Abstract
Current therapies for Renal Cell Carcinoma favor vascular endothelial growth factor receptor (VEGF-R) tyrosine kinase (TK) inhibitors (TKIs). In theory, these are most applicable in tumors that have lost VHL-with subsequent stabilization of HIF and upregulation of VEGF. A subset of patients harbor primary-refractory disease, as in this case, where there was no evidence for loss of VHL or chromosome 3p. We evaluated molecular targeted agents in viable tumor cells cultured from a patient’s clear cell renal cell carcinoma (RCC). Of 66 agents, only dasatinib, an inhibitor of Src tyrosine kinase, strongly reduced viability of the patient’s cultured kidney tumor cells. Immunostaining of the original primary tumor revealed strong positivity for VHL and Src protein expression. Functional evaluation of a patient’s tumor cells appears feasible in the setting of RCC.
Introduction
The success of imatinib in chronic myelogenous leukemia launched an era of rationally-designed molecular cancer therapies.Citation1 Standard of care in gastrointestinal stromal tumors, malignant melanoma, colorectal cancer, and lung cancer have been significantly altered by the development of therapies that target aberrations in signaling pathways resulting from oncogenic mutations. Since 2005, treatment of metastatic RCC has undergone a remarkable transformation with the availability of multiple effective new agents including VEGF-R TKIs, mTOR inhibitors, and anti-VEGF monocolonal antibody in combination with interferon. Despite the availability of these agents and their ability to induce partial responses and to stabilize disease, long-term survival remains poor due to nearly inevitable development of resistance. Some tumors, as in this case, appear to be primary refractory to currently available therapies. Effective therapeutics are particularly needed in this population of patients. Additionally, treatment of metastatic RCC remains relatively empiric, with no predictive markers yet established for choosing a therapy for an individual patient.
Recently, leukemia cells from individual patients are being subjected to functional assays of panels of small molecules that are already approved or in development for use in humans, in order to uncover sensitivities and potentially identify new targets for validation.Citation2-Citation4 Such a targeted approach remains in its infancy for epithelial solid tumors. We herein demonstrate the feasibility of identifying agents that could be effective in RCC differentially from VEGF-R inhibitors, through direct effects on tumor-derived cells.
Case Report
A 61-y-old male presented with gross hematuria. Imaging revealed a left renal mass and he underwent radical nephrectomy one month later, which revealed a 5.8 cm T1b clear cell renal cell carcinoma, Fuhrman grade 4. Distant metastatic workup was negative. Surveillance imaging at 5 mo after presentation revealed peritoneal nodules and mediastinal lymphadenopathy. Biopsy of an omental nodule was consistent with metastatic renal cell carcinoma. He began treatment with the VEGF-R TKI pazopanib at 6 mo, but received limited exposure due to significant transaminitis requiring dose interruption and reduction. Follow-up imaging at 7 mo revealed tumor progression. He began second line systemic therapy with the mTOR inhibitor everolimus at 8 mo, but follow-up imaging at 10 mo revealed progressive disease. The patient was aware of a publication regarding the potential utility of the Src inhibitor dasatinib in RCC.Citation5 He elected third line treatment with dasatinib which he began at 11 mo. CT scan after 6 weeks showed progressive disease with enlargement of pre-existing tumors and development of liver metastases. Despite subsequent treatment with the VEGF-R TKI sunitinib, the patient developed worsening symptomatic progression of metastatic disease, and died approximately one year and one month after his diagnosis.
Results and Discussion
Out of 66 agents tested in the initial screens (), Ki-CA tumor cells exhibited hypersensitivity only to dasatinib (hypersensitivity defined based on IC50 for Ki-CA that is 5-fold lower than the median IC50 observed for 150 other primary tumor specimens).Citation4 This was supported by response to PP2 (over 3-fold lower), recognized as a canonical Src inhibitor.
