Abstract
Ocimum genus (a.k.a holy basil or tulsi) is a dietary herb used for its multiple beneficial pharmacologic properties including anti-cancer activity. Here we show that crude extract of Ocimum gratissimum (OG) and its hydrophobic and hydrophilic fractions (HB and HL) differentially inhibit breast cancer cell chemotaxis and chemoinvasion in vitro and retard tumor growth and temporal progression of MCF10ADCIS.com xenografts, a model of human breast comedo-ductal carcinoma in situ (comedo-DCIS). OG-induced inhibition of tumor growth was associated with decreases in basement membrane disintegration, angiogenesis and MMP-2 and MMP-9 activities as confirmed by in situ gelatin zymography and cleavage of galectin-3. There was also decrease in MMP-2 and MMP-9 activities in the conditioned media of OG-treated MCF10AT1 and MCF10AT1-EIII8 premalignant human breast cancer cells as compared with control. The MMP-2 and MMP-9 inhibitory activities of OG were verified in vitro using gelatin, a synthetic fluorogenic peptide and recombinant galectin-3 as MMP substrates. Mice fed on OG-supplemented drinking water showed no adverse effects compared with control. These data suggest that OG is non-toxic and that the anti-cancer therapeutic activity of OG may in part be contributed by its MMP inhibitory activity.
Keywords: :
Introduction
Ocimum gratissimum belongs to the mint family Lameacea and since ancient times has been one of the most popular dietary herbs used for its numerous pharmacological properties. In recent years, resurging interest in its medicinal properties has led to several studies demonstrating its anti-carcinogenic, radiation-protecting and free-radical scavenging activities.Citation1 Aqueous extract of Ocimum gratissimum inhibits proliferation, migration, anchorage-independent growth, induction of COX-2 protein and three-dimensional growth and morphogenesis of breast cancer cells,Citation2 while others have shown that it suppressed A549 lung adenocarcinoma cells’ viability by activation of apoptotic signals and reducing the expression levels of Bcl-2.Citation3 Ethanolic extracts of Ocimum sanctum were reported to be cytotoxic to mouse Lewis lung carcinoma (LLC) cells and reduced number of tumor nodule formation in LLC-injected mice.Citation4 Essential oils isolated from Ocimum viride showed cytotoxic and apoptotic activities toward the colonic carcinoma cells COLO 205.Citation5 Previously, we compared three species of Ocimum, O. gratissimum, O. sanctum (green) and O. sanctum (purple) and examined their effect on breast cancer and endothelial cell migration. The results showed that aqueous extracts from all species exerted an inhibitory effect on tumor cell migration, but not significantly on endothelial cell migration. OG also reduced tumor size and neoangiogenesis in a MCF10ADCIS.com xenograft, a preclinical model of human breast ductal carcinoma in situ,Citation2 prompting the need for further evaluation of its breast cancer preventive and therapeutic properties. In this study, we have further analyzed the effect(s) of OG on tumor growth and progression with specific focus on its effects on MMP-2 and -9 activities.
MMPs are a family of at least 28 structurally and functionally related zinc dependent endoproteinases,Citation6,Citation7 which selectively degrade various components of extracellular matrix (ECM). Loss of ECM scaffold molecules leads to release of growth factors and cytokines to regulate cell function.Citation8,Citation9 MMPs also activate various latent growth factors, cytokines and chemokines and cleave cell surface proteins (cytokine receptors, cell adhesion molecules, urokinase receptor etc.Citation6,Citation7,Citation10,Citation11 Numerous reports have shown that MMPs are directly implicated in almost every biological process involving matrix remodeling throughout the mammalian life span, from embryo implantationCitation12 to cell death or necrosis.Citation13,Citation14 Roles of MMPs in tumor growth, apoptosis, vasculogenesis, lymphangiogenesis, neoplastic progression, invasion and metastasis have been reviewed extensively.Citation6,Citation15,Citation16
Of the various MMPs thought to be involved in cancer, MMP-2 and MMP-9, also known as gelatinases are the key members as they are overexpressed in a variety of malignant tumors and their expression and activities are often associated with tumor aggressiveness and a poor prognosis. Elevated levels of MMP-2 and/or MMP-9 are found in breast, brain, ovarian, pancreas, colorectal, bladder, prostate, lung cancers and melanoma.Citation7,Citation9,Citation17-Citation19 The collagen IV component of basement membrane is thought to be degraded mostly by MMP-2 and MMP-9; therefore they play a critical role in the conversion of in situ breast cancer to invasive lesions. While MMP-2 and MMP-9 are expressed by stromal cells, the MMP-2 protein is found both on stromal and cancer cell membranes.Citation20 MMP-9 has a distinct role in tumor angiogenesis: mainly regulating the bioavailability of vascular endothelial growth factor.Citation21 Earlier reports have indicated that gelatinases bind to collagen and fibronectin through their collagen binding domain.Citation22 They also bind to low density lipoprotein-related scavenger receptor (LRP), which is responsible for the internalization of various ligands including these enzymes.Citation23-Citation25 In addition, gelatinases also bind to other integral membrane proteins such as integrins (reviewed inCitation26). Consequently cell growth, migration and angiogenesis appear to depend on cell surface associations between gelatinases and these proteins. Numerous studies in preclinical cancer models demonstrated the ability of MMP inhibitors to delay primary tumor growth and block metastasis. However, in clinical trials these inhibitors failed due to significant toxicity and limited clinical efficacy. The paradigm shift for MMP functions, from destructive enzymes to cell signaling regulatorsCitation27 and disease anti targetsCitation28 suggests that the roles of MMPs in disease development are far more complex than originally thought. Hence in cancer MMP activities are probably more relevant during early stages of tumor development with MMP dependent signaling more relevant biologically than ECM degradation.
In the present study we report that Ocimum gtatissimum inhibits the progression of human breast cancer, which is partly due to its property as natural non-toxic inhibitor of MMP-2/-9 activities.
