Abstract
Cisplatin-based chemotherapy is considered the gold standard for patients with advanced bladder cancer. However, despite initial response, many patients will relapse; therefore, novel salvage treatment strategies are desperately needed. Herein, we studied a mechanism based treatment combination using a Smac mimetic with standard chemotherapy. Using a panel of 10 urothelial cancer cell lines, we exposed them to a combination of gemcitabine, cisplatin, and a Smac mimetic. Sensitivity was determined using a DNA fragmentation assay. We determined that three cell lines (UMUC-3, UMUC-13, and RT4v6) were considered sensitive to the combination of gemcitabine and cisplatin and an additional three cell lines were sensitized to gemcitabine and cisplatin with the addition of the Smac mimetic (UMUC-6, UMUC-12, and UMUC-18). We next explored the constitutive expression of selected members of the IAP family (XIAP, cIAP-1, cIAP-2, and Survivin), the BCL family (BCL-2, BCLXL, and BAX) and Smac using gene expression profiling and western blotting. We determined that RNA and protein expression of SMAC, selected members of the IAP family and members of the BCL family did not correlate to drug sensitivity. Lastly, using an in vivo mouse model, we determined that treatment with the Smac mimetic in combination with gemcitabine and cisplatin resulted in increased apoptosis, decreased microvessel density and decreased cellular proliferation. This novel treatment strategy may be effective in patients with advanced urothelial carcinoma and warrants further investigation.
Introduction
Cisplatin based chemotherapy is considered the gold standard for advanced bladder cancer. Methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) has demonstrated efficacy in the metastatic setting where response rates have been reported to be as high as 38‒72%.Citation1-Citation3 Because of a decreased side effect profile and similar efficacy, the combination of gemcitabine and cisplatin has also become a commonly used bladder cancer regimen.Citation4,Citation5 While initial response to chemotherapy is high, long-term progression free and overall survival are limited. Reports have consistently demonstrated a median overall survival of approximately one year.Citation1-Citation3 Therefore, effective salvage regimens are desperately needed.
Inhibitors of apoptosis (IAPs) are proteins that are capable of interacting with and inactivating caspases. There are eight total IAPs related by their functional protein domain, the baculovirus repeat domain (BIR). However, XIAP, cIAP-1, cIAP-2 and ML-IAP are considered the “classical” members.Citation6 Interestingly, XIAP is thought to be the only one that directly binds and inactivates caspases-3, -7, and -9.Citation7 Smac/DIABLO is a mitochondrial protein released into the cytosol upon apoptotic stimuli. Smac works by competitively binding to XIAP resulting in the release of caspases and allows the execution phase of apoptosis.Citation8 Dysregulation of IAPs and SMAC have been implicated in urologic malignancies.Citation9-Citation11
Recently, a class of drugs called Smac mimetics have become more extensively studied in several cancer models such as lung,Citation12 pancreas,Citation13-Citation15 and head and neck tumors.Citation16 Further, in each report, it has been demonstrated that a Smac mimetic enhances the efficacy of chemotherapy. We hypothesized that a Smac mimetic would be effective in overcoming a potential IAP mediated chemotherapy resistance in bladder cancer. Further, we investigated the feasibility of using constitutively expressed markers, as well as changes in their expression before and after therapy, to predict response. In our report, we demonstrate that a Smac mimetic, AZ58, is capable of overcoming resistance to standard bladder cancer chemotherapy in selected cell lines in vitro and produces increased apoptosis in vivo, in a bladder xenograft model both as a single agent and when added to chemotherapy.
Results
A Smac mimetic (AZ58) is capable of overcoming urothelial cancer cell line resistance to gemcitabine and cisplatin
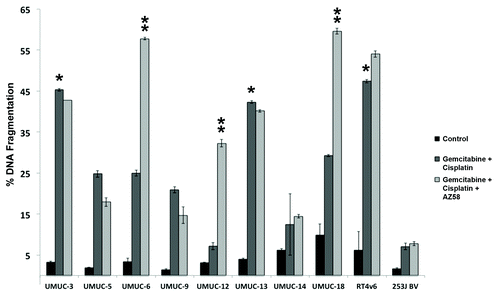
We exposed a panel of 10 distinct urothelial cancer cell lines () to established, standard bladder cancer chemotherapy.Citation4,Citation5 Gemcitabine and cisplatin dosages were fixed at 1 μM based on independent dose finding experiments (data not shown). A cut point of 30% DNA fragmentation on PI-FACS was used as an arbitrary cut point for sensitivity. Three cell lines, UMUC-3, UMUC-13, and RT4v6 (as indicated by single asterisk), were determined to be sensitive to chemotherapy alone (). All 10 urothelial cancer cell lines were further exposed to AZ58, a Smac mimetic, in addition to gemcitabine and cisplatin. A dose of 30 nM was used for all combination studies based on separate dose response studies (data not shown). The addition of the Smac mimetic enhanced DNA fragmentation and overcame chemotherapy resistance in three additional urothelial cancer cell lines: UMUC-6, UMUC-12, and UMUC-18 (as indicated by double asterisk). AZ58 demonstrated minimal single agent activity upon flow cytometry (DNA fragmentation 1‒18%). (Fig. S1A) However, UMUC-12 was extremely sensitive (13.1% of control) and UMUC-3 was moderately sensitive (56.7% of control) to single agent Smac mimetic in a cell proliferation assay (Fig. S1B) consistent with microscopic visualization (data not shown). Therefore, we considered UMUC-12 to be sensitive to single agent AZ58 for further experiments.
