Abstract
Background: Aberrant expression of the RON receptor tyrosine kinase is associated with tumor progression and carcinogenesis. The aims of this study were to determine the role and functional mechanisms of RON in Burkitt lymphoma (BL) and to document its potential as a therapeutic target. Methods: RON expression was determined in BL cell lines by western blot analysis and examined in human lymphoma specimens by both western blotting and immunohistochemistry. The correlation between RON expression and Epstein-Barr virus (EBV) infection was investigated. Raji cells were treated with the Zt/f2 anti-RON mAb and cell viability, colony formation, apoptosis and cell cycle arrest were measured in vitro using cell proliferation assays, colony-forming assays and flow cytometry. Downregulation of RON by Zt/f2 was validated in mice bearing Raji cell xenografts.
Results: Immunohistostaining showed a high frequency of RON+ cells in BL tissues and RON expression strongly correlated with EBV positivity. RON downregulation significantly decreased cell proliferation and colony formation via promotion of apoptosis and cell cycle arrest in Raji cells. The in vivo study showed that RON knockdown inhibits the tumorigenic potential of Raji cells in nude mice.
Conclusions: RON acts as an oncogene in the carcinogenesis and progression of BL and is therefore a potential target for therapeutic intervention.
Background
Burkitt’s lymphoma (BL) is a highly aggressive B-cell non-Hodgkin’s lymphoma (non-HL); it is the fastest growing human tumor and its etiology is thought to be multifactorial.Citation1 The Epstein-Barr virus (EBV) is known to play an important role in BL.Citation2 The EBV latent membrane protein 1 (LMP1) is considered to be the major EBV oncogene, as it is essential for B-lymphocyte transformation.Citation3 All forms of BL are characterized by a reciprocal chromosomal translocation between the Myc proto-oncogene and one of three immunoglobulin genes (Ig-Myc). The resulting deregulation of Myc has the dual effect of inducing cell proliferation and apoptosis.Citation4 In recent years, aberrant tyrosine kinase (TK) activities have been identified as a further pathogenic mechanism for B-cell lymphoma. Several studies revealed that RON is highly expressed in HL, suggesting that RON is involved in the pathogenesis of HL.Citation5,Citation6
RON belongs to the Met family of receptor tyrosine kinases (RTKs).Citation7 RON is a heterodimeric glycoprotein composed of a transmembrane β chain (which has TK activity) and a short extracellular α chain, linked by a single disulfide bond.Citation8 The RON ligand was identified as macrophage-stimulating protein (MSP), a member of the plasminogen-related growth factor family.Citation9 Induction of RON phosphorylation and kinase activity can be achieved through ligand-dependent and -independent mechanisms.Citation10 Aberrant RON expression has been implicated in the carcinogenesis and progression of many cancers, including those of the breast, colon and thyroid.Citation11-Citation13 Activated RON induces the activation of multiple oncogenic signaling pathways involved in cell growth, migration, apoptosis and survival,Citation14 including the mitogen-activated protein kinase (MAPK) pathway, the AKT pathway and the β-catenin-Myc pathway.Citation15-Citation17 LMP1-induced RON activation has been reported to mediate B-cell proliferation.Citation18 We found that RON is aberrantly overexpressed in BL. However, it was unclear whether RON plays an important role in the pathogenesis of BL and thus whether it could represent a target for therapeutic intervention.
The present study evaluated whether RON regulates tumor cell behavior and oncogenic signaling pathways in BL. The in vivo potential of RON as a drug target was also studied in a xenograft model. Through a series of experiments, we found that RON is highly expressed in BL tissues and its expression correlates with EBV positivity. RON knockdown significantly decreased cellular proliferation and colony formation in vitro by inducing apoptosis and G1-phase cell cycle arrest. In vivo analysis showed that treatment with a specific mAb suppresses Raji cell xenograft growth in mice and extends tumor latency. We investigated the potential mechanisms controlling apoptosis and cell cycle arrest and found that MSP-induced RON phosphorylation activates downstream signaling proteins, including Akt and ERK1/2. In contrast, RON knockdown inhibits signaling through these pathways.
Results
Distribution and expression of RON in lymphomas
We first analyzed RON expression in human leukemia/lymphoma cell lines and clinical specimens by western blotting. Our results showed that the Raji BL and L428 Hodgkin’s lymphoma cell lines expressed levels of RON protein similar to those found in tumor tissues (). We next investigated RON expression in different lymphoid tumor tissues by immunohistochemical (IHC) staining using a high-density tissue chip (). We found positive RON staining in about half of the BL and HL samples, in contrast to low or absent expression in normal lymph nodes and other lymphoma tissues. Semi-quantitative analysis of RON overexpression revealed that scores of ≥ 6 were only observed in BL and HL samples (). We also found a significant positive correlation between RON overexpression and EBV infection (). Among BL and HL cases, the percentage of RON+ cells was significantly higher in EBV+ cases compared with EBV- cases. These results demonstrate that there is significant heterogeneity in RON expression in lymphomas, with overexpression occurring in BL and HL. In addition, RON overexpression strongly correlated with EBV positivity.