Results
Effects of OG on chemotaxis, chemoinvasion and MMP-2/-9 activities
Preliminary fractionation of the aqueous extract (OG) was accomplished by extracting the lyophilized powder with methanol to yield the relatively hydrophobic (HB) and hydrophilic (HL) fractions. OG and its HB and HL fractions were analyzed for their ability to influence chemotaxis of human breast carcinoma cells MDA-MB-231 toward Matrigel. indicates that adding 75, 150, 200 or 300 µg/mL OG to Matrigel induced ~15, 20, 38 and 47% inhibition in chemotaxis respectively compared with vehicle control. HB did not show any significant inhibition, whereas HL showed approximately 8, 18, 21 and 52% inhibition at concentrations of 75, 150, 200 or 300 µg/mL respectively. These data suggest that chemotactic inhibitory activity is associated with the HL fraction.
Figure 1.(A) Effect of OG and its fractions on cell migration. *p < 0.001; *’p < 0.05 compared with control (vehicle alone). (B) Cleavage of galectin-3 by MMP-2 and -9 in the presence of OG, HB or HL. Band density was calculated using Odyssey Infrared Imaging System. % cleavage was calculated as % of cleaved galectin-3 to total protein (intact+ cleaved). (C) Effect of OG, HB or HL on chemoinvasion: The migrated cells were counted using the Cellsens software (Olympus). Three fields per sample were counted and average numbers were plotted (graph). Equal volume of vehicle was added in the control wells. The experiments were repeated thrice and representative experiments are depicted.
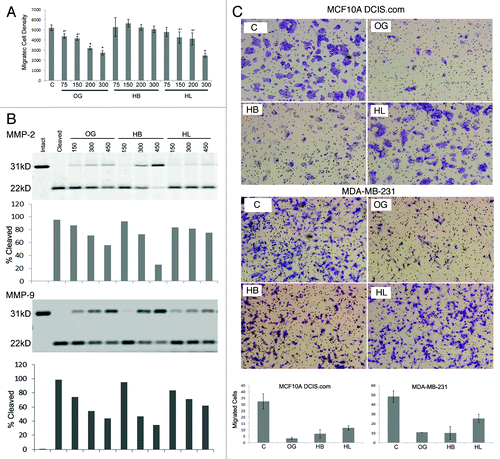
Next, we studied the effect of OG and its fractions on chemoinvasion of two breast cancer cell lines: MDA-MB-231 and MCF10ADCIS.com (). Data show an inhibition of chemoinvasion by OG and HB by approximately 90 and 80% that of control and 50–60% by HL in both the cell lines ().
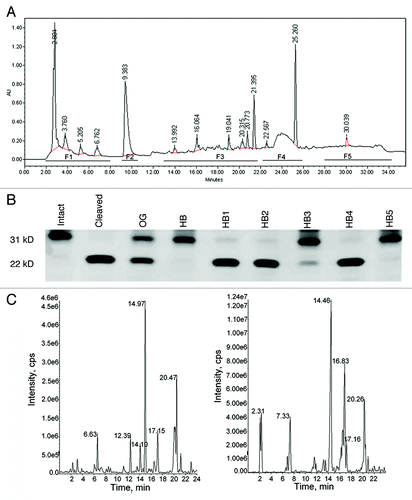
Since MMPs are implicated in chemoinvasion, next we studied the effect of OG and fractions on MMP-2 and -9 activities using three different substrates. Earlier we showed that active MMP-2 and MMP-9 cleave 31 kDa galectin-3 between Ala62-Tyr63 with resultant formation of a 22 kDa band.Citation29-Citation31 Galectin-3 cleavage by MMP-2 was inhibited in a dose dependent fashion by OG and HB and to a lesser extent by HL. HB was most effective showing an inhibition of ~85% at a concentration of 450 µg/mL. Lower concentrations of OG, HB and HL produced ~10–20% inhibition (). Similarly, galectin-3 cleavage by MMP-9 was effectively inhibited by OG and HB, whereas HL was less effective (). When the synthetic fluorogenic peptide (MMP-2 and -9 substrate) was incubated with MMP-2 or MMP-9, an increase in the relative fluorescence units (RFU) was observed linearly for the first hour, after which the activity slowed down reaching a plateau for the next 30 min. A dose dependent inhibition of MMP-2 activity was observed in the presence of different concentrations of extracts ranging from 12.5 to 300 µg/mL. Inhibition ranging from ~5 to 59% was observed with 12.5 to 300 µg/mL concentrations of OG respectively. A similar inhibition pattern was observed when the peptide was incubated with MMP-9 (data not shown). A comparison with equivalent concentrations of HB and HL showed that HB inhibited substrate cleavage more potently (~23 and 70% with HB vs ~14 and 54% with HL at 12.5 and 300 µg /mL concentrations respectively) (). The dose response curve () showed a dose dependent inhibition by all preparations with HB showing the strongest inhibition. HL was least effective; however, it exhibited inhibitory activity comparable to OG at 300 µg/mL.
Figure 2. Inhibition of MMP-2 activity by OG extract: (A–C) time dependent increase in RFU with different concentrations of OG, HB and HL ; (D) % decrease in RFU at 40 min relative to control values in the presence of various concentrations of extracts. (E) Gelatin zymography of APMA activated MMP-2 and –9 in the presence of OG, HB or HL. C indicates vehicle control. Band intensities were measured by ImageJ software.
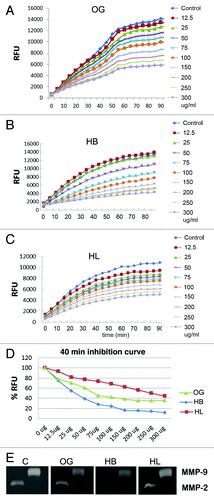
To analyze if the gelatinolytic activity of the MMPs was also susceptible to inhibition by OG, gelatin zymography was performed with recombinant MMP-2/-9 as described. The gels were incubated with 300 µg/mL OG, HB or HL fractions (). Densitometry scanning of the clear bands indicating collagenolytic activity showed that compared with control, OG and HB inhibited collagenolytic activities of both MMP-2 and MMP-9 with HB being more effective. A similar analysis with HL showed a limited inhibition. These data indicate that the MMP-2 and MMP-9 inhibitory activity is in the HB fraction.