Figure 1. AZ58 overcomes resistance to gemcitabine and cisplatin in select urothelial cancer cell lines. Ten urothelial cancer cell lines were exposed to 1 μM each of gemcitabine and cisplatin for 48 h. Cells were harvested for PI-FACS analysis. Asterisks indicate cell lines sensitive to the drug combination (30% DNA fragmentation). Error bars = standard error. The same 10 urothelial cancer cell lines were exposed to AZ58 at 30 nM in addition to gemcitabine and cisplatin. Double asterisks indicate cell lines sensitive to the drug combination (30% DNA fragmentation).
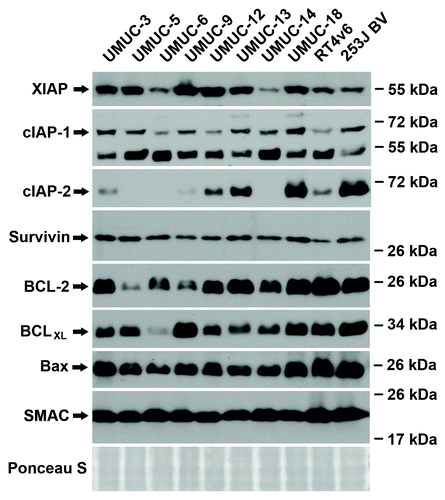
Constitutive RNA and protein expression of IAP family members, BCL family members, and Smac is not predictive of drug sensitivity
Gene expression profiling was performed on all ten urothelial cancer cell lines at baseline and a microarray was generated using probes for select members of the IAP family (XIAP, cIAP-1, cIAP-2, Survivin), BCL family (BCL-2, BCLXL, BAX), and SMAC (Fig. S2). RNA expression of XIAP, cIAP-1, Bcl-2, Bax, and Smac remained relatively constant between the cell lines. cIAP-2, Survivin, and BCLXL varied between the cell lines but did not correlate to drug sensitivity (gemcitabine and cisplatin or AZ58) or the ability of the Smac mimetic to overcome resistance to the chemotherapy. Western blotting analysis was performed on the same panel of urothelial cancer cell lines. () Protein expression of XIAP, cIAP-1, cIAP-2, Bcl-2, BCLXL, and Bax were variable between the 10 urothelial cancer cell lines. Survivin and total cellular Smac were relatively consistent between each cell line. Similar to the microarray data, levels of constitutive protein expression were not predictive of drug sensitivity.
AZ58 overcomes chemotherapy resistance through increased apoptosis
UMUC-12, UMUC-6, and UMUC-9 were identified as urothelial cancer cell lines, resistant to gemcitabine and cisplatin but with differential responses to the addition of the Smac mimetic (). UMUC-12 was considered sensitive to the Smac mimetic alone (based on microscopy and cell proliferation assay—data not shown) and in combination with chemotherapy. UMUC-6 was considered to be resistant to chemotherapy and to AZ58, separately. However, significant cell death occurred with the combination of chemotherapy and the Smac mimetic (57.8% DNA Fragmentation). UMUC-9 was considered relatively resistant to all conditions (although DNA fragmentation and caspase activation is demonstrated in AZ58 treated conditions). Cell lines were exposed to the conditions as indicated and proteins were harvested for western blotting. Corresponding Image J quantitation and PI-FACS data are demonstrated in .
Figure 3. AZ58 potentiates cell death through apoptosis. (A) Western blotting of the urothelial cancer cell lines considered resistant to gemcitabine and cisplatin (UMUC-12, UMUC-6, and UMUC-9). Treatment conditions are as labeled. (B) Image J quantitation of caspase cleavage products. Bar graphs demonstrate relative densities compared with the control. (C) Corresponding graphs of DNA fragmentation from PI-FACS are demonstrated.