Figure 1. RON is highly expressed in Burkitt’s lymphoma (BL) and Hodgkin’s lymphoma (HL) tissues and cells. (A) Western blots show RON expression in various human leukemia/lymphoma cell lines. Cellular protein samples (50 µg) were subjected to western blot analysis using rabbit immunoglobulin G to the RON C-terminus (R5029). 3T3-RON cells were used as positive controls. Actin served as a loading control. (B) RON expression in clinical samples was detected by western blot analysis. Lane 7, Burkitt’s lymphoma specimen; lanes 10 and 17, 3T3-RON cells; lanes 11 and 16, Hodgkin’s lymphoma specimens. (C) RON immunostaining and EBV in situ hybridization (ISH) in BL and HL lymphoma tissues. Upper panel, cases in which routine histological diagnosis was determined by H&E staining; middle panel, representative RON immunohistochemical (IHC) staining of BL and HL tissue samples; bottom panel, strong nuclear EBV ISH in BL and HL samples by EBER. Images were obtained using a BK40 Olympus microscope (magnification, ×40).
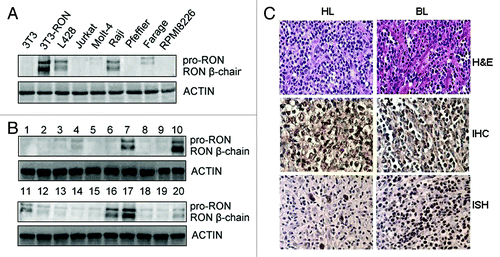
Table 1. RON expression in various types of lymphoma tissue, as assessed by immunohistochemical staining
Table 2. Correlation between RON overexpression (percentage) and EBV infection cases in BL and HL
RON regulates growth and survival in Raji cells
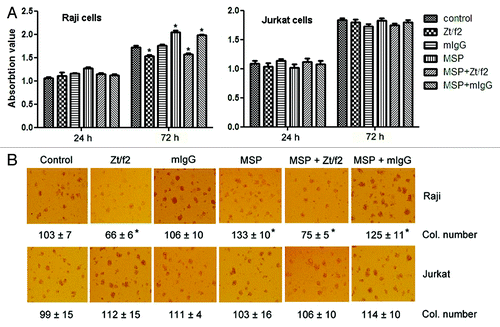
To explore the role of RON in cell proliferation, we used the MTT assay to analyze Raji and Jurkat cell proliferation following RON inhibition. Treatment of RON+ Raji cells with Zt/f2 for three days moderately inhibited cell growth compared with control or MSP-stimulated cells. Similarly, Zt/f2 moderately inhibited MSP-induced cell proliferation. However, there was no significant difference in the proliferation of Zt/f2- or MSP-treated RON˗ Jurkat cells (). Colony formation assays using Raji cells and Jurkat cells showed a clear increase in colony numbers in MSP-stimulated Raji cells, and Zt/f2 treatment moderately reduced the numbers of both spontaneous and MSP-stimulated colonies (). No such effect was observed in Jurkat cells. Our results suggest that RON regulates cell proliferation and migration and that RON overexpression may contribute to cancer development.
Figure 2. Effect of Zt/f2 treatment on Raji cell proliferation and survival in vitro. (A) Raji cell viability following Zt/f2 treatment was determined using an MTT assay. Exponentially growing cells were seeded into 96-well plates and triplicate samples were treated with macrophage-stimulating protein (MSP; 2 nM) in the presence or absence of Zt/f2 (2 nM) or control mouse immunoglobulin G (2 nM). Cell proliferation was determined using an MTT assay at different time points. Jurkat cells were used as negative controls. (B) Effect of Zt/f2 on Raji cell colony formation in methylcellulose. Cells (1 × 103/well) were seeded onto 0.8% methylcellulose and 20% FBS/RPMI-1640 in triplicate in 24-well plates and then MSP (2 nM), Zt/f2 (2 nM) or both were added. Jurkat cells were used as negative controls. Colony (Col.) numbers (for colonies containing ≥30 cells) were counted after 10 d; #, *, p < 0.05 vs. control group at 72 h.