In vivo inhibition of MMP-2/-9 activity by OG
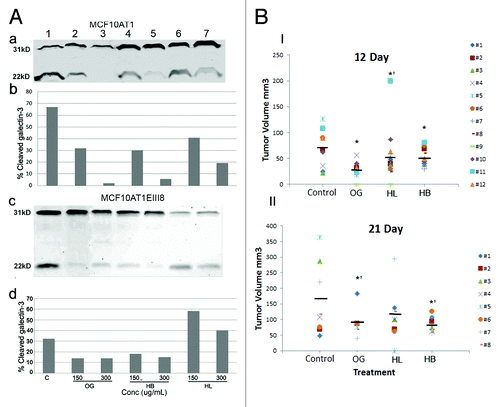
Previously we reported that galectin-3 cleavage is a surrogate marker for MMP activity in situ.Citation30 Therefore, we used cleavage of secreted galectin-3 by endogenous MMP-2/-9 as an indicator to verify in vivo MMP inhibitory activity of OG. We analyzed two breast cancer cell lines MCF10AT1 and MCF10AT1-EIII8 that secrete active MMP-2/-9 and the cleavable variant of galectin-3 H64. Untreated cells show the presence of 31 kDa and ~22 kDa galectin-3 in their conditioned media.Citation32 Comparison of the ratio between the intact 31 kDa and cleaved 22 kDa protein indicates that in MCF10AT1 cells 66.9% galectin-3 existed in cleaved form. Incubation with 150 µg/mL OG, HB or HL resulted in 31.7, 29.8 and 40.7% cleavage respectively, while treatment with 300 µg/mL OG, HB and HL resulted in 1.8, 5.6 and 19.1% cleavage indicating that OG and HB were more effective inhibitors of MMP activity in MCF10AT1 cells. HL fraction showed significant inhibition of cleavage only at higher concentration (, panel a,b). When MCF10AT1-EIII8 cells were similarly treated, 55, 15 and 61% of galectin-3 cleavage was observed with 150 µg/mL and 13, 8 and 50% galectin-3 cleavage was observed with 300 µg/ml OG, HB and HL respectively (, panel c and d). These data show that HB is equally or more effective than OG in inhibiting the MMP enzymatic activity.
Figure 3.(A) Effect of OG, HB or HL on the cleavage of secreted galectin-3: MCF10AT1 (a,b) and MCF10AT1-EIII8 (c,d) cells were incubated with 150 and 300 µg/mL OG (lanes 2,3), HB (lanes 4,5) or HL (lanes 6,7) respectively as indicated. Lane 1 is vehicle control. After 24 h pretreatment, the medium was replaced with fresh serum free medium with the extract and fractions. The conditioned media were collected after 24 h, separated on SDS-PAGE, stained with anti-galectin-3 pAb (a,c). Band intensities were measured using ImageJ software and % cleaved galectin-3 was calculated as the % age of cleaved protein in relation to the total (intact+cleaved) secreted protein (b,d).(B) Effect of OG, HB or HL on tumor growth in nude mice: Tumor volumes were calculated on day 12 (I) and 21 (II) using the formula: length x width × width/2. Bars represent mean tumor volume. P values were calculated using Student’s two tails t test. *p < 0.0005; *’p < 0.05
Inhibition of breast tumor growth and progression by OG and its fractions
MCF10ADCIS.com model of clinical comedo-DCIS
Among the several ductal carcinoma in situ (DCIS) subtypes of preinvasive breast cancer, comedo type DCIS or comedo-DCIS accounts for ~10% of all DCIS and confers the greatest risk for progression and post-operative recurrence.Citation33,Citation34 Comedo-DCIS tumors are easily distinguished from other DCIS by the characteristic central comedo-necrosisCitation35 that results from extensive spontaneous apoptosis.Citation35 MCF10ADCIS.com human breast cancer cells produce tumors that resemble clinical comedo-DCIS and recapitulate the temporal sequence of progression from in situ to invasive cancerCitation35-Citation37 when injected into nude mice.
Mice injected with MCF10ADCIS.com cells and fed with OG, HB and HL showed an inhibition of tumor growth. At day 12 after the injections, the average tumor volume in control mice was 75 ± 35 mm3, compared with 29.3 ± 14, 54.5 ± 50 and 50.6 ± 16.4 mm3 respectively in OG, HB and HL fed mice ( panel I). At day 21 the average tumor volumes were 161 ± 116, 90 ± 42, 110 ± 85 and 86 ± 26 mm3 in control, OG, HB and HL fed mice respectively (, panel II). Tumor volumes were not recorded on day 35 in part due to necrosis.
To analyze if OG affected only tumor size but also progression of MCF10ADCIS.com xenografts, tumors were harvested at day 12, 21 or 35 and analyzed for progression by morphology, p63 expression, blood vessel density, cell proliferation, basement membrane integrity and galectin-3 cleavage. Histological examination of day 12 control xenograft tumor showed cells organized as spheroids surrounded by a layer of myoepithelial cells (smooth muscle actin and p63 positive) (); the myoepithelial cells were not well defined in OG treatment (’ and B’). While 50–55% cells stained for p63 in control indicating an expansion of cells with basal lineage, only 3–7% cells were p63 positive in OG treated lesions at day 12. At day 35, the percentage of p63 positive cells was approximately 47% in control () compared with about 10% in OG treated lesions (’). In control 21 d lesions, the in situ lesions were surrounded by an intact basement membrane (), whereas lesions from OG treated mice displayed weakly developed basement membrane (’). At day 35, the basement membrane was disintegrated () and accompanied with high MMP-2/-9 activities in control lesions (), but was still intact in OG treated lesions (’), where MMP-2/-9 activity was not detected (’). The number of blood vessels () and proliferating tumor cells () was higher in control lesions compared with OG treated lesions (’ and H’, respectively). Approximately 12% cells showed positive PCNA staining in control compared with ~2% positive cells in OG treated lesions. Consistant with the MMP-2 and -9 data (’), lower levels of intact galectin-3 were observed in control lesions () as compared with OG treated lesions (’). HB and HL treated mice also showed inhibition of cancer progression compared with control, but these differences were not as pronounced as in the OG treated animals (’). Moreover, fewer in situ lesions progressed to comedo DCIS (’ and C) in OG treated mice (15–17%) compared with the control xenografts (60–69%). The number of comedo lesions with cores was counted independently by two investigators in 3 fields from 3 sections each from 21 d blocks.
