Abstract
Common cancer is an age-related disease. Slow aging is associated with reduced and delayed carcinogenesis. Calorie restriction (CR), the most studied anti-aging intervention, prevents cancer by slowing down the aging process. Evidence is emerging that CR decelerates aging by deactivating MTOR (Target of Rapamycin). Rapamycin and other rapalogs suppress cellular senescence, slow down aging and postpone age-related diseases including cancer. At the same time, rapalogs are approved for certain cancer treatments. Can cancer prevention be explained by direct targeting of cancer cells? Or does rapamycin prevent cancer indirectly through slowing down the aging process? Increasing evidence points to the latter scenario.
Cancer is an Age-Related Disease
Common cancers such as lung, breast, prostate, colon, gastric, esophageal, pancreatic, thyroid, brain and certain leukemias, are age-related diseases, meaning that their incidence is dramatically increased with aging and about one third of elderly people die from cancer. In rapidly aging mammals such as mice, cancer develops by the age of one to two years. In humans, common cancers are delayed and occur toward the end of the life, too. Centenarians, people who live more than 100 years, are especially protected from cancer.Citation1-Citation3 In long-lived mice, cancer is delayed.Citation4 In slow-aging naked mole-rats, cancer is uncommon despite high levels of oxidative damage.Citation5 Also CR slows down aging and delays cancer.Citation6-Citation14 In contrast, overeating, high caloric food and obesity accelerate cancer.Citation15
mTOR in Organismal Aging
So why do overeating, obesity and nutrients accelerate aging? One explanation is that nutrients and insulin activate the nutrient-sensing mTOR pathway. This pathway drives cellular mass growth.Citation16-Citation19 Growth factors, insulin and nutrients activate nutrient-sensing- and growth-promoting pathways, which in turn drive developmental growth. Later in life the same pathway drives aging, which is a continuation of developmental growth.Citation20-Citation22 In agreement, calorie restriction, which deactivates the TOR pathway, slows growth early in life and delays aging later in life.Citation10,Citation11 This is simple and straightforward. This predicts that rapamycin would slow down aging and extend life span.Citation23 In fact, rapamycin extends life span in yeast,Citation24 DrosophilaCitation25,Citation26 and mice.Citation27-Citation33 Also, genetic manipulations that decrease the activity of TOR in turn increase life span in diverse species.Citation34-Citation41
mTOR and Cellular Aging (Geroconversion)
In proliferating cells, growth factors (GFs) activate both the growth-promoting mTOR pathway and the cell cycle. Therefore cellular growth is balanced by cell division. When the cell cycle is blocked, yet mTOR is active, cells undergo gerogenic conversion or geroconversion.Citation42 At first, the arrested cell is not senescent. Overtime, the mTOR pathway converts arrest into senescence.Citation42-Citation44 Also activation of mTOR converts quiescence into senescence, when the cell cycle is still locked.Citation45-Citation47 This type of geroconversion may imitate physiological aging of post-mitotic cells in the organism. Aged cells are hypertrophic and hyper-functional.Citation48,Citation49 In turn, aging cells due to their hyper-functions cause diseases of aging such as obesity, pro-inflammatory syndrome, atherosclerosis, hypertension, neurodegeneration and osteoporosis.Citation50-Citation55It is very important to emphasize that geroconversion is not a transition from proliferation to arrest. Geroconversion is a transition from arrest and quiescence to senescence. In the young organism, post-mitotic cells are quiescent, becoming senescent over time. In theory, a proliferating cell may also undergo pro-gerogenic conversion. This condition may be manifested as cancer.