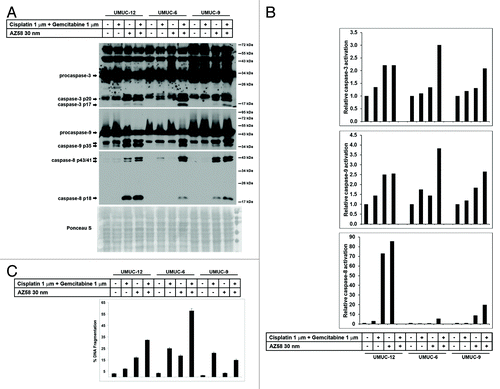
Cleaved caspase-3 is efficiently generated in the cell death conditions of UMUC-12 (Smac mimetic alone and combination) and UMUC-6 (combination), as expected. Caspase-3 activation is also evident in the combination condition in UMUC-9 consistent with the minimal DNA fragmentation seen on PI-FACS. Activation of caspase-9 is also observed in the cell death conditions of UMUC-12 and UMUC-6, while minimal generation of the p35 cleavage product is evident in the Smac mimetic treated conditions in UMUC-9 (). Cleavage products of caspase-8 are demonstrated in UMUC-9 (Smac mimetic and combination) along with the cell death conditions of UMUC-12 and UMUC-6. Taken together, these results show that AZ58 overcomes chemotherapy resistance through increased apoptosis. Further, even in a cell line considered to be “relatively resistant” to a combination of chemotherapy and the Smac mimetic (UMUC-9), cleavage products of caspases are present in the combination condition.
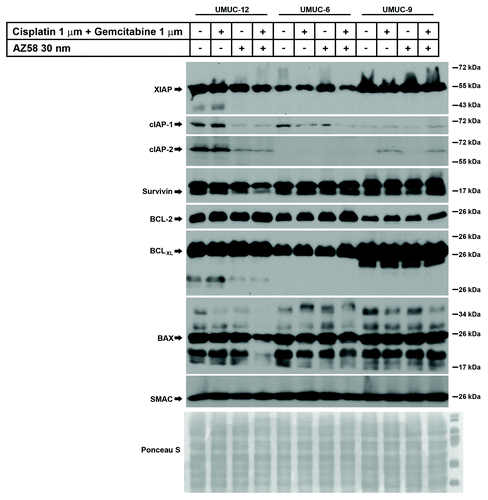
Downregulation of cIAP-1 is associated with cell death
UMUC-12, UMUC-6, and UMUC-9 were subjected to the same conditions as above (control, gemcitabine and cisplatin, Smac mimetic alone, and the combination) and lysed for western blotting. Blotting was performed for select members of the IAP family, BCL family, and SMAC. () XIAP was variably expressed in the treated conditions of UMUC-12 and UMUC-6 but relatively consistent in the UMUC-9 treated conditions. We demonstrate that XIAP expression was reduced in the cell death condition of UMUC-6 and to a lesser extent in UMUC-12 cells, but relatively unaffected in the UMUC-9 cells treated with gemcitabine, cisplatin, and AZ58. In UMUC-12 and UMUC-6, the downregulation of cIAP-1 correlated with the cell death conditions. This has been demonstrated to be one of the initial steps of Smac mimetic mediated cell death through the NFκB and TNF-α autocrine loop pathway.Citation17 While protein expression of cIAP-1 was low in UMUC-9, there were no obvious changes in any treatment conditions. Similar to cIAP-1, cIAP-2 was also degraded in the cell death conditions of UMUC-12. However, cIAP-2 was upregulated in the gemcitabine and cisplatin treated conditions of UMUC-9, irrespective of the presence or absence of AZ58. The relevance of these findings needs further exploration. Survivin degradation was evident in the cell death conditions of UMUC-12 but similar degradation was not seen in the other two cell lines. Bcl-2 and BCLXL expression remained consistent within each cell line; however, BAX demonstrated reduced expression in the combination treatment of UMUC-12. As expected, total cellular Smac expression was similar in all treated conditions.
Figure 4. Degradation of cIAP-1 corresponds to cell death conditions in urothelial cancer cell lines identified as resistant to gemcitabine and cisplatin. Western blotting of urothelial cancer cell lines resistant to gemcitabine and cisplatin were subjected to various conditions as indicated. Antibodies for selected members of the IAP family, BCL family, and Smac were immunoblotted.
AZ58 demonstrates a non-significant decrease in tumor volume
The UMUC-6 bladder cancer cell line was extremely sensitive to the combination of gemcitabine, cisplatin, and AZ58 despite demonstrating limited activity to the agents alone (). Therefore, a flank model of UMUC-6 was established in nude mice as described in the methods. Dosages for gemcitabine, cisplatin, and AZ58 were determined by 3 independent dose-determining studies (data not shown). While the average tumor sizes for the Smac mimetic alone (269.7 mm3) and the gemcitabine/cisplatin + Smac mimetic (294.7 mm3) were decreased compared with the control (625.5 mm3), the results were not statistically significant due to the significant variability of tumor growth within all of the groups ().