Figure 4. Immunohistochemical analysis of the MCF10ADCIS.com xenografts from water and OG fed mice. (A–I): water fed; (A’–I’): OG fed. A&B: 12 d; D: 21 d; C,E-I: 35day. (A,A’): smooth muscle actin, (B,B’): p63; (C,C’): p63; (D,D’): collagen IV. (E,E’): collagen IV; (F,F’): in situ zymography for MMP activity; (G,G)’: CD31; (H,H’): PCNA; I,I’:Intact Galectin-3. (A,B,G,H,I) × 200; (C,D,E) × 400. Arrows indicate positive staining.
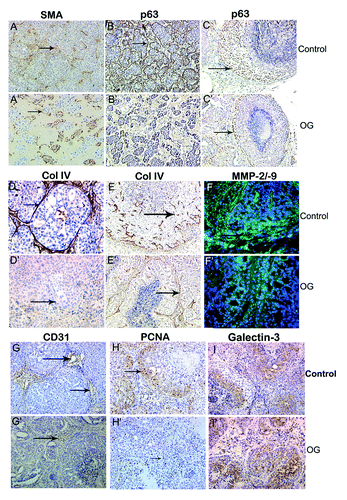
Figure 5. Immunohistochemical analysis of 35 d MCF10ADCIS.com xenografts from HB and HL fed mice. (A–D): HB fed; A’-D’: HL fed. (A,A’): CD31; (B,B’): p63; (C,C’): Collagen IV; (D,D’): PCNA. Brown color represents positive staining. (B,D): 200 × ; (A,C): 100 × . (E–H): Cleavage of galectin-3 in a 35 d xenograft. €: water fed; (F): OG fed,(G): HB fed, (H): HL fed. Red and yellow color indicates intact galectin-3 using mAb (arrowhead); Green color indicates cleaved galectin-3 using pAb (arrows). DAPI was used for nuclear staining. 200 ×
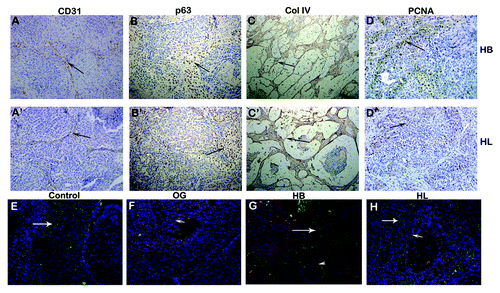
In situ analysis of galectin-3 cleavage by dual immunofluorescence stainingCitation32 showed that galectin-3 cleavage was seen in the control tumors but not in the OG treated tumors (arrow) () as well as in HB and HL treated mice, whereby a reduced cleavage was seen ().
Fractionation of HB by HPLC
In order to examine the effective constituents, we have fractionated HB by HPLC. HB fraction is a complex mixture of several compounds (). Absorbance at 220 nm showed the maximum number of peaks. However, since not all compounds are expected to have absorbance in the UV/Visible range, fractions were collected both when major peaks were observed as well as on a time basis when clusters of small peaks eluted. As shown in , further fractionation of HB resulted in five fractions. HB fractions 3 and 5 (HB-F3 and HB-F5) showed significant MMP-2 inhibitory activity by ~70 and 95% respectively, as measured by cleavage of galectin-3. Further resolution by LC-MS using a higher resolving power HPLC column produced M+H peaks 217, 244, 326, 328, 383 and 444 in HB-F3 (, left panel) and 179, 326, 383, 415 and 444 in HB-F5 (, right panel). Interestingly common molecular ions (383, 326 and 444) were identified in HB-F3 and HB-F5, however the identities of these compounds remain to be determined as we have not yet been able to identify the chemical structure and isolate enough material to examine the inhibitory activities of these components.
Figure 6.(A) HPLC fractionation of the HB fraction. The trace shown is at 220 nm, where most number of peaks were observed in the chromatogram. The horizontal bars below the chromatogram indicate time range of fractions collected. (B) Inhibition of galectin-3 cleavage with HB fractions 1–5 at final concentration of 200 µg/mL. (C) Active fractions of HB (F3 and F5) were further analyzed by LC-MS on a QTRAP5500 (ABSciex) mass analyzer coupled with Shimadzu XR HPLC system.
Discussion
In this study we have demonstrated that OG inhibits chemotaxis and chemoinvasion in vitro and breast cancer progression in vivo. Our data suggest that this is due in part to inhibition of MMP-2 and MMP-9 activities. Using solvent fractionation to obtain the relatively hydrophobic and hydrophilic fractions, we observed that HB and HL fractions contribute predominantly to the inhibition of MMP-2/-9 enzymatic activity and chemotaxis respectively. The OG crude extract, as expected, affects both processes. Natural product extracts are often complex mixtures that require extensive purification to identify the active compounds for the observed biological activities. While our preliminary efforts to purify the hydrophobic mixture by HPLC resulted in significant enrichment, the active fractions HB-F3 and HB-F5 still contain complex mixtures of phytochemicals that will require further purification for identication of the active component and its structural elucidation by mass spectrometry.
To compare the effect of OG and its fractions on breast cancer progression, we selected MCF10ADCIS.com cells, which produce DCIS-like lesions in nude mice and show a temporal progression from solid DCIS to comedo DCIS to undifferentiated carcinoma.Citation36 The data indicate that treatment with OG delays progression of MCF10ADCIS.com xenografts. Formation of basement membrane is the hallmark of in situ lesions. Progression from day 21 to day 35 showed that in OG treated lesions, formation of an intact basement membrane took longer and remained intact for longer periods of time. Thus it appears that OG helps to retard progression to invasive carcinoma. Although HB and HL treated mice showed decreased tumor growth, the inhibition of progression as measured by histology was not as pronounced and sustained as in OG treated mice.