mTOR in Cancer
The PI3K/mTOR is almost universally activated in cancerCitation56-Citation62 and is a promising therapeutic target.Citation63-Citation78 The similarity between cancer and aging is not coincidental. Aging can be viewed as “twisted growth,” when actual growth is precluded.Citation43 Cancer is actual growth and proliferation of pro-gerogenic cells (it is sufficient to ensure cell cycle arrest and then gerogenic conversion will occur). Oncogenic proteins such as growth factor-receptors, activated Ras, tyrosine (Src) and serine/threonine kinases, such as Raf, MEK, PI3K, Akt, all activate mTOR. Inactivation of tumor suppressors such as PTEN, NF-1, TSC2 activate the mTOR pathway.Citation56-Citation62 Inactivation of some tumor suppressors (Rb, p53 and p16) overcomes cell cycle arrest and prevents the senescent phenotype. P53 inhibits mTOR.Citation79-Citation81 Here it is important to emphasize that p53 not only causes arrest but also can suppress geroconversion (conversion from arrest to senescence), ensuring instead quiescence.Citation45,Citation82-Citation93 Therefore, p53 is so often inactivated in cancer cells, which can be viewed as proliferating senescent cells. In aging, the mTOR pathway is activated by signals from other cells and by feedback loops.Citation94 In cancer, the mTOR pathway is activated by mutations and other stable alterations in upstream oncoproteins/tumor suppressors. Multiple rounds of cellular proliferation and selection transform initially random mutations into non-random activation of the mTOR pathway. Growth-limiting conditions and senescence of normal cells provides such a selective advantage.Citation94-Citation97 Therapy further drives tumor progression.Citation98
Cancer Treatment with Rapamycin
Given the universal activation of the mTOR pathway in cancer, rapalogs are used for cancer treatment.Citation99-Citation102 Temsirolimus, a prodrug of rapamycin (Sirolimus), became the first rapalog approved for cancer treatment.Citation103 The uses of rapalogs in the therapies of cancer and other diseases are rapidly increasing.Citation104,Citation105 Hundreds if not thousands of clinical trials are under the way. Yet, rapalogs (rapamycin and its analogs) are not a panacea. Although effective for approved indications (otherwise the drugs would not be approved), rapalogs still play a modest role in cancer treatment. For one, rapalogs (rapamycin and its analogs) at relevant concentrations are not toxic drugs: they do not kill cells but rather they slow their growth. While this is a disadvantage of rapalogs as anticancer drugs, this is an advantage as cancer-preventive and anti-aging agents. Rapalogs can reverse cell senescence in cancer stroma,Citation106 potentially contributing to cancer treatment and prevention.Citation107,Citation108
Tumor Prevention by Rapalogs
While rapamycin and other rapalogs are modestly-potent anticancer drugs, they are effective cancer-preventive agents. Remarkably, their cancer-preventive effects were initially detected in humans. Patients, who received rapamycin due to renal transplantation, had a peculiar “side effect”: a decrease in cancer incidence.Citation109-Citation114 In cancer-prone mice, rapamycin dramatically delays cancer. For example, rapamycin decreased incidence and progression of tobacco carcinogen-induced lung tumors.Citation115 Also everolimus (rapalog), delayed tumor onset and progression in a transgenic mouse model of ovarian cancer.Citation116 Rapamycin inhibited multiple stages of tumor progression in a transgenic mouse model of HER2-positive breast cancer.Citation117 Rapamycin also prevents skin tumors induced by phorbol esters.Citation118 It was assumed that rapalogs delay cancer by targeting cancer cells directly. Yet, there were some indications that the effect might be indirect via targeting normal cells. Lkb1(+/−) mice were treated with rapamycin from the age of 8 weeks, well before the onset of polyposis. This decreased the number of gastric tumors. In the polyps from the treated mice, phosphorylation of S6 kinase (a marker of mTOR activity) was maintained.Citation119 Still, in these studies, the effect of rapamycin on overall longevity and rate of aging was not determined. When the rate of aging was measured, it was revealed that rapamycin at least “formally” decreased the rate of aging and increased maximal longevity in transgenic HER-2/neu cancer-prone mice.Citation120
Indirect “Anti-Aging” Model
It was suggested that cancer could be prevented by inhibiting aging with rapamycin or “by staying young.”Citation121 Rapamycin decreased cancer incidence in normal (non-cancer-prone) genetically heterogeneous miceCitation27,Citation28 and inbred mice.Citation29 These studies have been initiated in order to evaluate the effect of rapamycin on aging not cancer. Although retrospectively one may argue that life span was extended due to cancer prevention, this argument could (or not) be used to explain the life extending effect of calorie restriction as well. It is clear that dramatic difference in lifespan between man and C. elegans is not due cancer, but instead due to the speed of development and aging. Also in order to delay cancer and extend life span in p53+/− mice, treatment with rapamycin should be started earlier in life before tumors develop.Citation122 Perhaps rapamycin is less effective when cancer had developed already. CR delays cancer in p53−/− mice.Citation123-Citation125
How to Distinguish between Two Models of Cancer Prevention
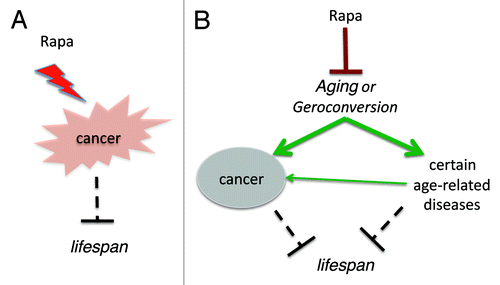
Two models of cancer prevention are very difficult to distinguish. One may believe that rapamycin prolongs life span by decreasing cancer (). Yet, I believe that rapamycin prevents cancer by suppressing aging (). It may be helpful to evaluate rapamycin in strains of mice with low tumor incidences. This might confirm that rapamycin increases life span independently from cancer prevention. In fact, it is already known that rapamycin delays most age-related diseases in rodents,Citation126,Citation32 including age-related retinopathyCitation127 and age-related obesity.Citation128 Second, we can test the cancer-preventive effect of rapamycin at low doses that do not inhibit cancer cell growth. Other approaches might be suggested by the readers of this paper (please address as letter to the editor).