Figure 5. AZ58 treatment results in reduced tumor burden; however, in a non-statistically significant fashion. (A) Microscopy of UMUC-6 cells demonstrating differential cell death in the various treatment conditions as labeled. Corresponding images show resultant graphs of PI-FACS demonstrating DNA fragmentation. (B) Female athymic nude mice were injected with UMUC-6 cells and treated as described in Materials and Methods. Tumor volumes were calculated and plotted. n = 6 mice for each condition. Error bars = standard error. (C) TUNEL staining of fragmented apoptotic DNA/nuclei to detect the efficacy of AZ58 and chemotherapy in vivo (green) and the total nuclei stained using propidium iodide (Red). Magnification 10 ×. (D) Mean number of TUNEL positive nuclei from 10 representative images.
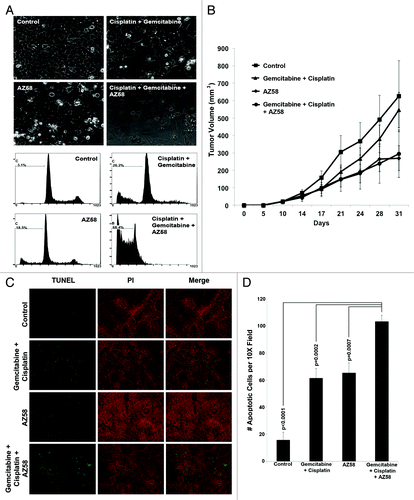
AZ58 in addition to chemotherapy increases apoptosis in tumor tissue
We performed Fluorescent terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining to determine the effects of apoptosis in tumor tissue. We found that both the chemotherapy alone and the AZ58 conditions each increased the number of apoptotic nuclei compared with the control (P < 0.0001 for both). Furthermore, the combination of chemotherapy and AZ58 increased apoptotic nuclei compared with the chemotherapy and AZ58 alone groups (P = 0.0002 and P = 0.0007, respectively). Representative images are demonstrated in with graphical representation of enumerated apoptotic cells displayed in . While the number of apoptotic cells reached statistical significance, this did not translate into a statistical difference in tumor volume. One potential explanation is the variable nature of the treatment effect and the fact that IHC was performed at only one time point. With further treatment and tumor sampling at multiple time points, a more definitive view of the apoptotic process over time could be investigated to explain the efficacy results. These experiments will need to be performed to confirm our hypotheses.
AZ58 in combination with chemotherapy produces decreased cellular proliferation and decreased microvessel density in xenograft tumors
We examined tumor sections for Ki-67 positivity using immunohistochemistry. Total Ki-67 positive cells were counted per ten random fields and averaged. Compared with the control condition, both the gemcitabine and cisplatin treated group and the combination treatment group inhibited Ki-67 to a significant level (P = 0.031 and P = 0.043, respectively). AZ58 alone treated tumors did not demonstrate a statistically significant reduction in Ki-67 positive cells. Representative images are displayed in with the associated graph in .
Figure 6. AZ58, in combination with chemotherapy, functions through decreased cellular proliferation and decreased microvessel density. (A) Immunohistochemistry with Ki-67 is displayed. Imaging was performed at 20 × magnification. Representative images are shown. (B) Positive Ki-67 nuclei were counted, averaged, and plotted on a bar graph. Error bars represent standard error. P values were calculated compared with the control condition. n = 10 images per treatment condition. (C) Immunohistochemistry using an antibody for CD-31 is displayed. Imaging was performed at 20 × magnification. Representative images are shown. (D) Positive foci of CD-31 were counted, averaged, and plotted on a bar graph. Error bars represent standard error. P values were calculated compared with the combination condition. n = 5 per treatment condition.
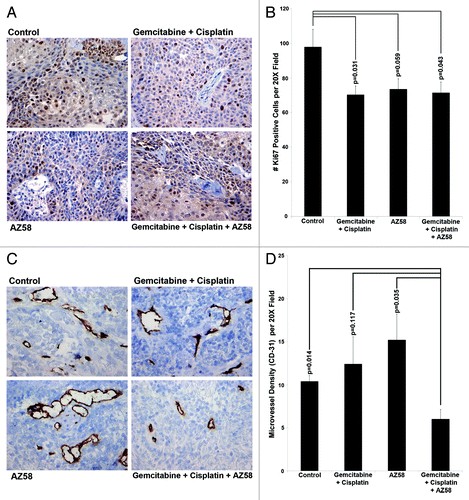
We next examined tumor tissue for microvessel density by CD-31 immunohistochemistry. CD-31 positive foci were counted in five random fields and averaged. The combination of gemcitabine, cisplatin and AZ58 reduced the amount of CD-31 positivity compared with all other conditions. The difference was statistically significant in comparison to the control condition and the AZ58 alone condition (P = 0.014 and P = 0.035, respectively). The decrease in foci compared with the chemotherapy alone group did not reach significance. Representative images are displayed in and averages of CD-31 positive foci are demonstrated in . The decrease in microvessel density has not been previously demonstrated by AZ58 and its mechanisms will have to be further investigated. Furthermore, it is worth mentioning that the IHC work was performed at a single time point (study endpoint) and the morphological changes are more likely on a continuum. Tumors likely adapt and change with the treatment of chemotherapy with and without AZ58. Future studies evaluating tissue protein expression at several time points will be very interesting.