We have previously shown strong expression of MMP-2, MMP-3, MMP-9 and MMP-11 in MCF10ADCIS.com lesions.Citation35 In situ gelatin zymography and galectin-3 cleavage of the MCF10ADCIS.com lesions confirmed that OG induced inhibition of tumor growth and progression is indeed partly due to inhibition of MMP-2 and -9 activities. However, at this stage it is not known whether it inhibits only MMP-2 and MMP-9 or other MMPs also. Various MMPs including MMP-2 and MMP-9 promote angiogenesis, cell growth, inflammation, cell invasion and cell survival (reviewed inCitation6). Many of these activities are inhibited by OG.Citation2,Citation38 As OG has been consumed by humans for centuries, and our in vivo data showed no toxic side effects in nude mice upto a dose equivalent of 800 mg/kg body weight, we propose that it has the potential to be developed into a suitable MMP inhibitor. We presume that these doses are effective as inhibition of tumor growth and progression to comedo along with reduced MMP-2 and MMP-9 activities, basement membrane disintegration, cell proliferation and angiogenesis in the xenografts are observed. Furthermore, since the activities are preserved in aqueous fractions, this would make it compatible for oral administration unlike the synthetic inhibitors. However, whether it can be used as a broad spectrum MMP inhibitor or a specific MMP inhibitor remains to be determined.
By using 3 different MMP substrates, a fluorogenic peptide, gelatin and galectin-3, we report here that the aqueous extract of Ocimum gratissimum inhibits the enzymatic activities of MMP-2 and MMP-9. Cleavage of a synthetic fluorescence quenched substrate showed that HB is most effective, followed by OG in inhibiting MMP-2/-9 activity. Galectin-3 is reported to be involved with tumor progression and metastasis.Citation39 The presence of cleaved protein is associated with a more progressive tumor phenotype.Citation29,Citation40 Incubation of galectin-3 with MMP-2 or MMP-9 in the presence of OG or its fractions resulted in reduced cleavage of galectin-3 by OG and HB, but not by HL suggesting the presence of MMP inhibitory activity mainly in the HB fraction. The data were also supported by zymogram and western blots of conditioned media from treated MCF10AT1 and MCF10T1-EIII8 cells suggesting that OG and HB can inhibit the enzymatic actvity of MMP-2 and MMP-9 regardless of the substrate used. When recombinant MMP-2 or MMP-9 were pre-incubated with OG and its fractions before loading on the zymogram, no difference in the activity was seen (not shown) indicating that the continuous presence of enzyme, inhibitor and the substrate is needed for inhibition of enzymatic activity.
Recently, some studies have reported MMP inhibition by natural compounds: e.g., turmeric curcuminoids were shown to inhibit secretion of MMP-3 in MDA-MB-231 cells;Citation41 anti-invasive effects of two active compounds from ginger namely 6-shogaol and 6-gingerol on human hepatoma cells were reported to be mediated by transcriptional inhibition of MMP-9.Citation42 EGCG, the major phytochemical in green tea was reported to inhibit bladder cancer cell invasion via suppression of MMP-9 expressionCitation43 and CL1 lung cancer cells through suppression of MMP-2 expression.Citation44 Hyperflorin induced a reduction of MMP-2 and -9 secretion in some human and murine cell lines.Citation45 OG aqueous extract was shown to reduce MMP-9 activity in male Wistar rats injected with carbon tetrachloride to induce liver injury.Citation46 Ethanol extracts of O. sanctum significantly inhibited cell adhesion and invasion as well as activities of MMP-9, but not MMP-2, in mouse Lewis lung carcinoma (LLC) cells.Citation4 However, in none of these studies an analysis of enzymatic activities of MMP-2 and MMP-9 was performed.
We show an inhibition of enzymatic activity using various substrates, however we did not see any difference in MMP-2 and MMP-9 at protein levels in the conditioned media or cell lysates of MCF10AT1 or MCF10T1-EIII8 cells (data not shown) indicating that the secretion and expression of MMP-2 and MMP-9 was not affected by OG and its fractions. Slight variations in the total amount of secreted galectin-3 were observed, which may be due to differences in loading or reduced secretion of galectin-3. However, the ratios of cleaved and intact protein showed patterns similar to those exhibited by recombinant protein i.e., an inhibition of cleavage was observed in OG and HB compared with control or HL.
Whereas OG and HB inhibited chemoinvasion more effectively than HL, HL more potently inhibited chemotaxis. While chemotaxis assay by Boyden chamber measures the migratory properties of cells toward a chemoattractant, chemoinvasion assay analyzes the invasion of cells through a Matrigel coated membrane. Thus, chemoinvasion can be related to the MMP activities of cells. In order for cells to invade through Matrigel as in chemoinvasion assays, the cells may need to express and activate MMP locally, which is inhibited by OG and HB. It is well known that the activities of plant materials may vary with the weather and soil conditions, which may be different from one place to another, it is therefore, important to extract active components from the crude extract to overcome these variations on one hand and to understand the molecular mechanisms of their action on the other hand. We used solvent extraction in an effort to enrich the inhibitory activities in OG sub-fractions. MMP-2 and MMP-9 inhibitory activities were found to be enriched in methanol extractable HB fraction, while the chemotaxis inhibitory activity was seen in the methanol non-extractable HL fraction.
As we have not yet identified the active component with MMP inhibitory activity, it is reasonable to assume at this stage, that the inhibitory effects of OG on DCIS progression could be due to effects on multiple processes including chemotaxis, chemoinvasion, cell growth, angiogenesis. However, inhibition of MMP-2 and MMP-9 activities appears to be a major effect of OG. To summarize, we report on the identification of a natural, non-toxic inhibitor of human breast carcinoma progression through inhibition of MMP-2 and MMP-9 activities. Efforts are underway for identification and characterization of compound(s) with MMP inhibitory activity.
Materials and Methods
Preparation of the aqueous extract and its fractions
Aqueous extract of Ocimum gratissimum was prepared and its activity was determined by chemotaxis inhibition assay as previously described.Citation2 Preliminary fractionation of the aqueous extract was accomplished by extracting the lyophilized powder with methanol to yield the HB and HL fractions. The lyophilized powders prepared from crude, HB and HL fractions were suspended in double distilled water at concentrations of 2 mg/mL for all assays to evaluate their anti-cancer activities.
Cell lines and culture
Human breast cancer cell line MDA-MB-231 (purchased from American Type Culture Collection) was maintained in Dulbecco’s Minimal Essential Medium (Invitrogen Corporation) containing 10% heat-inactivated fetal calf serum (FCS), essential and nonessential amino acids (Invitrogen), vitamins and antibiotics (Mediatech Cellgro) as described.Citation2 MCF10AT1 (Karmanos Cancer Institute Cell Core) and MCF10AT1-EIII8 cellsCitation47 were maintained in DMEM-F12 medium (Invitrogen) supplemented with 0.1 µg/ml cholera toxin (Sigma Chemical), 10 µg/ml insulin (Sigma), 0.5 µg/ml hydrocortisone (Sigma), 0.02 µg/ml epidermal growth factor (BD Biosciences), 100 IU/ml penicillin and 10% horse serum (Invitrogen). MCF10ADCIS.com cells (obtained from Cell Core, Karmanos Cancer Institute) were cultured in DMEM-F12 medium supplemented with 5% horse serum.Citation36,Citation37 All cells were maintained in a humidified chamber with 95% air and 5% CO2 at 37°C.
