Abstract
The KRAS oncogene is mutated in 40‒50% of colorectal cancers and confers resistance to EGFR-targeted therapy. In the clinic, agents such as cetuximab or panitumumab target the EGFR receptor for therapeutic benefit. Cetuximab was approved by the FDA in 2012 as first-line therapy for KRAS mutation-negative (wild-type), EGFR-expressing metastatic colorectal cancer, in combination with FOLFIRI (5-fluorouracil, irinotecan, leucovorin). Herein we report a case of metastatic colon cancer with conflicting testing results for the KRAS oncogene from two different reference laboratories. The discordant reports complicated the decision-making process regarding the administration of targeted anti-EGFR personalized therapy. As the second test result was wild-type from the same original pathological specimen, the patient was treated with cetuximab-containing combination chemotherapy and appeared to have a response after prior disease progression. It is unclear whether the observed response was fully due to regression of wild-type KRAS-containing tumor or any component of antibody-dependent cellular cytotoxicity to a heterogeneous tumor in this patient.
Introduction
Each year there is approximately 1.2 million new cases of colorectal cancer worldwide, resulting in 608 700 deaths per year.Citation1 In the United States, the incidence is decreasing due to enhanced screening for premalignant lesions; however, not every country has witnessed this decline.Citation2 Surgery alone is curative most patients with early stage disease and patients with stage III disease benefit from adjuvant chemotherapy with an approximate 6 y survival of 73% with oxaliplatin, fluoruracil, and leucovorin (FOLFOX) as was noted in the MOSAIC trial.Citation3 However, up to 20% of patients present with distant metastasis which has a 5 y overall survival of less than 10 percent.Citation4,Citation5 Clinical trials have shown that the addition of targeted agents have added additional benefit. Monoclonal antibodies that target the epidermal growth factor receptor (EGFR, cetuximab and panitumumab) and the vascular epidermal growth factor (VEGF, bevacizumab) have improved the overall survival in the metastatic setting beyond 20 mo.Citation6,Citation7 Although metastatic colorectal cancer has a poor overall prognosis, the introduction of targeted agents against EGFR and VEGF has led to improvements in response rate and survival.
Case Report
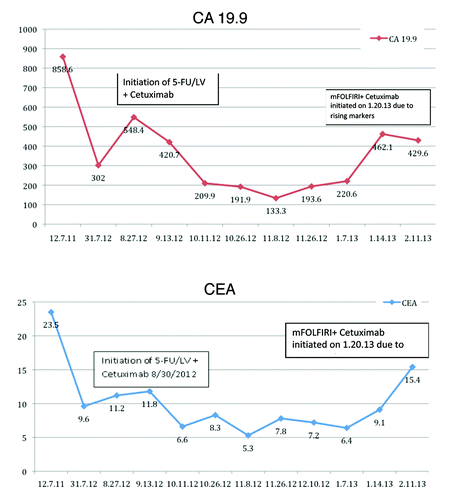
The patient is a 58-year-old woman who was diagnosed with a perforated T4, node-negative adenocarcinoma of the sigmoid colon. She had a sigmoid colon resection followed by 12 cycles of adjuvant chemotherapy with FOLFOX for her stage IIC disease. She was closely followed with CEA/CA19-9 levels and PET/CT scans that showed new lesions in her pelvis, colon, and lungs a year after completion of adjuvant therapy. A pelvic MRI confirmed the above findings. The case was discussed at a multidisciplinary tumor board conference and she underwent an ileocolectomy with side-to-side anastomosis, lower anterior resection with colostomy, bilateral ureterolysis, total abdominal hysterectomy, and bilateral salpingo-oophorectomy. Final pathology revealed invasive colorectal adenocarcinoma with local extension into the uterus, ileum, and ovary; one out of 12 lymph nodes was positive for metastatic carcinoma (). The paraffin block containing the tumor was sent to Response Genetics for mutational analysis. A KRAS Gly12Cys mutation was identified. This mutation strongly predicts lack of response to the EGFR inhibitors cetuximab and panitumumab.Citation1,Citation2 Post-operatively, a CT scan of the chest, abdomen, and pelvis demonstrated increase in size of her lung lesions and a CT-guided biopsy confirmed metastatic colon adenocarcinoma. She was started on FOLFIRI plus bevacizumab and a repeat PET/CT after three cycles showed disease progression. The same paraffin block was subsequently sent to Caris Target Now for further analysis. It came back with wild-type KRAS, which was paradoxical to the previous mutational analysis result and meant that EGFR inhibitors could be an option for this patient as opposed to hospice care. Based on this new result, the patient was started on combination therapy with 5-fluorouracil/leucovorin (5FU/LV) and cetuximab. She had a response to this regimen and her CEA levels decreased from 11.8 to 7.6 and her CA 19-9 levels decreased from 548 to 200 after just two cycles (). In addition, the patient had PET/CT scans done before the initiation of 5-FU/LV and cetuximab and during the treatment, which showed stable disease (). After 5 cycles of 5FU/LV with cetuximab the patient progressed with rising CEA level of 9.1 and CA 19-9 level of 462. Therapy was switched to 5FU/LV and Irinotecan (modified FOLFIRI) with cetuximab. She had one more cycle of chemotherapy when she was found to have extensive thrombosis and progressive disease. As her performance status deteriorated she was placed on hospice care.