To study the molecular mechanism(s) by which Zt/f2 inhibits Raji cell proliferation, we used flow cytometry to analyze the effects of Zt/f2 treatment on apoptosis and cell cycle progression. We observed that apoptosis rates increased moderately and cell cycle progression was significantly inhibited at the G1 phase after treatment with Zt/f2 for 3 d (). Western blot analysis supported the flow cytometric findings by demonstrating that Zt/f2 treatment activates two key molecules in the apoptosis pathway, namely, caspase-3 and poly (ADP-ribose) polymerase (PARP), significantly decreased cyclin D1, CDK4 and CDK6 protein expression and increased P27 levels in Raji cells (). These data suggest that Zt/f2 promotes apoptosis and induces G1-phase cell cycle arrest. These changes were statistically significant compared with the control group.
Figure 3. Zt/f2 treatment induces apoptosis and cell cycle arrest. Raji cells were treated with macrophage-stimulating protein (MSP; 2 nM) in the presence or absence of Zt/f2 (2 nM) or control mouse immunoglobulin G (2 nM) for 72 h. (A) The proportion of early apoptotic cells in each quadrant is indicated. Data show that Zt/f2 treatment alone moderately increases the percentage of early apoptotic cells. A slight increase in the proportion of apoptotic cells is observed following combined MSP and Zt/f2 treatment. (B) Zt/f2 treatment induces G1-phase cell cycle arrest in Raji cells. (C) Zt/f2 regulates the expression of apoptosis-related proteins in Raji cells. Treatment of Raji cells with Zt/f2 downregulates anti-apoptotic proteins (Mcl-1 and XIAP) and upregulates pro-apoptotic proteins (caspases 3 and 9, and poly [ADP-ribose] polymerase [PARP]). (D) Western blot analysis shows that expression of cyclins and cyclin-dependent kinases (cyclin D1, CDK4 and CDK6) are significantly reduced and p27 expression is significantly increased in Zt/f2-treated Raji cells.
![Figure 3. Zt/f2 treatment induces apoptosis and cell cycle arrest. Raji cells were treated with macrophage-stimulating protein (MSP; 2 nM) in the presence or absence of Zt/f2 (2 nM) or control mouse immunoglobulin G (2 nM) for 72 h. (A) The proportion of early apoptotic cells in each quadrant is indicated. Data show that Zt/f2 treatment alone moderately increases the percentage of early apoptotic cells. A slight increase in the proportion of apoptotic cells is observed following combined MSP and Zt/f2 treatment. (B) Zt/f2 treatment induces G1-phase cell cycle arrest in Raji cells. (C) Zt/f2 regulates the expression of apoptosis-related proteins in Raji cells. Treatment of Raji cells with Zt/f2 downregulates anti-apoptotic proteins (Mcl-1 and XIAP) and upregulates pro-apoptotic proteins (caspases 3 and 9, and poly [ADP-ribose] polymerase [PARP]). (D) Western blot analysis shows that expression of cyclins and cyclin-dependent kinases (cyclin D1, CDK4 and CDK6) are significantly reduced and p27 expression is significantly increased in Zt/f2-treated Raji cells.](/cms/asset/30ca95b9-18fc-47ca-92e2-14e453cfbe8b/kcbt_a_10923718_f0003.gif)
RON regulates multiple signaling cascades in Raji cells
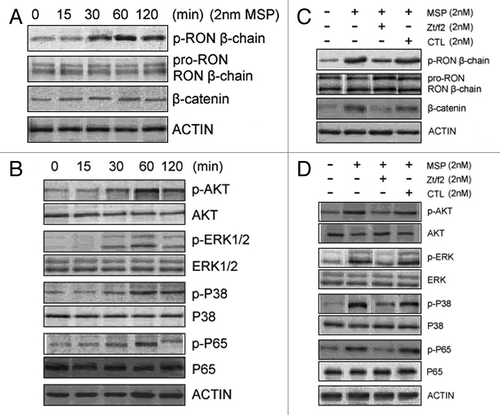
To explore the potential mechanisms involved in apoptosis and cell cycle arrest, we studied the role of RON in stimulating the intracellular signaling pathways leading to apoptosis and cell cycle arrest in Raji cells. RON was constitutively active in Raji cells and MSP stimulation further enhanced RON phosphorylation in a time-dependent manner (). In addition, β-catenin expression and Akt, ERK1/2, p38 and NF-kappa B phosphorylation increased in Raji cells after MSP treatment. However, the levels of total Akt, ERK1/2, p38 and NF-kappa B were unchanged (). In addition, β-catenin expression decreased in Raji cells. Combined treatment with MSP and Zt/f2 led to a clear decrease in Akt, Erk1/2, p38 and p65 phosphorylation ().