Chemotaxis
Chemotaxis was performed using a Boyden chamber (Neuroprobe) as described earlier.Citation2 In the lower chamber, 100 µg/mL Matrigel (BD Biosciences) alone or mixed with various concentrations of crude OG, HB or HL extracts or the appropriate vehicles were added. MDA-MB-231 (5 × 104) cells were loaded in the upper chamber. The migrated cell density was calculated using NIH Image Version 1.62. Each assay was performed in triplicates.
Chemoinvasion
Chemoinvasion was studied using a BD BioCoat Matrigel invasion chamber (BD Biosciences) according to manufacturer’s instructions in the presence of 150 µg/mL OG, HB or HL as described.Citation29 The migrated cells were photographed using the Zeiss Axiovert 35 microscope supporting Olympus DP72 imaging system and counted using the Cellsens software (Olympus). Three fields per sample were counted and average numbers were calculated.
Protein purification and cleavage of Galectin-3 by MMP-2 and MMP-9
Recombinant galectin-3 was expressed in E. coli, isolated as GST fusion proteins and cleaved by Precision plus protease using the manufacturer’s instructions (GE Healthcare).Citation30 Recombinant MMP-2 and MMP-9 were isolated and activated with 4-aminophenyl mercuric acetate (APMA) as described.Citation30 The purified galectin-3 (2 µg) was incubated with APMA activated recombinant MMP-2 or MMP-9 (2.5 ng) for 10 min, separated on a 12.5% polyacrylamide gel, stained with Coomassie brilliant blue. Full-length and cleaved galectin-3 was located as 31 and 22 kDa bands respectively.Citation30 To evaluate the inhibitory effects of OG and its HB and HL fractions, MMP-2 or MMP-9 were pre-incubated with the OG, fractions or vehicle control for 15 min on ice before incubation with galectin-3.
Proteolytic activity of MMP-2 and -9
The fluorescence-quenched substrate MOCAcPLGLA2pr(Dnp)AR-NH2 (7-methoxycoumarin-4-yl) acetyl-L-prolyl-L-glycyl-L-leucyl-[N3-(2,4-dinnitrophenol)-L-2,3-diaminopropionyl]-L-alanyl-l-arginine amide (Peptides International, Louisville, KY, USA) (20 mM) was incubated for 90 min at 37°C with 8 ng APMA activated recombinant MMP-2 or MMP-9 in a buffer consisting of 50 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM CaCl2, 0.01% Brij-35, 1% Me2SO in the presence of different concentrations of OG and its fractions. The increase in the fluorescence intensity with substrate cleavage was analyzed at excitation and emission wavelengths of 328 and 393 nm respectively using Spectramax Gemini Microplate Spectrofluorometer as described.Citation48,Citation49
Gelatin zymography
APMA activated recombinant MMP-2 and MMP-9 (5 ng each) were electrophoresed on an 8% SDS-polyacrylamide gel containing 1 mg/ml gelatin. The gel was washed three times with 2.5% Triton X-100 for 10 min and 10 mM TRIS-HCl (pH 8.0) for 10 min each. Then, the gel was incubated in 50 mM TRIS-HCl (pH 8.0) with 5 mM CaCl2 for 16 h at 37°C in the presence of various extracts as described. The control was treated with the solvent (water). The gel was stained with 1% Coomassie brilliant blue solution. After destaining, sharp transparent bands indicating gelatinolytic activity were visualized in blue background. Density of each band was quantified using ImageJ software.
Western blot analysis
MCF10AT1 and MCF10AT1-EIII8 cells (2 × 106) were seeded in 100 mm tissue culture dishes. The conditioned media were collected as described in figure legends and equal amounts of total proteins were subjected to SDS-PAGE and western blot analysis with a 1:2,000 dilution of anti–galectin-3 polyclonal antibody (Zymed Laboratories).
Tumor growth in nude mice
One × 106 MCF10ADCIS.com cells suspended in 0.1 ml of Matrigel were injected s.c. at 2 sites near each of no. 5 mammary gland nipple of NCR nu/nu female mice (Taconic) as previously described.Citation2,Citation36
One week before the injections, mice were randomly divided into 4 groups of 6 mice each and fed ad libitum with drinking water supplemented with 4 mg/mL lyophilized OG, HB or HL. The control group received regular drinking water. Tumor volumes were measured twice a week. The xenografts were harvested at 12, 21 and 35 d. The tumors were weighed, fixed in 10% buffered formalin and processed for immunohistochemical staining using anti galectin-3 mono and polyclonal antibodies, anti-collagen IV (Dako), p63 (AbCam), CD31 (AbCam) and pCNA (Sigma) antibodies as described. The animal experiments were performed according to the guidelines provided by Institutional Animal Care and Usage Committee, Wayne State University.
Immunohistochemical analysis and in situ gelatin zymography
Four micrometer tissue sections were de-parafinized, rehydrated and microwaved on high for 5 min twice in 1 mM sodium citrate buffer, pH 6.0 and stained as described.Citation30 Visualization and documentation were accomplished with an OLYMPUS BX40 microscope supporting a Olympus DP72 3CCD video camera and quantified with the Olympus Cellsens Imaging software.
Dual immunofluorescent labeling was applied to paraffin sections according to the protocol described for the M.O.M. Basic kit from Vector Laboratories. Briefly, the sections were first stained with anti galectin-3 monoclonal antibody (recognizing intact protein) and then with the polyclonal antibody (recognizing cleaved and intact protein). Each primary antibody was tagged by red (Texas Red) or green (fluorescein) conjugated secondary antibody. The slides were mounted and coverslipped with Vectashield mounting medium (Vector Laboratories) containing 4',6-diamidino-2-phenylindole (DAPI).