Figure 1. Functional assays of Ki-CA patient tumor derived cells’ response to molecular targeted small-molecule kinase inhibitors. Two thousand cells in medium without EGF were added to 96-well plates containing each small-molecule inhibitor at four serial dilutions spanning a concentration range that includes the predicted IC50, incubated at 37°C for three days and assayed by MTS (Promega). The viability data were adjusted for wells with no cells/inhibitor, and normalized to untreated wells of the cultured cells (OD value for wells with cells without drug treatment = 100% cell viability) and to a database of cell lines and tumor samplesCitation4 to yield responses and the IC50 in nM for each agent. IC50 values less than 20% of the global median (shown as 100%) are considered responses and are well within clinically achievable concentrations.
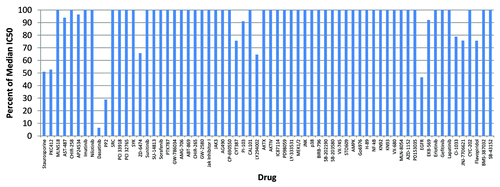
Dasatinib has been tested in the clinic, and it is approved for resistant chronic myelogenous leukemia (CML). While both dasatinib and its parent drug imatinib inhibit cAbl kinase, imatinib was not effective, suggesting pathways discordant between dasatinib and imatinib are driving growth of the Ki-CA tumor, one of which is the Src kinase, affected by dasatinib but not imatinib.
Further dose response studies were performed with dasatinib; inhibitors targeting epidermal growth factor receptor including EKB-569 and lapatinib because of slight differences in response of cells cultured in the presence (data not shown) or absence of EGF; the mTOR inhibitor rapamycin, belonging to the same class as everolimus, because it is used as second line therapy in RCC patients; and pazopanib,Citation6 a TKI used in first line treatment of RCC, including in this patient ().
Figure 2. Dose responses of Ki-CA patient derived cell line to selected drugs. Cells were added to 96-well plates containing each small-molecule inhibitor at 11 serial dilutions spanning a concentration range that includes the predicted IC50 and evaluated as in . Values shown are mean ± SD for triplicate wells. Best fit curves were generated using GraphPad Prism software.
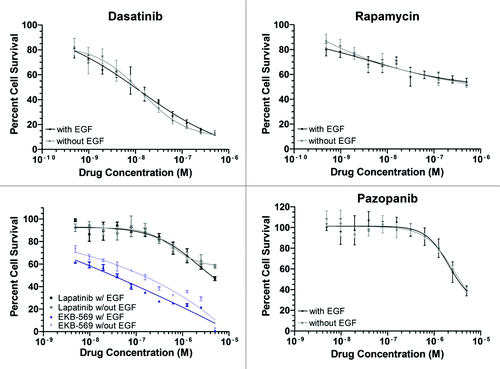
The dasatinib IC50 in Ki-CA cells was 10nM compared with 401 nM globally established median IC50. IC50s for the irreversible inhibitor of EGFR EKB-569 was 40 nM in the absence of EGF compared with 2193 nM globally established median.Citation4 The reversible EGFR targeted agent lapatinib was ineffective, with IC50 of 6476 nM in the absence of EGF, 3-fold higher than in the presence of EGF. Ki-CA cells showed detectable sensitivity to rapamycin at the lowest dose tested, with an IC20 of 0.4 nM; however rapamycin barely reached an IC50 at 500 nM, which is less than the globally established median. Rapamycin inhibits mTOR, a regulator of hypoxia inducible factor (HIF). Hif1a/2a is commonly activated in RCC, associated with loss/inactivation of the VHL gene that occurs in ~60% of all RCC, most of which are the clear cell type, and providing the rationale for treatment of RCC with angiogenesis inhibitors. Treatments used in RCC showed no effect, such as sunitinib, a multitargeted TKI of VEGF-R (Ki-CA IC50 equivalent to globally established median 1000nM), and pazopanibCitation6 (10,000 nM equivalent to globally established median). However the inhibitor assays are expected to detect tumor cell-autonomous effects, or effects not requiring the complex tumor environment, such as anti-angiogenesis targeted by VEGF-R TKIs.