Figure 1. Two models of cancer-prevention by rapalogs. (A) Direct anticancer effect. Rapalogs (Rapa) suppress cancer cells, prevent cancer and thus extend lifespan. (B) Indirect anticancer effect due to aging-suppression. Rapalogs (Rapa) suppress aging (gerosuppression) and thus prevent cancer and other age-related diseases, extending lifespan.
Rapalogs for Cancer Prevention
For cancer prevention via inhibiting the aging process, rapamycin (and other rapalogs) could be used at lower doses in order to target normal cells, which are very sensitive to rapamycin.Citation129 There is no need to kill any cells. Furthermore, there is no need to inhibit mTOR completely: it could be inhibited just slightly to slow down aging. Second, administration of rapamycin can be intermittent, like intermittent fasting. In low doses or intermittent schedules, rapalogs may have no side effects. Rapamycin prevents cancer in p53-/+ mice, which could be viewed as a model of Li-Fraumeni syndrome. Currently, there is no clinically-available therapy to prevent cancer in patients with Li-Fraumeni syndrome.Citation130-Citation132 Definitely, there is an excellent opportunity to start cancer prevention by rapalogs.
Selective Protection of Normal Cells from Therapy Induced Cell Death and Senescence
Numerous studies have demonstrated that rapalogs potentiate chemotherapy against cancer cells. In theory, rapalogs could protect normal cells from chemotherapy and to increase therapeutic index.Citation63 In cell culture, rapamycin in combination with the p53-inducing agent Nutlin-3 and metformin can selectively protect normal cells from chemotherapy.Citation133-Citation135 Remarkably, in mice rapamycin prevented epithelial stem cell senescence and protects from radiation-induced mucositis.Citation136, Citation137 Like rapamycin, fasting inhibits the mTOR pathway. Fasting also increases therapeutic window and decreases side effects chemotherapy in animals and humans.Citation138-Citation141
Conclusions
There are several lines of reasoning suggesting that the effects of rapamycin on cancer prevention are indirect. First, while rapalogs (as monotherapy) are relatively modest anti-cancer drugs, these drugs are potent cancer-preventive and anti-aging agents. Second, typical anti-cancer drugs do not and cannot be used for cancer prevention. For example, radiation, paclitaxel and doxorubicin, just to mention a few, have no cancer-preventive effects in animals. In contrast, these treatments tend to cause secondary cancer in both animals and humans. Direct anticancer drugs are rather carcinogenic. Third, an anti-aging effect is sufficient to explain cancer-preventive effects. Yet, we might never indisputably distinguish between the anti-cancer and anti-aging effects of rapamycin because both cancer and aging share the activation of a common signaling pathway, and this pathway is targeted by rapamycin.