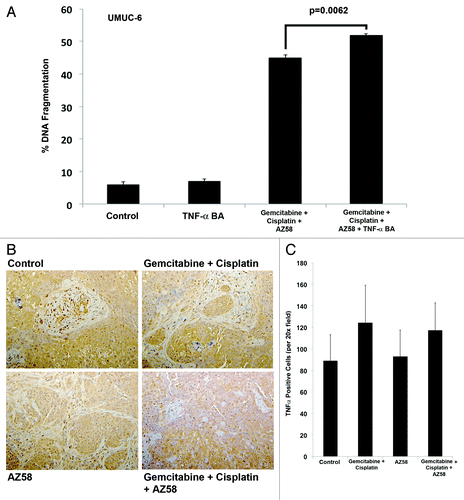
Cell death in UMUC-6 from AZ58 is mediated through a TNF-α-independent mechanism
Smac mimetic treatment has been demonstrated to induce degradation of cIAP-1 and activation of the canonical and non-canonical NFκB pathway; activation of this pathway results in cell death through a TNF-α-dependent autocrine loop.Citation17-Citation19 We demonstrated a downregulation of cIAP-1 in cell death conditions; therefore, we investigated whether TNF-α played an essential role in the cell death of UMUC-6 with the Smac mimetic. We found that DNA fragmentation in UMUC-6 exposed to gemcitabine, cisplatin, and the Smac mimetic did not rely on TNF-α using a blocking antibody (). We then investigated the expression of TNF-α in tumor tissue using IHC. We demonstrate that there is no significant difference in expression between treatment conditions () concluding that the cell death demonstrated in the UMUC-6 animal model is independent of a TNF-α mechanism.
Figure 7. Cell death in UMUC-6 from AZ58 is mediated through a TNF-α-independent mechanism. (A) The effects on UMUC-6 of chemotherapy and AZ58 were not mitigated by the TNF-α blocking antibody. (B) Representative images of IHC for TNF-α demonstrate no difference in expression between treatment conditions. (C) Ennumeration with cell counting confirms the lack of a statistical difference in TNF-α expression.
Discussion
In our report, we have demonstrated the effectiveness of a Smac mimetic in conjunction with what is considered to be a standard regimen in bladder cancer chemotherapy. In vitro, we show that 3 of 10 urothelial cancer cell lines are sensitive to gemcitabine and cisplatin at baseline. However, the Smac mimetic is able to overcome resistance in 3 additional cell lines. Microarray data and constitutive protein expression of IAP family, BCL2 family, and Smac were not able to predict for sensitivity to the chemotherapy or the Smac mimetic. However, degradation of cIAP-1 correlated with cell death induction by AZ58 in UMUC-12 and UMUC-6. Additionally, in an in vivo mouse model of bladder cancer, treatment with a Smac mimetic alone and in combination with chemotherapy resulted in smaller average tumor volumes than the control or chemotherapy alone groups. The increased apoptosis in the tumor sections of combination treated conditions reached significance compared with all other conditions. Furthermore, we demonstrate that the combination treated conditions had decreased tumor Ki-67 positivity and decreased CD-31 compared with the control untreated group. We also determined that the cell death in the UMUC-6 cell line both in vitro and in vivo were independent of a TNF-α mechanism.
Smac mimetics have been shown to have activity in other tumors. For instance, in pancreatic cancer, a Smac mimetic was effective in enhancing the tumoricidal capacity of multiple chemotherapeutics.Citation13 Drug combinations including the Smac mimetic were able to decrease cellular proliferation and increase apoptosis in vitro while decreasing tumor size and prolonged survival in mouse models in vivo.Citation13,Citation15 Likewise in lung cancer, a Smac mimetic was also able to sensitize cells to chemotherapy in a synergistic fashion. Further, in combination with chemotherapy, the Smac mimetic was able to increase tumor inhibition and increase survival in a xenograft model of lung cancer.Citation12
Recent evidence has demonstrated the importance of IAPs in bladder cancer. Li et al. examined tumor tissue from 176 patients with NMIBC for expression of XIAP. Using immunohistochemistry, they divided the group into positive (108 patients) and negative (68) for XIAP. They determined that patients negative for XIAP had a statistically significant improvement in recurrence free survival compared with the cohort who had positive staining of XIAP. Furthermore, of the patients who recurred, 12 patients progressed to a more advanced stage and all came from the XIAP positive group. XIAP status was significant on multivariate analysis even after controlling for stage, grade, size of tumor and multiplicity.Citation20 It has also been demonstrated that both cIAP-1 and cIAP-2 expression are higher in bladder cancer tissue compared with normal bladder controls, and nuclear cIAP-1 expression has been shown to be predictive of overall survival even after controlling for stage and grade in a multivariable model.Citation21
Smac expression has also demonstrated significance in bladder cancer. Mizutani et al. reported Smac expression in tumors of 84 patients with bladder cancer by western blotting. They found that Smac expression was almost universal in Ta and T1 tumors but expressed in less than half of muscle invasive tumor samples. Further, more vs. less expression of Smac in the NMIBC cohort predicted for recurrence free survival. In the muscle-invasive cohort, Smac expression predicted for disease specific survival.Citation11 These data demonstrate the significance of IAPs and Smac in the pathogenesis of bladder cancer. Therefore, Smac mimetics may be significant in the treatment of urothelial carcinoma.