Figure 1. Pathology showed a moderately differentiated adenocarcinoma in the rectum with direct extension to the ovary and uterus at the time of recurrence (H&E, 200× original magnification).
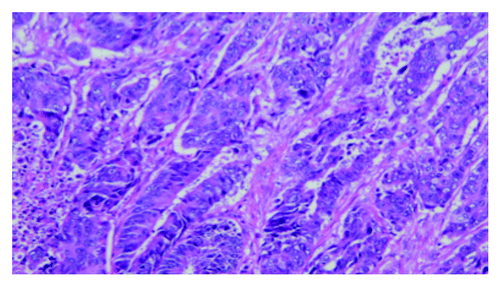
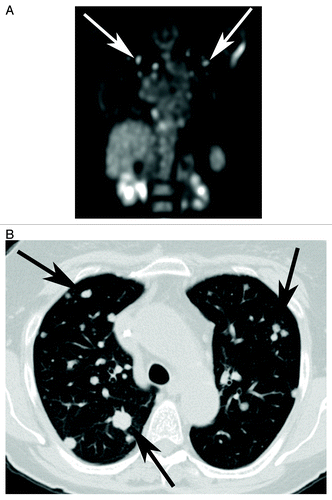
Figure 3. (A) Coronal PET image shows multiple fluoro-18-deoxyglucose (FDG) avid nodules (white arrows) scattered throughout both lungs and right hila region, consistent with thoracic metastatic disease. (B) Axial CT scan obtained at the same time show multiple bilateral pulmonary nodules (black arrows) of various sizes, ranging from a few millimeters to a couple of centimeters, consistent with pulmonary metastasis form colorectal cancer. There was no appreciable change in lesion size or FDG avidity with treatment.
Discussion
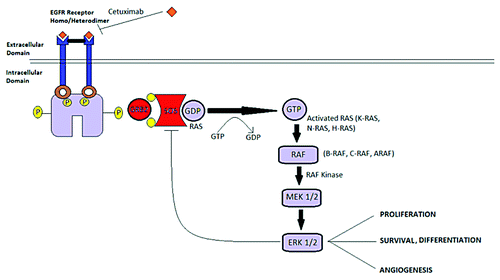
The past decade has witnessed an exponential growth of targeted agents for colorectal cancer which has led to personalized medicine. Roughly 40 percent of advanced colon cancers harbor activating KRAS mutations, which are associated with a poor outcome.Citation3 Ras signaling pathways are linked to proliferation, survival, angiogenesis and differentiation. Ras is activated when it binds to guanosine triphosphate (GTP) and inactivated when GTPase-activating proteins (GAPs) hydrolyses it to Ras-guanosine diphosphate (GDP). In the active form, RAS proteins are capable of activating downstream signaling cascades such as the Raf-MEK-ERK pathway (). Activating RAS mutations and its regulators can lead to aberrant cell signaling and eventually result in tumorgenesis.Citation4 Due to the high frequency and importance of RAS mutations in cancer it has become a key target in clinical practice, among which therapies directed at KRAS have proven to be beneficial in metastatic colorectal cancer.