References
- Hitt R, Young-Xu Y, Silver M, Perls T. Centenarians: the older you get, the healthier you have been. Lancet 1999; 354:652; http://dx.doi.org/10.1016/S0140-6736(99)01987-X; PMID: 10466675
- Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci 2003; 58:232 - 7; http://dx.doi.org/10.1093/gerona/58.3.M232; PMID: 12634289
- Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci 2012; 67:395 - 405; http://dx.doi.org/10.1093/gerona/glr223; PMID: 22219514
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci 2003; 58:291 - 6; http://dx.doi.org/10.1093/gerona/58.4.B291; PMID: 12663691
- Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 2005; 60:1369 - 77; http://dx.doi.org/10.1093/gerona/60.11.1369; PMID: 16339321
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science 2010; 328:321 - 6; http://dx.doi.org/10.1126/science.1172539; PMID: 20395504
- Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci 2010; 31:89 - 98; http://dx.doi.org/10.1016/j.tips.2009.11.004; PMID: 20097433
- Sharp ZD. Aging and TOR: interwoven in the fabric of life. Cell Mol Life Sci 2011; 68:587 - 97; http://dx.doi.org/10.1007/s00018-010-0542-0; PMID: 20960025
- Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci 2010; 65:695 - 703; http://dx.doi.org/10.1093/gerona/glq042; PMID: 20371545
- Blagosklonny MV. Linking calorie restriction to longevity through sirtuins and autophagy: any role for TOR. Cell Death Dis 2010; 1:e12; http://dx.doi.org/10.1038/cddis.2009.17; PMID: 21364614
- Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans). Cell Cycle 2010; 9:683 - 8; http://dx.doi.org/10.4161/cc.9.4.10766; PMID: 20139716
- Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011; 3:1051 - 62; PMID: 22166724
- Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2010; 2:361 - 8; PMID: 20606248
- Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY) 2011; 3:374 - 9; PMID: 21483032
- Blagosklonny MV. NCI’s provocative questions on cancer: some answers to ignite discussion. Oncotarget 2011; 2:1352 - 67; PMID: 22267462
- Inoki K, Guan KL. Complexity of the TOR signaling network. Trends Cell Biol 2006; 16:206 - 12; http://dx.doi.org/10.1016/j.tcb.2006.02.002; PMID: 16516475
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124:471 - 84; http://dx.doi.org/10.1016/j.cell.2006.01.016; PMID: 16469695
- Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol 2011; 23:744 - 55; http://dx.doi.org/10.1016/j.ceb.2011.09.003; PMID: 21963299
- Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009; 1:357 - 62, 20; PMID: 20157523
- Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 2010; 9:3151 - 6; http://dx.doi.org/10.4161/cc.9.16.13120; PMID: 20724817
- Blagosklonny MV. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging (Albany NY) 2010; 2:265 - 73; PMID: 20519781
- Blagosklonny MV. Why the disposable soma theory cannot explain why women live longer and why we age. Aging (Albany NY) 2010; 2:884 - 7; PMID: 21191147
- Blagosklonny MV. Rapamycin and quasi-programmed aging: four years later. Cell Cycle 2010; 9:1859 - 62; http://dx.doi.org/10.4161/cc.9.10.11872; PMID: 20436272
- Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev 2006; 20:174 - 84; http://dx.doi.org/10.1101/gad.1381406; PMID: 16418483
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 2010; 11:35 - 46; http://dx.doi.org/10.1016/j.cmet.2009.11.010; PMID: 20074526
- Moskalev AA, Shaposhnikov MV. Pharmacological inhibition of phosphoinositide 3 and TOR kinases improves survival of Drosophila melanogaster. Rejuvenation Res 2010; 13:246 - 7; http://dx.doi.org/10.1089/rej.2009.0903; PMID: 20017609
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460:392 - 5; PMID: 19587680
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011; 66:191 - 201; http://dx.doi.org/10.1093/gerona/glq178; PMID: 20974732
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 2011; 10:4230 - 6; http://dx.doi.org/10.4161/cc.10.24.18486; PMID: 22107964
- Khanna A, Kapahi P. Rapamycin: killing two birds with one stone. Aging (Albany NY) 2011; 3:1043 - 4; PMID: 22170738
- Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011; 3:1039 - 40; PMID: 22147496
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell 2012; 11:675 - 82; http://dx.doi.org/10.1111/j.1474-9726.2012.00832.x; PMID: 22587563