Newer drug classes such as Smac mimetics are attractive, especially compared with non-specific cytotoxic chemotherapy agents. However, with all treatments, identifying patients most apt to respond is of the utmost importance for clinicians and scientists alike. Herein, we identify a novel drug combination that may produce a benefit in patients with advanced bladder cancer. Further work is necessary to elucidate an exact mechanism of action and to identify predictive markers to treat patients most likely to respond to therapy.
Materials and Methods
Cell lines and reagents
All urothelial carcinoma cell lines were obtained and fingerprinted by the specimen core of the MD Anderson GU SPORE. The RT4v6 cell line was developed from the RT4 bladder cancer cell line through six serial passages through nude mice to isolate a more rapidly growing subset. The urothelial cell line 253J BV was generated by five passages of the 253J parental cell line. All cells were cultured in MEM supplemented with 10% FBS, penicillin, streptomycin, vitamins, l-glutamine, nonessential amino acids and pyruvate supplements.
AZ58 is a Smac mimetic developed by AstraZeneca. Antibodies used for western blot analysis were purchased from the following manufacturers: XIAP, cIAP-1, cIAP-2, caspase-9, caspase-8 (Cell Signaling Technology); caspase-3, BCLXL, BCL-2, survivin, Bax (Santa Cruz Biotechnology); and Smac (R&D Systems). The anticancer drugs used in the study were cisplatin (PCH Pharmachemie) and gemcitabine (SAGENT Pharmaceuticals).
Analysis of apoptosis
3‒5 × 104 cells were plated on 6-well plates depending on proliferation rates of the urothelial cancer cell line. After 24 h of incubation, cell lines were treated with varying drug dosages as indicated in the figures. After 48 h of treatment (unless indicated otherwise), both attached and floating cells were harvested by trypsinization and pelleted using centrifugation. Cells were resuspended in PBS containing propidium iodide (50 mg/mL), 0.1% Triton X-100, and 0.1% sodium citrate. PI fluorescence was analyzed by fluorescence activated cell sorting (Becton Dickinson). The sub-G0 phase cells, indicating DNA fragmentation, were considered apoptotic. All PI-FACS analysis was performed in triplicate.
Western blotting
After plating cells and treating as indicated in the figures, cells were scraped in media at 24 h, pelleted at 3500 rpm (five minutes), washed once with ice cold PBS and re-pelleted at 3500 rpm (5 min). The pellets were lysed (lysis buffer: 50 mM TRIS-HCl, pH 7.4; 150 mM NaCl; 5 mM EDTA; 25 mM NaF; 1% Triton-X 100; 1% NP-40; 0.1 mM Na3VO4; 12.5 mM β-glycerophosphate; 1 mM PMSF and complete protease inhibitors (Roche) and clarified at 13 000 rpm for 10 min at 4 °C. The lysates were separated on 15% SDS-PAGE and western blotted using nitrocellulose membranes. The blots were developed using ECL reagent (GE Healthcare). Equal protein loading was confirmed using Ponceau-S staining and a representative image is provided in each panel of western blots. Ponceau-S for each individual immunoblot is available. Select western blots were quantified using Image J software (NIH).
Animals
Female Nude Mice (athymic NCr-nu/nu; age 3‒5 weeks) were purchased from the National Cancer Institute. All animal experiments were performed in accordance with the MD Anderson Institutional Animal Care and Use Committee (IACUC) approved protocol (11-10-10131).
