
- Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell 2007; 27:992 - 1004; http://dx.doi.org/10.1016/j.molcel.2007.07.031; PMID: 17889671
- Raina D, Kharbanda S, Kufe D. The MUC1 oncoprotein activates the anti-apoptotic PI3K/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem 2004; 279:20607 - 12; http://dx.doi.org/10.1074/jbc.M310538200; PMID: 14999001
- Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 2005; 7:167 - 78; http://dx.doi.org/10.1016/j.ccr.2005.01.008; PMID: 15710329
- Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, et al. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Res 2009; 69:7013 - 21; http://dx.doi.org/10.1158/0008-5472.CAN-09-0523; PMID: 19706766
- Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene 2003; 22:6107 - 10; http://dx.doi.org/10.1038/sj.onc.1206732; PMID: 12955090
- Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, et al. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem 2007; 282:19321 - 30; http://dx.doi.org/10.1074/jbc.M703222200; PMID: 17500061
- Wei X, Xu H, Kufe D. Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. Cancer Res 2007; 67:1853 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-06-3063; PMID: 17308127
- Ren J, Agata N, Chen D, Li Y, Yu W-H, Huang L, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 2004; 5:163 - 75; http://dx.doi.org/10.1016/S1535-6108(04)00020-0; PMID: 14998492
- Ren J, Bharti A, Raina D, Chen W, Ahmad R, Kufe D. MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene 2006; 25:20 - 31; PMID: 16158055
- Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T, et al. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res 2009; 69:5133 - 41; http://dx.doi.org/10.1158/0008-5472.CAN-09-0854; PMID: 19491255
- Zhou J, Rajabi H, Kufe D. MUC1-C oncoprotein is a target for small molecule inhibitors. Mol Pharm 2011; 79:886 - 93; http://dx.doi.org/10.1124/mol.110.070797
- Raina D, Ahmad R, Rajabi H, Panchamoorthy G, Kharbanda S, Kufe D. Targeting cysteine-mediated dimerization of the MUC1-C oncoprotein in human cancer cells. Int J Oncol 2012; 40:1643 - 9; PMID: 22200620
- Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, et al. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther 2011; 10:806 - 16; http://dx.doi.org/10.1158/1535-7163.MCT-10-1050; PMID: 21421804
- Joshi MD, Ahmad R, Yin L, Raina D, Rajabi H, Bubley G, et al. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol Cancer Ther 2009; 8:3056 - 65; http://dx.doi.org/10.1158/1535-7163.MCT-09-0646; PMID: 19887552
- Raina D, Ahmad R, Kumar S, Ren J, Yoshida K, Kharbanda S, et al. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. EMBO J 2006; 25:3774 - 83; http://dx.doi.org/10.1038/sj.emboj.7601263; PMID: 16888623
- Amm HM, Zhou T, Steg AD, Kuo H, Li Y, Buchsbaum DJ. Mechanisms of drug sensitization to TRA-8, an agonistic death receptor 5 antibody, involve modulation of the intrinsic apoptotic pathway in human breast cancer cells. Mol Cancer Res 2011; 9:403 - 17; http://dx.doi.org/10.1158/1541-7786.MCR-10-0133; PMID: 21357440
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22:27 - 55; http://dx.doi.org/10.1016/0065-2571(84)90007-4; PMID: 6382953
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 2010; 70:440 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-09-1947; PMID: 20068163
- Yin L, Li Y, Ren J, Kuwahara H, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem 2003; 278:35458 - 64; http://dx.doi.org/10.1074/jbc.M301987200; PMID: 12826677
- Yin L, Kharbanda S, Kufe D. Mucin 1 oncoprotein blocks hypoxia-inducible factor 1alpha activation in a survival response to hypoxia. J Biol Chem 2007; 282:257 - 66; http://dx.doi.org/10.1074/jbc.M610156200; PMID: 17102128
- Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein promotes autophagy in a survival response to glucose deprivation. Int J Oncol 2009; 34:1691 - 9; PMID: 19424588
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res 2005; 65:10413 - 22; http://dx.doi.org/10.1158/0008-5472.CAN-05-2474; PMID: 16288032
- Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi M, et al. MUC1-C oncoprotein induces TCF7L2 activation and promotes cyclin D1 expression in human breast cancer cells. J Biol Chem 2012; 287:10703 - 13; http://dx.doi.org/10.1074/jbc.M111.323311; PMID: 22318732
- Ahmad R, Alam M, Rajabi H, Kufe D. The MUC1-C oncoprotein binds to the BH3 domain of the pro-apoptotic BAX protein and blocks BAX function. J Biol Chem 2012; 287:20866 - 75; http://dx.doi.org/10.1074/jbc.M112.357293; PMID: 22544745