- Spong A, Bartke A. Rapamycin slows aging in mice. Cell Cycle 2012; 11:845; http://dx.doi.org/10.4161/cc.11.5.19607; PMID: 22356747
- Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005; 310:1193 - 6; http://dx.doi.org/10.1126/science.1115535; PMID: 16293764
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 2004; 14:885 - 90; http://dx.doi.org/10.1016/j.cub.2004.03.059; PMID: 15186745
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009; 326:140 - 4; http://dx.doi.org/10.1126/science.1177221; PMID: 19797661
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta 2009; 1790:1067 - 74; http://dx.doi.org/10.1016/j.bbagen.2009.06.007; PMID: 19539012
- Lee JH, Bodmer R, Bier E, Karin M. Sestrins at the crossroad between stress and aging. Aging (Albany NY) 2010; 2:369 - 74; PMID: 20606249
- Ha CW, Huh WK. The implication of Sir2 in replicative aging and senescence in Saccharomyces cerevisiae. Aging (Albany NY) 2011; 3:319 - 24; PMID: 21415463
- Pani G. P66SHC and ageing: ROS and TOR?. Aging (Albany NY) 2010; 2:514 - 8; PMID: 20689155
- Stipp D. A new path to longevity. Sci Am 2012; 306:32 - 9; http://dx.doi.org/10.1038/scientificamerican0112-32; PMID: 22279832
- Blagosklonny MV. Cell cycle arrest is not senescence. Aging (Albany NY) 2011; 3:94 - 101; PMID: 21297220
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle 2008; 7:3355 - 61; http://dx.doi.org/10.4161/cc.7.21.6919; PMID: 18948731
- Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle 2009; 8:1896 - 900; http://dx.doi.org/10.4161/cc.8.12.8809; PMID: 19478560
- Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany, NY Online) 2010; 2:892 - 3
- Wesierska-Gadek J. mTOR and its link to the picture of Dorian Gray—reactivation of mTOR promotes aging. Aging 2010; 2:924 - 35; PMID: 21212465
- Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle 2012; 11:2391 - 401; http://dx.doi.org/10.4161/cc.20683; PMID: 22627671
- Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle 2009; 8:1883 - 7; http://dx.doi.org/10.4161/cc.8.12.8815; PMID: 19448395
- Gems DH, Guardia YD. Alternative Perspectives on Aging in Caenorhabditis elegans: Reactive Oxygen Species or Hyperfunction?. Antioxid Redox Signal 2012; http://dx.doi.org/10.1089/ars.2012.4840; PMID: 22870907
- Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle 2009; 8:4055 - 9; http://dx.doi.org/10.4161/cc.8.24.10310; PMID: 19923900
- Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol 2012; 181:1142 - 6; http://dx.doi.org/10.1016/j.ajpath.2012.06.024; PMID: 22841821
- Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans 2011; 39:460 - 5; http://dx.doi.org/10.1042/BST0390460; PMID: 21428920
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol 2012; 22:R741 - 52; http://dx.doi.org/10.1016/j.cub.2012.07.024; PMID: 22975005
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell 2012; 11:326 - 35; http://dx.doi.org/10.1111/j.1474-9726.2011.00791.x; PMID: 22212527
- Tacutu R, Budovsky A, Yanai H, Fraifeld VE. Molecular links between cellular senescence, longevity and age-related diseases - a systems biology perspective. Aging (Albany NY) 2011; 3:1178 - 91; PMID: 22184282
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004; 10:789 - 99; http://dx.doi.org/10.1038/nm1087; PMID: 15286780
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57 - 70; http://dx.doi.org/10.1016/S0092-8674(00)81683-9; PMID: 10647931
- Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell 2002; 13:2276 - 88; http://dx.doi.org/10.1091/mbc.01-12-0584; PMID: 12134068
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell 2007; 12:487 - 502; http://dx.doi.org/10.1016/j.devcel.2007.03.020; PMID: 17419990
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 2007; 13:252 - 9; http://dx.doi.org/10.1016/j.molmed.2007.04.002; PMID: 17452018
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 2010; 40:310 - 22; http://dx.doi.org/10.1016/j.molcel.2010.09.026; PMID: 20965424
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12:21 - 35; http://dx.doi.org/10.1038/nrm3025; PMID: 21157483
- Blagosklonny MV, Darzynkiewicz Z. Four birds with one stone: RAPA as potential anticancer therapy. Cancer Biol Ther 2002; 1:359 - 61; http://dx.doi.org/10.4161/cbt.1.4.6; PMID: 12432246
- Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther 2003; 2:Suppl 1 S169 - 77; PMID: 14508096
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 2006; 6:184 - 92; http://dx.doi.org/10.1038/nrc1819; PMID: 16453012
- Dbouk HA, Backer JM. A beta version of life: p110β takes center stage. Oncotarget 2010; 1:729 - 33; PMID: 21321382
- Hart JR, Vogt PK. Phosphorylation of AKT: a mutational analysis. Oncotarget 2011; 2:467 - 76; PMID: 21670491
- Garrett JT, Chakrabarty A, Arteaga CL. Will PI3K pathway inhibitors be effective as single agents in patients with cancer?. Oncotarget 2011; 2:1314 - 21; PMID: 22248929
- Bhattacharya B, Akram M, Balasubramanian I, Tam KK, Koh KX, Yee MQ, et al. Pharmacologic synergy between dual phosphoinositide-3-kinase and mammalian target of rapamycin inhibition and 5-fluorouracil in PIK3CA mutant gastric cancer cells. Cancer Biol Ther 2012; 13:34 - 42; http://dx.doi.org/10.4161/cbt.13.1.18437; PMID: 22336586
- Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget 2010; 1:530 - 43; PMID: 21317449
- Major P. Potential of mTOR inhibitors for the treatment of subependymal giant cell astrocytomas in tuberous sclerosis complex. Aging (Albany NY) 2011; 3:189 - 91; PMID: 21415462
- Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget 2010; 1:89 - 103; PMID: 20671809
- Li H, Gao Q, Guo L, Lu SH. The PTEN/PI3K/Akt pathway regulates stem-like cells in primary esophageal carcinoma cells. Cancer Biol Ther 2011; 11:950 - 8; http://dx.doi.org/10.4161/cbt.11.11.15531; PMID: 21467840
- Barger JF, Gallo CA, Torni KA, Merk L, Seibel WL, Nelson S, et al. Identification of Akt-selective cytotoxic compounds that enhance cytotoxic responses to rapamycin. Cancer Biol Ther 2011; 10:1256 - 61; PMID: 20935504
- Schmidt-Kittler O, Zhu J, Yang J, Liu G, Hendricks W, Lengauer C, et al. PI3Kα inhibitors that inhibit metastasis. Oncotarget 2010; 1:339 - 48; PMID: 21179398
- Sokolosky ML, Stadelman KM, Chappell WH, Abrams SL, Martelli AM, Stivala F, et al. Involvement of Akt-1 and mTOR in sensitivity of breast cancer to targeted therapy. Oncotarget 2011; 2:538 - 50; PMID: 21730367
- Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther 2006; 5:1065 - 73; http://dx.doi.org/10.4161/cbt.5.9.3175; PMID: 16969122
- Weber GL, Parat MO, Binder ZA, Gallia GL, Riggins GJ. Abrogation of PIK3CA or PIK3R1 reduces proliferation, migration, and invasion in glioblastoma multiforme cells. Oncotarget 2011; 2:833 - 49; PMID: 22064833
- Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A 2005; 102:8204 - 9; http://dx.doi.org/10.1073/pnas.0502857102; PMID: 15928081
- Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev 2006; 20:267 - 75; http://dx.doi.org/10.1101/gad.1363206; PMID: 16452501
- Matthew EM, Hart LS, Astrinidis A, Navaraj A, Dolloff NG, Dicker DT, et al. The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions. Cell Cycle 2009; 8:4168 - 75; http://dx.doi.org/10.4161/cc.8.24.10800; PMID: 20054236
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A 2010; 107:9660 - 4; http://dx.doi.org/10.1073/pnas.1002298107; PMID: 20457898
- Leontieva OV, Gudkov AV, Blagosklonny MV. Weak p53 permits senescence during cell cycle arrest. Cell Cycle 2010; 9:4323 - 7; http://dx.doi.org/10.4161/cc.9.21.13584; PMID: 21051933
- Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY). 2010;2:344-352.
- Blagosklonny MV. The power of chemotherapeutic engineering: arresting cell cycle and suppressing senescence to protect from mitotic inhibitors. Cell Cycle 2011; 10:2295 - 8; http://dx.doi.org/10.4161/cc.10.14.16819; PMID: 21715978
- Maki CG. Decision-making by p53 and mTOR. Aging (Albany NY) 2010; 2:324 - 6; PMID: 20603526
- Serrano M. Shifting senescence into quiescence by turning up p53. Cell Cycle 2010; 9:4256 - 7; http://dx.doi.org/10.4161/cc.9.21.13785; PMID: 20980826
- Darzynkiewicz Z. Another “Janus paradox” of p53: induction of cell senescence versus quiescence. Aging (Albany NY) 2010; 2:329 - 30; PMID: 20603525
- Galluzzi L, Kepp O, Kroemer G. TP53 and MTOR crosstalk to regulate cellular senescence. Aging (Albany NY) 2010; 2:535 - 7; PMID: 20876940