In the metastatic colorectal cancers, epidermal growth factor inhibitors, such as cetuximab and panitumumab, have shown to increase response rate, progression-free survival, and overall survival when given as either monotherapy or in combination with chemotherapy.Citation5-Citation7 Studies have shown the KRAS mutations at Codon 12 and 13 predict a lack of response to EGFR directed therapy. The American Society for Clinical Oncology (ASCO) Provisional Opinion and the National Comprehensive Cancer Network (NCCN) recommend testing patients with metastatic colon cancer for KRAS mutation in a CLIA-certified laboratory and if mutations in codon 12 or 13 are detected, anti-EGFR monoclonal therapies should not be considered as a treatment option.Citation8,Citation9 This data was supported by a systemic review by Dehabreh et al.Citation10 However, some studies have questioned or given conflicting results regarding KRAS mutations. De Roock et al. showed that the KRAS G13D mutation had better overall survival when treated with cetuximab vs. best supportive care, compared with other types of KRAS mutated tumors.Citation11 Our case illustrates that KRAS testing is not always straightforward and can greatly impact patient care. If the Caris Target Now had not been sent, the likely next step would have been hospice care. However, the patient was subsequently found to be wild-type by Caris Target Now analysis. She went on to receive targeted therapy with cetuximab and achieved a good response with decreasing tumor marker levels and improved quality of life with minimal toxicity.
The etiology of the discordant results remains unknown but several possibilities are plausible. First, it is known that intratumor heterogeneity exists. As noted by Gerlinger et al., when they looked at multiple spatially separated samples of four patients with metastatic renal cell carcinoma; they found that up to 69% of somatic mutations were not detectable across all tumor regions.Citation12 It is conceivable that different parts of the sample tissue were micro-dissected in two different laboratories leading to the discordant results; however, this information is not available to us. Second, in most cases, routine surgical specimen does not contain PURE tumor cells, which means there is always some normal tissue present. Thus, most laboratories require a minimum amount of tumor present on the sections (e.g., >25%) to avoid contamination by normal tissue. In this case, the pathologist reviewed the slides and picked a tumor-rich block, and the same block was tested in two different molecular laboratories sequentially. Without knowing the detailed methodology and protocol in each laboratory, we cannot exclude the possibility of tissue contamination in one of the two laboratories. Third, it is also possible that the tumor tissue was partially exhausted after the first round of testing and most of the tissue remained in the block was normal tissue that was subsequently tested in the second laboratory. However, this does not fit the clinical scenario.
Now with routine genetic testing and available targeted therapy, it is imperative to obtain accurate testing results on oncogene mutations to avoid overtreatment or undertreatment. If there is any doubt, confirmatory testing by a second molecular laboratory and/or method may be considered.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 2008; 26:374 - 9; http://dx.doi.org/10.1200/JCO.2007.12.5906; PMID: 18202412
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:1626 - 34; http://dx.doi.org/10.1200/JCO.2007.14.7116; PMID: 18316791
- Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol 2009; 27:5931 - 7; http://dx.doi.org/10.1200/JCO.2009.22.4295; PMID: 19884549
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 2007; 7:295 - 308; http://dx.doi.org/10.1038/nrc2109; PMID: 17384584
- Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:2311 - 9; http://dx.doi.org/10.1200/JCO.2007.13.1193; PMID: 18390971
- Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007; 357:2040 - 8; http://dx.doi.org/10.1056/NEJMoa071834; PMID: 18003960
- Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007; 25:1658 - 64; http://dx.doi.org/10.1200/JCO.2006.08.1620; PMID: 17470858
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009; 27:2091 - 6; http://dx.doi.org/10.1200/JCO.2009.21.9170; PMID: 19188670
- Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, et al, National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw 2009; 7:778 - 831; PMID: 19755046
- Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: Anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med 2011; 154:37 - 49; http://dx.doi.org/10.7326/0003-4819-154-1-201101040-00006; PMID: 21200037
- De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 2010; 304:1812 - 20; http://dx.doi.org/10.1001/jama.2010.1535; PMID: 20978259
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366:883 - 92; http://dx.doi.org/10.1056/NEJMoa1113205; PMID: 22397650