Functional assessment of cell lines derived from the primary tumor in this case showed no direct anti-tumor cell effects of pazopanib and everolimus (rapamycin), used in first and second line treatment of this patient. Consistent with efficacy of dasatinib in the Ki-CA cells, immunohistochemistry performed on formalin fixed paraffin embedded (FFPE) sections of the primary Ki-CA tumor revealed strong positivity for Src and VHL (), characterizing this patient’s tumor as one of a subset of RCC that is VHL positive/Src positive. Src/VHL positivity are two features of a subset of ~20% of 346 patient RCC specimens, recently associated with dasatinib efficacy through a Src and VHL-dependent mechanism in human RCC cell lines and xenografts in mice.Citation5 Sensitivity to dasatinib has also been reported in established RCC cell lines.Citation7 In keeping with the positive immunohistochemical staining of VHL shown in , chromosomal analysis supports that VHL is still present (see Materials and Methods).
Figure 3. Primary Ki-CA patient tumor immunohistochemistry. Tumor exhibited both Src positivity (A) and VHL positivity (B). Power: 20 × ; Scale bar: 20 μM
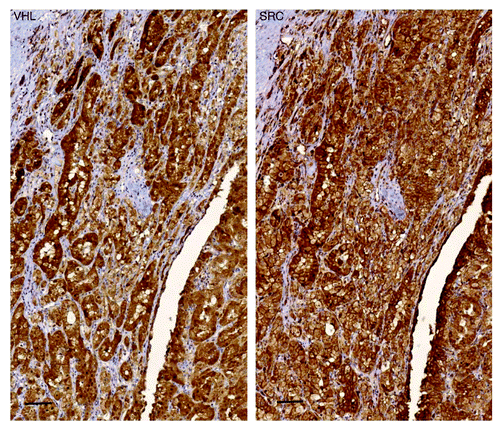
Clinical trials that are designed to investigate the predictive value of immunohistochemical and (epi)genetic signatures are of high interest. Given that Src is associated with invasion and metastasis, trials of dasatinib as first or second line therapy, or as adjuvant therapy based upon functionally and immunohistochemically-defined predictive signatures are worthy of consideration.
The long latencies of RCC and most solid tumors suggest etiology through accumulation of multiple oncological events, such as deregulated cell cycle control and cell death mechanisms, ability to invade, travel through and extravasate from blood and lymph vessels and establish in inappropriate sites throughout the body, implying complexity both intrinsic and extrinsic to the epithelial tumor cells themselves.Citation8,Citation9 Given the multi-factor etiology of solid tumors, it is possible that single agents may have limited potential, and rational combinations of therapies may be required for effective treatment. Mechanistic support for combination treatment comes from a study showing that transcription factors EGFR, Src and the signal transducer and activator of transcription (Stat)3 proteins form a heterotrimeric complex on the c-myc promoter in pancreatic cancer Panc-1 and Colo-357 cell lines. Functional concurrent inhibition of any two of these TKs (Stat3 and EGFR, Stat3 and Src, or EGFR and Src), but not any one alone, strongly suppressed c-Myc expression.Citation10 Our dose response studies suggested sensitivity to irreversible EGFR inhibition in the Ki-CA tumor cells. Numerous clinical trials of EGFR inhibitors in RCC have failed to show meaningful activity.Citation11 Preclinical RCC models suggest that the order of administration of the EGFR inhibitor or whether the inhibitor was reversible or irreversible determined its effects in combination with the proteasome inhibitor bortezomib, dependent upon efficient blockade of constitutive nuclear factor kappa B (NFκB) activity.Citation12 Irreversible EGFR inhibitor MP-412 or CI-1033 but not reversible inhibitors could induce ubiquitination, internalization, and degradation of ErbB2 (a member of the same family of receptors as EGFR) in several human breast cancer cell lines.Citation13,Citation14
The study of tumor autonomous effects in cell culture may require broadening to assess more complex tumor biology that may affect responses to molecular targeted agents, such as assessment of tumor-derived stromal cells and the patient’s metabolic status, circulating cytokines and other factors that may interfere with tumor autonomous sensitivity.Citation9 Ongoing studies at our institute are directed at defining engineered culture cell environments that predict tumor responses to current and promising new molecular targeted therapies for RCC, and could have the collateral benefit of defining conditions for RCC progenitor cell culture and characterization (James Korkola, personal communication).