In vivo evaluation of cisplatin, gemcitabine, and AZ58 against urothelial cancer cells
UMUC-6 cells were trypsinized at 70–80% confluence and washed (1100 rpm for 5 min) once with 10% FBS containing MEM and once with PBS. The cells were resuspended in PBS and counted. 5 × 105 cells in 100 μL PBS were injected subcutaneously in the flank region. Mice were grouped into the following treatment conditions (n = 6): control, gemcitabine, and cisplatin (25 mg/kg and 2.5 mg/kg, respectively) dosed by intraperitoneal injection once weekly, Smac mimetic (2 mg/kg) dosed by tail vein injection once weekly and the combination of gemcitabine, cisplatin, and Smac mimetic. All treatments were performed on the same day and initiated on post-tumor injection day 10 when tumors were visible. AZ58 was diluted in Captisol (Ligand Pharmaceuticals) for animal experiments. The primary endpoint of the experiment was when any of the mice reached the IACUC guideline limit on tumor burden of 1.5 cm in diameter. Mice and tumors were inspected every 3‒4 d and measurements were taken using Vernier calipers. Tumor volumes were calculated as described previously with 0.5 × α × β2 where α represents the longest dimension and β is perpendicular to α.Citation22 Error bars represent standard error.
TUNEL staining of tumor sections
Formalin-fixed, paraffin-embedded tumor sections were deparaffinized, fixed in paraformaldehyde and apoptotic cells were stained using a dead end fluorescent terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) kit (G3250) as per manufacturer’s instructions (Promega). Total nuclei were stained using propidium iodide. Sections were imaged using a Zeiss Plan-Neofluar lens on an epifluorescence microscope (Olympus). Representative images were taken using a 10 × objective lens. The total number of TUNEL positive nuclei was enumerated from ten random fields per treatment condition and counted. Mean and standard errors were calculated.
Proliferating cells (Ki-67), microvessel density (CD-31) and TNFα expression in vivo
Formalin-fixed, paraffin-embedded sections were deparaffinized, fixed in paraformaldehyde and stained with anti-Ki-67 antibody (DAKO) as previously reported.Citation23 Ten random images were taken using a 20x objective lens. Ki-67 positive nuclei were counted, averaged and plotted. The same protocol was replicated for TNFα expression using anti-TNFα antibody (Genzyme). For CD-31 (BD Biosciences), cryosections of OCT preserved tumor samples were sectioned and stained with diaminobenzidine (DAB). Five random images were obtained using a 20x objective lens. Foci of CD-31 positive areas were enumerated, averaged and plotted.
Microarray analysis
Total RNA was isolated from each of the ten cell lines using the MirVana RNA Isolation kit (Ambion). Quality control for the RNA was determined with the Experion Bioanalyzer (Bio-Rad). Total RNA was labeled, subjected to biotinylated cRNA synthesis using the Illumina RNA amplification kit, and hybridized to the Illumina Human HT12 v3 chips (Illumina). Bead Chips were scanned with Genome Studio (Illumina) and data were normalized using the quantile normalization method in the LIMMA (Linear Models for Microarray Data) package in the R language environment.Citation24 The heat map was generated after adjusting each gene value to a mean of zero.
Evaluation of the TNF-α contribution to cell death
TNFα Blocking antibody was purchased from R&D Systems. After cells were plated for 24–36 h (50‒70% confluence), treatment media and cells were treated with TNF-α blocking antibody (1 μg/mL). After one hour, the TNF-α blocking antibody media was replaced with the treatment media (treatment condition + TNF-α blocking antibody). After 24 h of treatment, cells were trypsinized and stained for PI-FACS analysis.
Cell proliferation assay
3–5 × 104 cells were plated on 96-well plates according to the differing proliferation rates to obtain a 50‒70% confluence at 24 h. Treatment commenced with varying drug dosages as indicated in figures. After 48 h of incubation, MTT solution (methyl-thiazolyldiphenyl-tetrazolium bromide) was added to the cells for 2 h and the crystals were dissolved using DMSO (100 μL). Quantitation of formazan crystals was performed by spectrophotometric measurements by the difference in optical density at 570 nm between the sample and the blank.
Statistical analyses
All statistical analyses for relevance were performed using the Student t test (two-tailed distribution, two-sample unequal variance) in Microsoft Excel 2010. The P value less than 0.05 were considered significant.
Conclusion
A Smac mimetic, AZ58, is able to overcome resistance to chemotherapy in selected bladder cancer cells through induction of apoptosis. This treatment strategy merits clinical evaluation, especially in patients with urothelial cancer who are resistant to chemotherapy.
Additional material
Download Zip (148 KB)Disclosure of Potential Conflicts of Interest
This study was funded in part by AstraZeneca.