Figure 4. Zt/f2 inhibits RON phosphorylation and downstream signaling. (A, B) Macrophage-stimulating protein (MSP)-induces phosphorylation of RON and downstream signaling proteins in Raji cells. Cells were stimulated with MSP (2 nM) for different periods of time. Phosphorylation of RON at tyrosine residues was detected by western blotting with the mAb PY-100, which is specific for phospho-tyrosine residues. β-Catenin and phosphorylated/total Akt, ERK1/2, p38 and p65 were also measured by western blotting using specific antibodies. Actin was used as a loading control. (C, D) RON inhibition by Zt/f2 inhibits phosphorylation of Akt, Erk1/2, P38 and P65 in Raji cells. Cells were treated with 2 nM MSP, 2 nM Zt/f2 or both for 60 min followed by western blot analysis using antibodies specific to phosphorylated and total RON, Akt, Erk1/2, P38, P65 and β-catenin.
Therapeutic effect of Zt/f2 on Raji cell xenograft growth
The results from our in vitro studies prompted us to validate the therapeutic activity of Zt/f2 in vivo using a Raji cell xenograft tumor model. In this model, Zt/f2 treatment was started three days after cell inoculation. The tumor size increased in a time-dependent manner (growth index was 1.00 at day 45) in control mice. However, weekly tumor volume measurements indicated that Zt/f2 treatment significantly inhibited Raji cell tumor growth (); this effect could be observed as early as four days after Zt/f2 administration. An average of a 40% reduction in tumor volume was observed following Zt/f2 treatment, compared with tumors formed in control mice ().
Figure 5. Zt/f2 treatment inhibits Raji cell xenograft growth. (A) Zt/f2 treatment significantly suppresses the growth of Raji cell xenografts in athymic nude mice. Tumor-bearing mice were randomized into different groups (three mice per group). Animals were treated with Zt/f2, PBS or control mouse IgG at the doses and schedule defined in the Materials and Methods section. For each treatment group, the tumor volume was measured weekly. Tumor xenograft tissues were harvested after 7 weeks. (B) The tumor growth index in control mice was set to 1.00 and used to make comparisons among groups. (C, D) Immunohistochemical detection of TUNEL-positive and RON+ cells in tumor xenograft tissues. The percentage of positively stained cells in the tissues of each group is shown. TUNEL-positive and RON+ cells were counted in four or five different fields, the data are summarized as the mean of percentage positive cells in three xenograft samples. The proportion of apoptotic cells was significantly increased and there was a clear downregulation of RON in Zt/f2-treated samples. *, p < 0.05 vs. PBS control group.
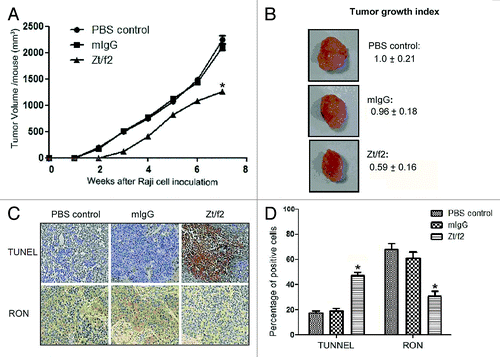
We further evaluated the effect of Zt/f2 treatment on the apoptotic index of tumor cells in xenograft samples using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Immunohistochemical analysis revealed a greater number of TUNEL-positive cells in xenograft samples from mice treated with Zt/f2 compared with those from control mice. Immunohistochemical analysis of xenograft samples revealed that the numbers of RON+ cells were lower in tumors from Zt/f2-treated mice than in controls ().
Discussion
RON is a typical RTK and was originally identified as a member of the c-MET proto-oncogene family. Aberrant expression of RON has been implicated in the pathogenesis of epithelial tumors.Citation19 Recent studies demonstrate that six RTKs, including RON, are expressed in Hodgkin/Reed-Sternberg HL cells and suggest that RON may be involved in the pathogenesis of HL.Citation20 Our results indicate that RON expression is significantly higher in BL and HL tissues than in normal lymph node and other lymphoma tissues. IHC staining showed that RON staining intensity and the number of positive cells are significantly higher in EBV+ patients than in EBV- patients. Therefore, there seems to be a close correlation between the expression of RON and EBV in the present study.
In most tumors with aberrant TK activity, usually one TK allele is aberrantly activated.Citation21 Deregulation of several EBV-regulated RTKs is well-documented in lymphoma;Citation22,Citation23 therefore, it is interesting to determine whether and how frequently RON is aberrantly overexpressed and activated in BL. Chou demonstrated that LMP1 induces RON activation in lymphoblastoid cell lines through NF-kappa β signaling.Citation24 Our next study will explore the precise mechanisms of EBV-induced RON activation in BL.