In situ gelatin zymography was performed on the fresh frozen MCF10ADCIS.com xenografts as described by Mook et al.Citation50
High performance liquid chromatography
High Performance Liquid Chromatography (HPLC) was performed on a Waters Alliance 2695 system equipped with a diode-array detector (2998). HB fraction was injected on to a Sunfire C18 column (5µ, 3 × 250 mm, Waters) and eluted with a linear gradient of methanol and water at a flow rate of 0.4 ml/min (methanol gradient: 0–5 min: isocratic at 20%, 5–25 min: 20–90% linear gradient and 25–35 min: isocratic at 90%). HPLC eluent from repeated injection of the HB fraction was collected into five fractions: Fraction 1: 2–8 min; Fraction 2: peak at 9.383 min; Fraction 3: 13–22 min; Fraction 4: 22–26 min; and Fraction 5: 28–34 min retention time. These HPLC fractions were dried under vacuum and resuspended in water at concentrations of 2 mg/mL. MMP-2 inhibitory activity in fractions 1–5 was determined at final concentrations of 200 µg/mL as described earlier. Active fractions (HB-F3 and HB-F5) were further analyzed by liquid chromatography–mass spectrometry (LC-MS) on a QTRAP5500 (ABSciex) mass analyzer coupled with Shimadzu XR HPLC system. HPLC was performed on a Luna C18 column (5µ, 2 × 150 mm) using a linear gradient of Water:Acetonitrile with 0.1% formic acid (acetonitrile gradient: 0–20 min 30–100% followed by isocratic at 100% until 25 min). The eluent was directly introduced to QTRAP5500 and scanned for m/z 150 to 1000 in the positive ion mode and information dependent enhanced product ion spectra were recorded.
Acknowledgments
We thank Mr. Victor Hogan for help in manuscript preparation. Financial support by National Institute of Health (R37CA46120–19 to A.R.) is thankfully acknowledged. H.L. was a visiting scholar funded by China Scholarship Council (CSC) and Chongqing University of Education.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol 2002; 40:765 - 73; PMID: 12597545
- Nangia-Makker P, Tait L, Shekhar MP, Palomino E, Hogan V, Piechocki MP, et al. Inhibition of breast tumor growth and angiogenesis by a medicinal herb: Ocimum gratissimum. Int J Cancer 2007; 121:884 - 94; http://dx.doi.org/10.1002/ijc.22733; PMID: 17437270
- Chen HM, Lee MJ, Kuo CY, Tsai PL, Liu JY, Kao SH. Ocimum gratissimum Aqueous Extract Induces Apoptotic Signalling in Lung Adenocarcinoma Cell A549. Evid Based Complement Alternat Med 2011; 2011; http://dx.doi.org/10.1155/2011/739093; PMID: 20953389
- Kim SC, Magesh V, Jeong SJ, Lee HJ, Ahn KS, Lee HJ, et al. Ethanol extract of Ocimum sanctum exerts anti-metastatic activity through inactivation of matrix metalloproteinase-9 and enhancement of anti-oxidant enzymes. Food Chem Toxicol 2010; 48:1478 - 82; http://dx.doi.org/10.1016/j.fct.2010.03.014; PMID: 20233602
- Sharma M, Agrawal SK, Sharma PR, Chadha BS, Khosla MK, Saxena AK. Cytotoxic and apoptotic activity of essential oil from Ocimumviride towards COLO 205 cells. Food Chem Toxicol 2010; 48:336 - 44; http://dx.doi.org/10.1016/j.fct.2009.10.021; PMID: 19852999
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141:52 - 67; http://dx.doi.org/10.1016/j.cell.2010.03.015; PMID: 20371345
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011; 41:271 - 90; http://dx.doi.org/10.1007/s00726-010-0689-x; PMID: 20640864
- Hatfield KJ, Reikvam H, Bruserud O. The crosstalk between the matrix metalloprotease system and the chemokine network in acute myeloid leukemia. Curr Med Chem 2010; 17:4448 - 61; http://dx.doi.org/10.2174/092986710794183033; PMID: 21062258
- Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol 2009; 27:5287 - 97; http://dx.doi.org/10.1200/JCO.2009.23.5556; PMID: 19738110
- Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol 2007; 42:113 - 85; http://dx.doi.org/10.1080/10409230701340019; PMID: 17562450
- Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta 2010; 1803:39 - 54; http://dx.doi.org/10.1016/j.bbamcr.2009.09.015; PMID: 19800373
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, et al. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development 1996; 122:1723 - 36; PMID: 8674412
- Currie JC, Fortier S, Sina A, Galipeau J, Cao J, Annabi B. MT1-MMP down-regulates the glucose 6-phosphate transporter expression in marrow stromal cells: a molecular link between pro-MMP-2 activation, chemotaxis, and cell survival. J Biol Chem 2007; 282:8142 - 9; http://dx.doi.org/10.1074/jbc.M610894200; PMID: 17229722
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2:161 - 74; http://dx.doi.org/10.1038/nrc745; PMID: 11990853
- Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta 2010; 1803:103 - 20; http://dx.doi.org/10.1016/j.bbamcr.2009.09.017; PMID: 19800930
- Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol 2012; 181:1895 - 9; http://dx.doi.org/10.1016/j.ajpath.2012.08.044; PMID: 23063657
- Rydlova M, Holubec L Jr., Ludvikova M Jr., Kalfert D, Franekova J, Povysil C, et al. Biological activity and clinical implications of the matrix metalloproteinases. Anticancer Res 2008; 28:2B 1389 - 97; PMID: 18505085
- Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 2005; 87:287 - 97; http://dx.doi.org/10.1016/j.biochi.2005.01.014; PMID: 15781315
- Yu XF, Han ZC. Matrix metalloproteinases in bone marrow: roles of gelatinases in physiological hematopoiesis and hematopoietic malignancies. Histol Histopathol 2006; 21:519 - 31; PMID: 16493582
- Polette M, Gilbert N, Stas I, Nawrocki B, Nöel A, Remacle A, et al. Gelatinase A expression and localization in human breast cancers. An in situ hybridization study and immunohistochemical detection using confocal microscopy. Virchows Arch 1994; 424:641 - 5; http://dx.doi.org/10.1007/BF01069745; PMID: 8055158