References
- Sternberg CN, Yagoda A, Scher HI, Watson RC, Geller N, Herr HW, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer 1989; 64:2448 - 58; http://dx.doi.org/10.1002/1097-0142(19891215)64:12<2448::AID-CNCR2820641209>3.0.CO;2-7; PMID: 2819654
- Logothetis CJ, Dexeus FH, Finn L, Sella A, Amato RJ, Ayala AG, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 1990; 8:1050 - 5; PMID: 2189954
- Loehrer PJ Sr., Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1992; 10:1066 - 73; PMID: 1607913
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18:3068 - 77; PMID: 11001674
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23:4602 - 8; http://dx.doi.org/10.1200/JCO.2005.07.757; PMID: 16034041
- Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 2012; 11:109 - 24; http://dx.doi.org/10.1038/nrd3627; PMID: 22293567
- Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 2006; 7:988 - 94; http://dx.doi.org/10.1038/sj.embor.7400795; PMID: 17016456
- Chen DJ, Huerta S. Smac mimetics as new cancer therapeutics. Anticancer Drugs 2009; 20:646 - 58; http://dx.doi.org/10.1097/CAD.0b013e32832ced78; PMID: 19550293
- Mizutani Y, Nakanishi H, Li YN, Matsubara H, Yamamoto K, Sato N, et al. Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosis. Int J Oncol 2007; 30:919 - 25; PMID: 17332931
- Mizutani Y, Nakanishi H, Yamamoto K, Li YN, Matsubara H, Mikami K, et al. Downregulation of Smac/DIABLO expression in renal cell carcinoma and its prognostic significance. J Clin Oncol 2005; 23:448 - 54; http://dx.doi.org/10.1200/JCO.2005.02.191; PMID: 15572731
- Mizutani Y, Katsuoka Y, Bonavida B. Prognostic significance of second mitochondria-derived activator of caspase (Smac/DIABLO) expression in bladder cancer and target for therapy. Int J Oncol 2010; 37:503 - 8; http://dx.doi.org/10.3892/ijo_00000699; PMID: 20596678
- Greer RM, Peyton M, Larsen JE, Girard L, Xie Y, Gazdar AF, et al. SMAC mimetic (JP1201) sensitizes non-small cell lung cancers to multiple chemotherapy agents in an IAP-dependent but TNF-α-independent manner. Cancer Res 2011; 71:7640 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-10-3947; PMID: 22049529
- Awasthi N, Kirane A, Schwarz MA, Toombs JE, Brekken RA, Schwarz RE. Smac mimetic-derived augmentation of chemotherapeutic response in experimental pancreatic cancer. BMC Cancer 2011; 11:15; http://dx.doi.org/10.1186/1471-2407-11-15; PMID: 21226944
- Stadel D, Cristofanon S, Abhari BA, Deshayes K, Zobel K, Vucic D, et al. Requirement of nuclear factor κB for Smac mimetic-mediated sensitization of pancreatic carcinoma cells for gemcitabine-induced apoptosis. Neoplasia 2011; 13:1162 - 70; PMID: 22241962
- Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res 2010; 70:2852 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-09-3892; PMID: 20332237
- Sun Q, Zheng X, Zhang L, Yu J. Smac modulates chemosensitivity in head and neck cancer cells through the mitochondrial apoptotic pathway. Clin Cancer Res 2011; 17:2361 - 72; http://dx.doi.org/10.1158/1078-0432.CCR-10-2262; PMID: 21242120
- Vince JE, Wong WW-L, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007; 131:682 - 93; http://dx.doi.org/10.1016/j.cell.2007.10.037; PMID: 18022363
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131:669 - 81; http://dx.doi.org/10.1016/j.cell.2007.10.030; PMID: 18022362
- Probst BL, Liu L, Ramesh V, Li L, Sun H, Minna JD, et al. Smac mimetics increase cancer cell response to chemotherapeutics in a TNF-α-dependent manner. Cell Death Differ 2010; 17:1645 - 54; http://dx.doi.org/10.1038/cdd.2010.44; PMID: 20431601
- Li M, Song T, Yin ZF, Na YQ. XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancer. Chin Med J (Engl) 2007; 120:469 - 73; PMID: 17439739
- Che X, Yang D, Zong H, Wang J, Li X, Chen F, et al. Nuclear cIAP1 overexpression is a tumor stage- and grade-independent predictor of poor prognosis in human bladder cancer patients. Urol Oncol 2012; 30:450 - 6; PMID: 21795072
- Metwalli AR, Khanbolooki S, Jinesh G, Sundi D, Shah JB, Shrader M, et al. Smac mimetic reverses resistance to TRAIL and chemotherapy in human urothelial cancer cells. Cancer Biol Ther 2010; 10:885 - 92; http://dx.doi.org/10.4161/cbt.10.9.13237; PMID: 20814238
- Scott IS, Morris LS, Bird K, Davies RJ, Vowler SL, Rushbrook SM, et al. A novel immunohistochemical method to estimate cell-cycle phase distribution in archival tissue: implications for the prediction of outcome in colorectal cancer. J Pathol 2003; 201:187 - 97; http://dx.doi.org/10.1002/path.1444; PMID: 14517835
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003; 19:185 - 93; http://dx.doi.org/10.1093/bioinformatics/19.2.185; PMID: 12538238