To elucidate the role of RON in BL tumorigenesis, we employed a loss of function approach via RON inhibition using the Zt/f2 mAb in Raji cells. Notably, we found that RON downregulation inhibited Raji cell proliferation. In addition, tight regulation of apoptosis and the cell cycle is crucial for correct development and homeostasis. A potential function of RON in apoptosis and cell cycle progression has been investigated in several cancer cell lines.Citation25-Citation27 Our experiments showed that RON knockdown causes G1 cell cycle arrest and increases apoptosis in Raji cells in vitro.
To explore the potential mechanisms mediating these effects, we studied the effect of altered RON activity on intracellular signaling pathways, apoptosis and cell cycle progression. Akt functions as a critical regulator of cell apoptosis and proliferation and is commonly deregulated in human malignancies.Citation28 Inhibition of the PI3K/Akt signaling pathway is reported to suppress growth and induce apoptosis in BL cell lines.Citation29 Moreover, the MAPK signaling cascade is activated by a wide variety of receptors during cell growth and differentiation. In addition, many studies suggest that activation of the β-catenin cascade mediates RON-induced tumorigenesis.Citation10,Citation15 We showed that RON downregulation not only blocks the Akt and MAPK signaling cascades, but also β-catenin expression. These data suggest that RON modulates the activity of mitogenic signaling cascades, thus influencing BL cell tumorigenicity.
Finally, we employed a xenograft nude mice model to investigate whether RON contributes to BL development in vivo and found that RON downregulation suppresses tumor growth and extends tumor latency in tumor-bearing mice. These data indicate that RON promotes the BL cell tumorigenicity in vivo.
In summary, we report that RON acts as an oncogene to promote BL carcinogenesis and functions by inhibiting apoptosis and cell cycle arrest. These findings indicate that inhibition of RON may provide a therapeutic benefit in BL.
Material and Methods
Cell lines and reagents
The Raji BL cell line, Molt-4 and Jurkat human T-cell lymphocytic leukemia cell lines, L428HL cell line, Farage and Pfeiffer diffuse large B-cell lymphoma cell lines, and the RPMI8226 multiple myeloma cell line were purchased from American Type Culture Collection. NIH3T3 cells expressing RON and mouse mAb specific to RON extracellular domains (Zt/g4 and Zt/f2)Citation30 were kindly supplied by Prof. Wang (Laboratory of Cancer Biology and Therapeutics, First Affiliated Hospital, Zhejiang University School of Medicine). RPMI-1640, fetal bovine serum (FBS), penicillin and streptomycin were purchased from GIBCO (Invitrogen Corporation). Human mature MSP was obtained from R&D Systems; 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and propidium iodide (PI) were purchased from Sigma Corporation. An Annexin V/PI Kit was purchased from KeyGen. The EBER detection kit was obtained from Triplex International Biosciences. All antibodies were purchased from Santa Cruz Biotechnology. All other reagents were procured locally.
Clinical samples and tissue microarray
We retrospectively collected 89 cases whose diagnosis was identified by pathology in the First Affiliated Hospital of College of Medicine of Zhejiang University during the period from 2009 to 2011, including seven inflammatory patients, 21 DLBCL patients, 15 T cell lymphoma patients, 3 BL patients, 17 HL patients, 15 chronic lymphocyte lymphoma patients, seven multiple myeloma patients and four normal cases. 125 cases of tissue microarrays were purchased from US Biomax and probed for RON using Zt/f2 as the primary antibody.
Immunohistochemistry
The tissue of microarray of RON expression was evaluated by immunohistochemistry (IHC). Following deparaffinization, endogenous peroxidase activity was inhibited by incubating tissue sections in 0.3% H202 for 10 min at room temperature. Following antigen retrieval, slides were rinsed in distilled water and then in phosphate buffered saline (PBS). All incubations were performed at room temperature. After incubation with the Zt/f2 primary antibody, the streptavidin peroxidase method was used for signal detection.Citation31 Diaminobenzidine tetrachloride (DAB) was applied for 10 min and then cells were counterstained with hematoxylin. All slides were scored by two independent observers in a blinded fashion. A manual scoring method was used to assess the levels of receptor expression. This method combines scores for the staining intensity (0–3) and the proportion of stained cells (0–4). A combined score ≥ 6 was considered to signify RON overexpression.Citation32
In situ hybridization
All tissue sections were also subjected to conventional in situ hybridization using the EBER detection kit, according to the manufacturer’s recommendations. Slides were deparaffinized, predigested with pepsin for 30 min, hybridized with an EBER oligonucleotide probe for 8 h or 16 h at 37°C and then incubated with horseradish peroxidase-conjugated-streptavidin for 30 min at 37°C. The DAB substrate was used as chromogen (1–5 min incubation at 37°C). Cells were counterstained with hematoxylin. Brown nuclear staining indicated a positive hybridization signal.