The present study used specimens from the primary RCC tumor for functional analysis. Currently tissue samples from metastatic disease judged as inoperable are limited or unavailable. Characterization of primary cells by cell culture could be validated for relevance to treatment of metastatic disease by functional assessment and comparison of immunohistochemical or (epi)genetic signatures in metastatic tissue samples, including upon autopsy.
Integration of functional studies and rational molecular assays should be prospectively studied in the setting of RCC and may accelerate progress toward stratification of patients for molecular targeted drug therapies beneficial to them. New agents are being continuously added to the panel of inhibitors that promise to expand options for RCC treatment, and help to guide pre-clinical and clinical studies for RCC treatment.
Materials and Methods
The kidney cancer primary tumor (designated Ki-CA) cells were initiated by explant outgrowth culture in DMEM/F-12 medium with 15% iron-supplemented bovine calf serum (Hyclone), antibiotic/antimycotic (Invitrogen), 0.4ug/ml hydrocortisone and 10ng/ml EGF by Jim Rheinwald of the Harvard Skin Disease Research Center, Boston, essentially as described.Citation15-Citation17 Chromosomal analysis of Ki-CA tumor derived cells revealed abnormal karyotypes such as trisomies 7 and 10 and loss of Y (data not shown) reportedly common in renal cell carcinomaCitation18,Citation19 and renal tissue,Citation18 but no detectable loss of chromosome 3p, a common feature in RCC associated with VHL loss/inactivation. Cells at passage 4, with or without 10 ng/ml EGF were plated for functional screens and dose response studies. Functional screens of small molecules, mostly tyrosine kinase inhibitors, were performed and evaluated for ability to reduce survival of Ki-CA cells according to methods established for leukemias.Citation2-Citation4 Drugs selected for follow-up dose response studies were dasatinib (Sprycel, LC Labs), EKB-569 (Pelitinib, Exclusive Chemistry), rapamycin (rapamycin-like compound everolimus Afinitor, LC Labs), lapatinib (Tykerb, LC Labs), and pazopanib (Votrient, LC Labs). Immunostaining for Src, VHL and EGFR was done as describedCitation5 using commercially available antibodies (Src: Cell Signaling Technologies; VHL: BD Biosciences; EGFR: Dako).
| Abbreviations: | ||
| VEGF-R | = | vascular endothelial growth factor receptor |
| TKI | = | tyrosine kinase inhibitor |
| VHL | = | von Hippel-Lindau tumor suppressor |
| HIF | = | hypoxia-inducible factor |
| RCC | = | renal cell carcinoma |
| mTOR | = | mammalian target of rapamycin |
| CT | = | computed tomography |
| IC50 | = | half maximal inhibitory concentration |
| CML | = | chronic myelogenous leukemia |
| EGFR | = | epithelial growth factor receptor |
| Ki-CA | = | kidney cancer primary tumor cells |
Disclosure of Potential Conflicts of Interest
C.R. communicates potential conflicts of interest with regard to his role as consultant for Aveo, GSK, Bayer and Onyx and as a consultant and honoraria for Pfizer and Novartis. No potential conflicts of interest were disclosed by other authors.
Acknowledgments
We acknowledge the patient, and Dr. James Rheinwald of the Harvard Skin Diseases Research Center. We are also grateful to Dr. Toni Choueiri, Dr. Christopher Doyle, Dr. Michelle Hirsch and Dr. Jeanne Shen and the Brigham and Women’s Hospital. Aaron Wortham is a predoctoral fellow of the National Institutes of Health under award number T32CA106195. The OHSU Knight Cancer Institute is supported by P30 CA069533.