- Chao SK, Horwitz SB, McDaid HM. Insights into 4E-BP1 and p53 mediated regulation of accelerated cell senescence. Oncotarget 2011; 2:89 - 98; PMID: 21399233
- Lane DP, Verma C, Fang CC. The p53 inducing drug dosage may determine quiescence or senescence. Aging (Albany NY) 2010; 2:748; PMID: 21068468
- Poyurovsky MV, Prives C. P53 and aging: A fresh look at an old paradigm. Aging (Albany NY) 2010; 2:380 - 2; PMID: 20657036
- Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010; 2:471 - 4; PMID: 20729567
- Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011; 3:1130 - 41; PMID: 22246147
- Blagosklonny MV. Oncogenic resistance to growth-limiting conditions. Nat Rev Cancer 2002; 2:221 - 5; http://dx.doi.org/10.1038/nrc743; PMID: 11990858
- Greaves M. Leukemogenesis and ageing: 'fit for transformation'?. Aging (Albany, NY Online) 2011; 3:79 - 80
- Henry CJ, Marusyk A, DeGregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what’s the connection?. Aging (Albany NY) 2011; 3:643 - 56; PMID: 21765201
- Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell 2004; 5:13 - 7; http://dx.doi.org/10.1016/S1535-6108(03)00336-2; PMID: 14749122
- Garber K. Rapamycin’s resurrection: a new way to target the cancer cell cycle. J Natl Cancer Inst 2001; 93:1517 - 9; http://dx.doi.org/10.1093/jnci/93.20.1517; PMID: 11604470
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4:335 - 48; http://dx.doi.org/10.1038/nrc1362; PMID: 15122205
- Choo AY, Blenis J. TORgeting oncogene addiction for cancer therapy. Cancer Cell 2006; 9:77 - 9; http://dx.doi.org/10.1016/j.ccr.2006.01.021; PMID: 16473275
- Kwitkowski VE, Prowell TM, Ibrahim A, Farrell AT, Justice R, Mitchell SS, et al. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist 2010; 15:428 - 35; http://dx.doi.org/10.1634/theoncologist.2009-0178; PMID: 20332142
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al, Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356:2271 - 81; http://dx.doi.org/10.1056/NEJMoa066838; PMID: 17538086
- Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget 2011; 2:510 - 7; PMID: 21680954
- Sacco A, Roccaro A, Ghobrial IM. Role of dual PI3/Akt and mTOR inhibition in Waldenstrom’s Macroglobulinemia. Oncotarget 2010; 1:578 - 82; PMID: 21317453
- Mercier I, Camacho J, Titchen K, Gonzales DM, Quann K, Bryant KG, et al. Caveolin-1 and accelerated host aging in the breast tumor microenvironment: chemoprevention with rapamycin, an mTOR inhibitor and anti-aging drug. Am J Pathol 2012; 181:278 - 93; http://dx.doi.org/10.1016/j.ajpath.2012.03.017; PMID: 22698676
- Lewis DA, Travers JB, Machado C, Somani AK, Spandau DF. Reversing the aging stromal phenotype prevents carcinoma initiation. Aging (Albany NY) 2011; 3:407 - 16; PMID: 21515933
- Smit MA, Peeper DS. Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging (Albany NY) 2010; 2:735 - 41; PMID: 20975209
- Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004; 18:446 - 9; http://dx.doi.org/10.1111/j.1399-0012.2004.00188.x; PMID: 15233824
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med 2005; 352:1317 - 23; http://dx.doi.org/10.1056/NEJMoa042831; PMID: 15800227
- Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol 2006; 17:581 - 9; http://dx.doi.org/10.1681/ASN.2005090993; PMID: 16434506
- Cullis B, D’Souza R, McCullagh P, Harries S, Nicholls A, Lee R, et al. Sirolimus-induced remission of posttransplantation lymphoproliferative disorder. Am J Kidney Dis 2006; 47:e67 - 72; http://dx.doi.org/10.1053/j.ajkd.2006.01.029; PMID: 16632009
- Yakupoglu YK, Buell JF, Woodle S, Kahan BD. Individualization of immunosuppressive therapy. III. Sirolimus associated with a reduced incidence of malignancy. Transplant Proc 2006; 38:358 - 61; http://dx.doi.org/10.1016/j.transproceed.2006.01.019; PMID: 16549120
- Zmonarski SC, Boratyńska M, Rabczyński J, Kazimierczak K, Klinger M. Regression of Kaposi’s sarcoma in renal graft recipients after conversion to sirolimus treatment. Transplant Proc 2005; 37:964 - 6; http://dx.doi.org/10.1016/j.transproceed.2004.12.172; PMID: 15848592
- Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, et al. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res 2007; 13:2281 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-06-2570; PMID: 17404113
- Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res 2007; 67:2408 - 13; http://dx.doi.org/10.1158/0008-5472.CAN-06-4490; PMID: 17363557
- Mosley JD, Poirier JT, Seachrist DD, Landis MD, Keri RA. Rapamycin inhibits multiple stages of c-Neu/ErbB2 induced tumor progression in a transgenic mouse model of HER2-positive breast cancer. Mol Cancer Ther 2007; 6:2188 - 97; http://dx.doi.org/10.1158/1535-7163.MCT-07-0235; PMID: 17699716
- Checkley LA, Rho O, Moore T, Hursting S, DiGiovanni J. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev Res (Phila) 2011; 4:1011 - 20; http://dx.doi.org/10.1158/1940-6207.CAPR-10-0375; PMID: 21733825
- Robinson J, Lai C, Martin A, Nye E, Tomlinson I, Silver A. Oral rapamycin reduces tumour burden and vascularization in Lkb1(+/-) mice. J Pathol 2009; 219:35 - 40; http://dx.doi.org/10.1002/path.2562; PMID: 19434632
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 2010; 176:2092 - 7; http://dx.doi.org/10.2353/ajpath.2010.091050; PMID: 20363920
- Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther 2008; 7:1520 - 4; http://dx.doi.org/10.4161/cbt.7.10.6663; PMID: 18769112
- Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, et al. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging (Albany, NY Online) 2012; 4
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 2002; 23:817 - 22; http://dx.doi.org/10.1093/carcin/23.5.817; PMID: 12016155
- Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci U S A 1994; 91:7036 - 40; http://dx.doi.org/10.1073/pnas.91.15.7036; PMID: 8041741
- Hursting SD, Perkins SN, Brown CC, Haines DC, Phang JM. Calorie restriction induces a p53-independent delay of spontaneous carcinogenesis in p53-deficient and wild-type mice. Cancer Res 1997; 57:2843 - 6; PMID: 9230186
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 2006; 5:2087 - 102; http://dx.doi.org/10.4161/cc.5.18.3288; PMID: 17012837
- Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol 2012; 181:472 - 7; http://dx.doi.org/10.1016/j.ajpath.2012.04.018; PMID: 22683466
- Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 2012; 75:425 - 36; http://dx.doi.org/10.1016/j.neuron.2012.03.043; PMID: 22884327
- Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: preliminary proposal. Cell Cycle 2010; 9:4788 - 94; http://dx.doi.org/10.4161/cc.9.24.14360; PMID: 21150328
- Sherif ZA, Sultan AS. Divergent control of Cav-1 expression in non-cancerous Li-Fraumeni syndrome and human cancer cell lines. Cancer Biol Ther 2012; 14:11; PMID: 23114650
- Herbert BS, Chanoux RA, Liu Y, Baenziger PH, Goswami CP, McClintick JN, et al. A molecular signature of normal breast epithelial and stromal cells from Li-Fraumeni syndrome mutation carriers. Oncotarget 2010; 1:405 - 22; PMID: 21311097
- Glazer RI, et al. A new therapeutic basis for treating Li-Fraumeni Syndrome breast tumors expressing mutated TP53. Oncotarget 2010; 1:470 - 1; PMID: 21317445
- Darzynkiewicz Z, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Novel strategies of protecting non-cancer cells during chemotherapy: are they ready for clinical testing?. Oncotarget 2011; 2:107 - 8; PMID: 21487161
- van Leeuwen IM, Laín S. Pharmacological manipulation of the cell cycle and metabolism to protect normal tissues against conventional anticancer drugs. Oncotarget 2011; 2:274 - 6; PMID: 21512204
- Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 2012; 11:401 - 14; http://dx.doi.org/10.1016/j.stem.2012.06.007; PMID: 22958932
- Iglesias-Bartolome R, Gutkind JS. Exploiting the mTOR paradox for disease prevention. Oncotarget 2012; 3:1061 - 3; PMID: 23085637
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009; 1:988 - 1007; PMID: 20157582
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 2010; 70:1564 - 72; http://dx.doi.org/10.1158/0008-5472.CAN-09-3228; PMID: 20145127
- Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle 2010; 9:4474 - 6; http://dx.doi.org/10.4161/cc.9.22.13954; PMID: 21088487
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 2012; 4:24ra27; http://dx.doi.org/10.1126/scitranslmed.3003293; PMID: 22323820
- Blagosklonny MV. Carcinogenesis, cancer therapy and chemoprevention. Cell Death Differ 2005; 12:592 - 602; http://dx.doi.org/10.1038/sj.cdd.4401610; PMID: 15818400