Immunoprecipitation and western blot analysis
Cellular proteins from cell lines and clinical samples immunoprecipitated using the Zt/g4 mAb, as described by Padhye et al.Citation33 Protein complex components were separated by 10% or 12% SDS-PAGE under reducing conditions and transferred to nitrocellulose membranes. Rabbit IgG specific to the RON or to other proteins were used as the primary antibody, followed by horseradish peroxidase-coupled secondary antibodies. Membranes were reprobed with an anti-actin antibody to show sample loading.
Assays for cell viability and colony formation
Raji and Jurkat cells were plated onto 96-well plates at 2.0 × 105 cells/well and treated with MSP in the presence or absence of Zt/f2 mAb at the indicated concentrations for 24 h or 72 h. Normal mouse IgG was used as a negative control. Cell viability was measured by MTT colorimetry. Colony formation by Raji or Jurkat cells was performed in 0.8% methylcellulose and 20% FBS/RPMI-1640. Colonies containing more than 30 cells after 10 d were counted and photographed.
Assessment of apoptosis
Apoptosis of Raji cells was quantitated using Annexin V/PI double staining and flow cytometry. Cells were washed twice with PBS and resuspended in 1× binding buffer. Allophycocyanin conjugate was added first, then Annexin V and PI were added and cells were gently vortexed and incubated in the dark for 20 min at room temperature. After the addition of 400 µL 1× binding buffer, cells were analyzed using a FACScan flow cytometer and CellQuest software. The expression of apoptosis-related proteins was determined by western blotting.
Cell cycle analysis
Following PI staining, cell cycle distribution was monitored by flow cytometry (Becton Dickinson), according to the manufacturer’s protocol. Cells were harvested, washed with PBS and fixed with 2% paraformaldehyde at room temperature for 30 min. After removal of paraformaldehyde, the cell pellets were resuspended in 70% ethanol and stored at -20°C for 2 h. Following this, cells were sedimented, resuspended in PBS containing 200 µg/ml RNase A and incubated at 37°C for 1 h. PI was added to a final concentration of 50 µg/ml and incubated for 2 h before flow cytometric analysis. The expression of cell cycle proteins was determined using western blotting.
Mouse experiments
Animal experiments were approved by the Institute Animal Care and Use Committee. Raji cells (5 × 106 cells per mouse) were injected subcutaneously into the subscapularis muscle of 6-week-old female BALB/c nude mice (National Rodent Laboratory Animal Resources). Mice were then randomized into three experimental groups (three mice per group). Zt/f2 (0.5 mg/mouse) was injected intraperitoneally twice weekly starting from three days after cell inoculation.Citation33 Normal mouse IgG was used as a negative control. Tumors were measured weekly and tumor volumes were calculated by the modified ellipsoidal formula: length × width2 × 0.5. Animals were sacrificed six weeks after the initiation of treatment, and xenografts were surgically excised and weighed. A portion of each tumor was paraffin-embedded and used for immunohistochemical analysis.
Statistical analysis
Results are presented as mean ± SD and differences between groups were determined by the Student’s t-test. Correlations between RON expression and EBV infection were evaluated using the Chi-square test. Data were analyzed using SPSS 11.0 software; p < 0.05 was considered to be significant.