References
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344:1031 - 7; http://dx.doi.org/10.1056/NEJM200104053441401; PMID: 11287972
- Tyner JW, Erickson H, Deininger MW, Willis SG, Eide CA, Levine RL, et al. High-throughput sequencing screen reveals novel, transforming RAS mutations in myeloid leukemia patients. Blood 2009; 113:1749 - 55; http://dx.doi.org/10.1182/blood-2008-04-152157; PMID: 19075190
- Tyner JW, Fletcher LB, Wang EQ, Yang WF, Rutenberg-Schoenberg ML, Beadling C, et al. MET receptor sequence variants R970C and T992I lack transforming capacity. Cancer Res 2010; 70:6233 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-10-0429; PMID: 20670955
- Tyner JW, Yang WF, Bankhead A, Fan G, Fletcher LB, Bryant J, et al. Kinase Pathway Dependence in Primary Human Leukemias Determined by Rapid Inhibitor Screening. Cancer Res 2012; http://dx.doi.org/10.1158/0008-5472.CAN-12-1906; PMID: 23087056
- Suwaki N, Vanhecke E, Atkins KM, Graf M, Swabey K, Huang P, et al. A HIF-regulated VHL-PTP1B-Src signaling axis identifies a therapeutic target in renal cell carcinoma. Sci Transl Med 2011; 3:85ra47; http://dx.doi.org/10.1126/scitranslmed.3002004; PMID: 21632985
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010; 28:1061 - 8; http://dx.doi.org/10.1200/JCO.2009.23.9764; PMID: 20100962
- Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012; 483:570 - 5; http://dx.doi.org/10.1038/nature11005; PMID: 22460902
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309 - 22; http://dx.doi.org/10.1016/j.ccr.2012.02.022; PMID: 22439926
- Jaganathan S, Yue P, Paladino DC, Bogdanovic J, Huo Q, Turkson J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells. PLoS One 2011; 6:e19605; http://dx.doi.org/10.1371/journal.pone.0019605; PMID: 21573184
- Dancey JE. Epidermal growth factor receptor and epidermal growth factor receptor therapies in renal cell carcinoma: do we need a better mouse trap?. J Clin Oncol 2004; 22:2975 - 7; http://dx.doi.org/10.1200/JCO.2004.04.934; PMID: 15210735
- An J, Rettig MB. Epidermal growth factor receptor inhibition sensitizes renal cell carcinoma cells to the cytotoxic effects of bortezomib. Mol Cancer Ther 2007; 6:61 - 9; http://dx.doi.org/10.1158/1535-7163.MCT-06-0255; PMID: 17237266
- Akbulut T, Regner KR, Roman RJ, Avner ED, Falck JR, Park F. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol Renal Physiol 2009; 297:F662 - 70; http://dx.doi.org/10.1152/ajprenal.00146.2009; PMID: 19570883
- Suzuki T, Fujii A, Ochi H, Nakamura H. Ubiquitination and downregulation of ErbB2 and estrogen receptor-alpha by kinase inhibitor MP-412 in human breast cancer cells. J Cell Biochem 2011; 112:2279 - 86; http://dx.doi.org/10.1002/jcb.23147; PMID: 21503962
- Kulesz-Martin MF, McLimans WF. Steady-state cultures of human skin. Exp Cell Biol 1982; 50:195 - 206; PMID: 6749572
- Rheinwald JG, Jorgensen JL, Hahn WC, Terpstra AJ, O’Connell TM, Plummer KK. Mesosecrin: a secreted glycoprotein produced in abundance by human mesothelial, endothelial, and kidney epithelial cells in culture. J Cell Biol 1987; 104:263 - 75; http://dx.doi.org/10.1083/jcb.104.2.263; PMID: 3543023
- Rheinwald JG, O’Connell TM. Intermediate filament proteins as distinguishing markers of cell type and differentiated state in cultured human urinary tract epithelia. Ann N Y Acad Sci 1985; 455:259 - 67; http://dx.doi.org/10.1111/j.1749-6632.1985.tb50416.x; PMID: 3866508
- Casalone R, Granata Casalone P, Minelli E, Portentoso P, Righi R, Meroni E, et al. Significance of the clonal and sporadic chromosome abnormalities in non-neoplastic renal tissue. Hum Genet 1992; 90:71 - 8; http://dx.doi.org/10.1007/BF00210747; PMID: 1427791
- van den Berg E, van der Hout AH, Oosterhuis JW, Störkel S, Dijkhuizen T, Dam A, et al. Cytogenetic analysis of epithelial renal-cell tumors: relationship with a new histopathological classification. Int J Cancer 1993; 55:223 - 7; http://dx.doi.org/10.1002/ijc.2910550210; PMID: 8370620