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000; 2:737 - 44; http://dx.doi.org/10.1038/35036374; PMID: 11025665
- Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta 2005; 1755:37 - 69; PMID: 15907591
- Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem 2001; 276:15498 - 503; http://dx.doi.org/10.1074/jbc.M100121200; PMID: 11279011
- Van den Steen PE, Van Aelst I, Hvidberg V, Piccard H, Fiten P, Jacobsen C, et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J Biol Chem 2006; 281:18626 - 37; http://dx.doi.org/10.1074/jbc.M512308200; PMID: 16672230
- Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem 2001; 276:8403 - 8; http://dx.doi.org/10.1074/jbc.M008925200; PMID: 11113133
- Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta 2012; 1825:29 - 36; PMID: 22020293
- Overall CM. Dilating the degradome: matrix metalloproteinase 2 (MMP-2) cuts to the heart of the matter. Biochem J 2004; 383:e5 - 7; http://dx.doi.org/10.1042/BJ20041433; PMID: 15508185
- Balbín M, Fueyo A, Tester AM, Pendás AM, Pitiot AS, Astudillo A, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet 2003; 35:252 - 7; http://dx.doi.org/10.1038/ng1249; PMID: 14517555
- Nangia-Makker P, Wang Y, Raz T, Tait L, Balan V, Hogan V, et al. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer 2010; 127:2530 - 41; http://dx.doi.org/10.1002/ijc.25254; PMID: 20162566
- Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res 2007; 67:11760 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-3233; PMID: 18089806
- Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, et al. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 1994; 33:14109 - 14; http://dx.doi.org/10.1021/bi00251a020; PMID: 7947821
- Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res 2007; 67:11760 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-3233; PMID: 18089806
- Fisher ER, Land SR, Saad RS, Fisher B, Wickerham DL, Wang M, et al. Pathologic variables predictive of breast events in patients with ductal carcinoma in situ. Am J Clin Pathol 2007; 128:86 - 91; http://dx.doi.org/10.1309/WH9LA543NR76Y29J; PMID: 17580274
- Yang M, Moriya T, Oguma M, De La Cruz C, Endoh M, Ishida T, et al. Microinvasive ductal carcinoma (T1mic) of the breast. The clinicopathological profile and immunohistochemical features of 28 cases. Pathol Int 2003; 53:422 - 8; http://dx.doi.org/10.1046/j.1440-1827.2003.01498.x; PMID: 12828606
- Shekhar MP, Tait L, Pauley RJ, Wu GS, Santner SJ, Nangia-Makker P, et al. Comedo-ductal carcinoma in situ: A paradoxical role for programmed cell death. Cancer Biol Ther 2008; 7:1774 - 82; http://dx.doi.org/10.4161/cbt.7.11.6781; PMID: 18787417
- Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 2000; 92:1185 - 6; http://dx.doi.org/10.1093/jnci/92.14.1185A; PMID: 10904098
- Tait LR, Pauley RJ, Santner SJ, Heppner GH, Heng HH, Rak JW, et al. Dynamic stromal-epithelial interactions during progression of MCF10DCIS.com xenografts. Int J Cancer 2007; 120:2127 - 34; http://dx.doi.org/10.1002/ijc.22572; PMID: 17266026
- Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol 2005; 49:125 - 31; PMID: 16170979
- Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta 2006; 1760:616 - 35; http://dx.doi.org/10.1016/j.bbagen.2005.12.020; PMID: 16478649
- Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol 2009; 174:1515 - 23; http://dx.doi.org/10.2353/ajpath.2009.080816; PMID: 19286570
- Boonrao M, Yodkeeree S, Ampasavate C, Anuchapreeda S, Limtrakul P. The inhibitory effect of turmeric curcuminoids on matrix metalloproteinase-3 secretion in human invasive breast carcinoma cells. Arch Pharm Res 2010; 33:989 - 98; http://dx.doi.org/10.1007/s12272-010-0703-6; PMID: 20661707
- Weng CJ, Wu CF, Huang HW, Ho CT, Yen GC. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol Nutr Food Res 2010; 54:1618 - 27; http://dx.doi.org/10.1002/mnfr.201000108; PMID: 20521273
- Qin S, Alcorn JF, Craigo JK, Tjoeng C, Tarwater PM, Kolls JK, et al. Epigallocatechin-3-gallate reduces airway inflammation in mice through binding to proinflammatory chemokines and inhibiting inflammatory cell recruitment. J Immunol 2011; 186:3693 - 700; http://dx.doi.org/10.4049/jimmunol.1002876; PMID: 21307292
- Deng YT, Lin JK. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J Agric Food Chem 2011; 59:13318 - 27; http://dx.doi.org/10.1021/jf204149c; PMID: 22082235
- Donà M, Dell’Aica I, Pezzato E, Sartor L, Calabrese F, Della Barbera M, et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res 2004; 64:6225 - 32; http://dx.doi.org/10.1158/0008-5472.CAN-04-0280; PMID: 15342408
- Chiu CC, Huang CY, Chen TY, Kao SH, Liu JY, Wang YW, et al. Beneficial Effects of Ocimum gratissimum Aqueous Extract on Rats with CCl(4)-Induced Acute Liver Injury. Evid Based Complement Alternat Med 2012; 2012:736752; http://dx.doi.org/10.1155/2012/736752; PMID: 22792126
- Shekhar MP, Werdell J, Tait L. Interaction with endothelial cells is a prerequisite for branching ductal-alveolar morphogenesis and hyperplasia of preneoplastic human breast epithelial cells: regulation by estrogen. Cancer Res 2000; 60:439 - 49; PMID: 10667599
- Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang M, Fridman R, et al. Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem 2005; 280:33992 - 4002; http://dx.doi.org/10.1074/jbc.M504303200; PMID: 16046398
- Olson MW, Bernardo MM, Pietila M, Gervasi DC, Toth M, Kotra LP, et al. Characterization of the monomeric and dimeric forms of latent and active matrix metalloproteinase-9. Differential rates for activation by stromelysin 1. J Biol Chem 2000; 275:2661 - 8; http://dx.doi.org/10.1074/jbc.275.4.2661; PMID: 10644727
- Mook OR, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem 2003; 51:821 - 9; http://dx.doi.org/10.1177/002215540305100613; PMID: 12754293