| Abbreviations: | ||
| ATCL | = | adult T-cell lymphoma |
| BL | = | Burkitt’s lymphoma |
| CDK | = | cyclin-dependent kinase |
| DAB | = | diaminobenzidine tetrachloride |
| EBV | = | Epstein-Barr virus |
| HL | = | Hodgkin’s lymphoma |
| IHC | = | immunohistochemistry |
| LPL | = | lymphoplasmacytoid lymphoma |
| MALT | = | mucosa-associated lymphoid tissue marginal zone B-cell lymphoma |
| MAPK | = | mitogen-activated protein kinase |
| MCL | = | mantle cell lymphoma |
| MSP | = | macrophage-stimulating protein |
| MTT | = | 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| PARP | = | poly (ADP-ribose) polymerase |
| PBS | = | phosphate buffered saline |
| PI | = | propidium iodide |
| RTK | = | receptor tyrosine kinase |
| TK | = | tyrosine kinase |
| TUNEL | = | terminal deoxynucleotidyl transferase dUTP nick end labeling |
Acknowledgments
The authors thank Professor Yongqing Zhou (Department of Neurosurgery, First Affiliated Hospital, Zhejiang University College of Medicine, Hangzhou, Zhejiang, P. R. China). This work was supported in part by grants from National Science Fund of China (81172250 and 30972777), National Science Fund of China (LY12H16015) and Scientific Foundation of Zhejiang (2012C13021–3).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Wright DH. Burkitt’s lymphoma: a review of the pathology, immunology, and possible etiologic factors. [J] Pathol Annu 1971; 6:337 - 63; PMID: 4342309
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. [J] Lancet 1964; 1:702 - 3; http://dx.doi.org/10.1016/S0140-6736(64)91524-7; PMID: 14107961
- Kaye KM, Izumi KM, Li H, Johannsen E, Davidson D, Longnecker R, et al. An Epstein-Barr virus that expresses only the first 231 LMP1 amino acids efficiently initiates primary B-lymphocyte growth transformation. [J] J Virol 1999; 73:10525 - 30; PMID: 10559372
- Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. [J] Nat Rev Cancer 2002; 2:764 - 76; http://dx.doi.org/10.1038/nrc904; PMID: 12360279
- Renné C, Martín-Subero JI, Hansmann ML, Siebert R. Molecular cytogenetic analyses of immunoglobulin loci in nodular lymphocyte predominant Hodgkin’s lymphoma reveal a recurrent IGH-BCL6 juxtaposition. [J] J Mol Diagn 2005; 7:352 - 6; http://dx.doi.org/10.1016/S1525-1578(10)60564-8; PMID: 16049307
- Renné C, Hinsch N, Willenbrock K, Fuchs M, Klapper W, Engert A, et al. The aberrant coexpression of several receptor tyrosine kinases is largely restricted to EBV-negative cases of classical Hodgkin’s lymphoma. [J] Int J Cancer 2007; 120:2504 - 9; http://dx.doi.org/10.1002/ijc.22511; PMID: 17330841
- Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. [J] Oncogene 1993; 8:1195 - 202; PMID: 8386824
- Montero-Julian FA, Dauny I, Flavetta S, Ronsin C, André F, Xerri L, et al. Characterization of two monoclonal antibodies against the RON tyrosine kinase receptor. [J] Hybridoma 1998; 17:541 - 51; http://dx.doi.org/10.1089/hyb.1998.17.541; PMID: 9890710
- Wang MH, Ronsin C, Gesnel MC, Coupey L, Skeel A, Leonard EJ, et al. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. [J] Science 1994; 266:117 - 9; http://dx.doi.org/10.1126/science.7939629; PMID: 7939629
- Wang MH, Wang D, Chen YQ. Oncogenic and invasive potentials of human macrophage-stimulating protein receptor, the RON receptor tyrosine kinase. [J] Carcinogenesis 2003; 24:1291 - 300; http://dx.doi.org/10.1093/carcin/bgg089; PMID: 12807733
- Xu XM, Zhou YQ, Wang MH. Mechanisms of cytoplasmic beta-catenin accumulation and its involvement in tumorigenic activities mediated by oncogenic splicing variant of the receptor originated from Nantes tyrosine kinase. [J] J Biol Chem 2005; 280:25087 - 94; http://dx.doi.org/10.1074/jbc.M414699200; PMID: 15878878
- Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP, et al. β-Catenin is required for Ron receptor-induced mammary tumorigenesis. [J] Oncogene 2011; 30:3694 - 704; http://dx.doi.org/10.1038/onc.2011.86; PMID: 21423209
- Wang MH, Lee W, Luo YL, Weis MT, Yao HP. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. [J] J Pathol 2007; 213:402 - 11; http://dx.doi.org/10.1002/path.2245; PMID: 17955509
- Danilkovitch-Miagkova A. Oncogenic signaling pathways activated by RON receptor tyrosine kinase. [J] Curr Cancer Drug Targets 2003; 3:31 - 40; http://dx.doi.org/10.2174/1568009033333745; PMID: 12570659
- Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. [J] Cancer Res 2007; 67:6075 - 82; http://dx.doi.org/10.1158/0008-5472.CAN-06-4128; PMID: 17616662
- Lu Y, Yao HP, Wang MH. Significance of the entire C-terminus in biological activities mediated by the RON receptor tyrosine kinase and its oncogenic variant RON160. [J] J Exp Clin Cancer Res 2008; 27:55; http://dx.doi.org/10.1186/1756-9966-27-55; PMID: 18950514
- Wang J, Rajput A, Kan JL, Rose R, Liu XQ, Kuropatwinski K, et al. Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma. [J] J Biol Chem 2009; 284:10912 - 22; http://dx.doi.org/10.1074/jbc.M809551200; PMID: 19224914
- Chou YC, Lin SJ, Lu J, Yeh TH, Chen CL, Weng PL, et al. Requirement for LMP1-induced RON receptor tyrosine kinase in Epstein-Barr virus-mediated B-cell proliferation. [J] Blood 2011; 118:1340 - 9; http://dx.doi.org/10.1182/blood-2011-02-335448; PMID: 21659546
- Wang MH, Lee W, Luo YL, Weis MT, Yao HP. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. [J] J Pathol 2007; 213:402 - 11; http://dx.doi.org/10.1002/path.2245; PMID: 17955509
- Renné C, Willenbrock K, Martin-Subero JI, Hinsch N, Döring C, Tiacci E, et al. High expression of several tyrosine kinases and activation of the PI3K/AKT pathway in mediastinal large B cell lymphoma reveals further similarities to Hodgkin lymphoma. [J] Leukemia 2007; 21:780 - 7; http://dx.doi.org/10.1038/sj.leu.2404594; PMID: 17375124
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. [J] Nature 2001; 411:355 - 65; http://dx.doi.org/10.1038/35077225; PMID: 11357143
- Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. [J] J Virol 2000; 74:10838 - 45; http://dx.doi.org/10.1128/JVI.74.22.10838-10845.2000; PMID: 11044134
- Renné C, Hinsch N, Willenbrock K, Fuchs M, Klapper W, Engert A, et al. The aberrant coexpression of several receptor tyrosine kinases is largely restricted to EBV-negative cases of classical Hodgkin’s lymphoma. [J] Int J Cancer 2007; 120:2504 - 9; http://dx.doi.org/10.1002/ijc.22511; PMID: 17330841
- Chou YC, Lin SJ, Lu J, Yeh TH, Chen CL, Weng PL, et al. Requirement for LMP1-induced RON receptor tyrosine kinase in Epstein-Barr virus-mediated B-cell proliferation. [J] Blood 2011; 118:1340 - 9; http://dx.doi.org/10.1182/blood-2011-02-335448; PMID: 21659546
- Cho SB, Park YL, Song YA, Kim KY, Lee GH, Cho DH, et al. Small interfering RNA-directed targeting of RON alters invasive and oncogenic phenotypes of human hepatocellular carcinoma cells. [J] Oncol Rep 2011; 26:1581 - 6; PMID: 21874262
- Chung CY, Park YL, Song YA, Myung E, Kim KY, Lee GH, et al. Knockdown of RON inhibits AP-1 activity and induces apoptosis and cell cycle arrest through the modulation of Akt/FoxO signaling in human colorectal cancer cells. [J] Dig Dis Sci 2012; 57:371 - 80; http://dx.doi.org/10.1007/s10620-011-1892-7; PMID: 21901254
- Song YA, Park YL, Kim KY, Myung E, Chung CY, Cho SB, et al. RON is associated with tumor progression via the inhibition of apoptosis and cell cycle arrest in human gastric cancer. [J] Pathol Int 2012; 62:127 - 36; http://dx.doi.org/10.1111/j.1440-1827.2011.02765.x; PMID: 22243783
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. [J] J Cell Mol Med 2005; 9:59 - 71; http://dx.doi.org/10.1111/j.1582-4934.2005.tb00337.x; PMID: 15784165
- Huang Y, Hu J, Zheng J, Li J, Wei T, Zheng Z, et al. Down-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin. [J] J Exp Clin Cancer Res 2012; 31:48; http://dx.doi.org/10.1186/1756-9966-31-48; PMID: 22607709
- Yao HP, Luo YL, Feng L, Cheng LF, Lu Y, Li W, et al. Agonistic monoclonal antibodies potentiate tumorigenic and invasive activities of splicing variant of the RON receptor tyrosine kinase. [J] Cancer Biol Ther 2006; 5:1179 - 86; http://dx.doi.org/10.4161/cbt.5.9.3073; PMID: 16880737
- Yao HP, Zhou YQ, Ma Q, Guin S, Padhye SS, Zhang RW, et al. The monoclonal antibody Zt/f2 targeting RON receptor tyrosine kinase as potential therapeutics against tumor growth-mediated by colon cancer cells. [J] Mol Cancer 2011; 10:82; http://dx.doi.org/10.1186/1476-4598-10-82; PMID: 21749705
- Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. [J] Oncogene 2003; 22:186 - 97; http://dx.doi.org/10.1038/sj.onc.1206075; PMID: 12527888
- Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang R, Wang MH. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. [J] Mol Pharm 2011; 8:2310 - 9; http://dx.doi.org/10.1021/mp200193u; PMID: 22014215