Abstract
γ-secretase inhibitors (GSIs), the indirect inhibitors of Notch, are emerging as a new class of anticancer agents for the treatment of solid and hematological malignancies, but little is known about their effects on gastric cancer. In this study, we demonstrate that DAPT, a potent GSI, was effective to inhibit γ-secretase activity in gastric cancer (GC) cell lines that contained a fragment with approximately the size of the Notch1 intracellular domain (NICD), but was limited in their ability to induce apoptosis. However, activation of extracellular signal-regulated kinase (ERK)1/2 upon DAPT treatment was detected. Selective inhibition of ERK1/2 activation dramatically sensitized GC cells to apoptosis via downregulating β-catenin signaling in these GC cells. Notably, in a xenograft mouse tumor model, combination therapy using ERK inhibitor PD98059 plus DAPT yielded additive antitumor effects as compared with either agent alone. Taken together, these data demonstrated that γ-secretase inhibition combined with ERK1/2 inhibitor enhances cell death in GC cells partly through downregulation of WNT/β-catenin pathways.
Introduction
Notch signaling has been known to coordinate a wide range of fundamental cellular processes including cell proliferation, apoptosis, differentiation, migration and carcinogenesis.Citation1 The canonical Notch signaling is initiated by binding of the extracellular domain of transmembrane Notch receptors to their ligands and followed by cytoplasmic cleavage of the Notch intracellular domain (NICD) by γ-secretase. NICD translocates to the nucleus rapidly and interacts with the DNA-binding protein CSL (Rbp-Jκ in mice; CBF1 in humans) to affect transcriptional responses.Citation2 Notch pathway has strong ability to cross-talk with all the other major signaling pathways in cell fate decision.Citation3 Over the past few years, Notch pathway has been justified as a therapeutic target for cancers.Citation4 As for gastric cancer (GC), it has been reviewed by Katoh et al. that γ-secretase inhibitors (GSIs) may be used as anti-cancer drugs.Citation5 N-[N-(3,5-difluorophenacetyl)-l-ananyl]-S-phenyglycine t-butyl ester (DAPT), one kind of GSIs, is widely used to inhibit Notch activation, suggesting that DAPT may be useful in treating GC. However, there are conflict results of Notch1 expression in GC,Citation6,Citation7 and the therapeutic effect of DAPT on GC remains unclear.
ERK pathway is known to be a positive regulator of cellular processes and contribute to tumorigenesis. Recently, high throughput kinase inhibitor screens revealed ERK signaling to be the fundamental Notch regulators in breast cancer,Citation8 and the Notch pathway regulates ERK activation in non-small cell lung carcinoma,Citation9 indicating there may exist cross talk between Notch and ERK pathways. Therefore, to verify the related roles of Notch and ERK pathways in GC will be helpful to understand the potential therapeutic effect of DAPT on GC.
In the present study, we investigated the therapeutic effect of DAPT on GC, and the results showed that concurrent treatment of GC cell lines in vitro and in vivo with DAPT and the ERK1/2 inhibitor PD98059 results in dramatically increased cytotoxicity than observed with either agent alone. This additive lethality appears dependent upon the regulation of β-catenin and its downstream targets c-Myc and cyclin D1.
Results
Notch1 activation in GC tissues and cells
GC tissue array (n = 71, ) showed that activated Notch1, the cleaved intracellular (activated) form (Notch1 intracellular domain, NICD) which is generated by γ-secretase cleavage of Notch, were expressed in both tumor tissues and their paired corresponding adjacent normal tissues, but compared with their paired normal gastric mucosa, 54.93% (39/71) of GC tissue expressed positive Notch1, while 15.49% (11/71) of GC tissue manifested as negative (), suggesting activated Notch1 may play an important role in gastric carcinogenesis.
Table 1. Clinical characteristics of activated Notch1 tissue specimens
Figure 1. Analysis of activated Notch1 expression in human GC tissues and cells. Immunohistochemical staining for activated Notch1 in gastric adenocarcinoma and their normal counterparts (n = 71) was detected using polyclonal antibody to human activated Notch1 (1:200 dilution). The results of immunohistochemical staining for activated Notch1 were scored and relative expression of activated Notch1 in 71 primary gastric cancer tissues compared with their pair-matched adjacent non-tumor tissues were illustrated (A). The histograms above the x-axis means positive expression of activated Notch1. The brown signals represent positive staining for activated Notch1 (original magnifications ×200) (B). Activated Notch1 expression levels in the cellular total protein were determined by western blotting. Each blot was stripped after being detected with activated Notch1 and reprobed with GAPDH to ensure equal protein loading (C). Band densities were normalized to GAPDH. Intensities of the immunoreactive bands were quantified by densitometric scanning.
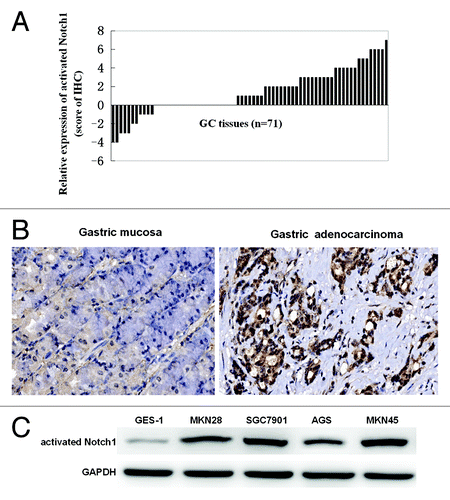
To identify gastric cancer cells with or without activated Notch1 signaling, western blot analysis was performed using an antibody raised against activated Notch1. We determined activated Notch1 expressions in gastric cancer cell lines MKN28, SGC7901, AGS, MKN45, and GES-1, an immortalized human gastric mucosal epithelial cell. Although the expression level varied with the GC cell lines, Notch1 signaling was activated in all five cell lines, whereas GES-1 weakly expressed activated Notch1 ().
Effect of DAPT on ERK1/2 pathway activity
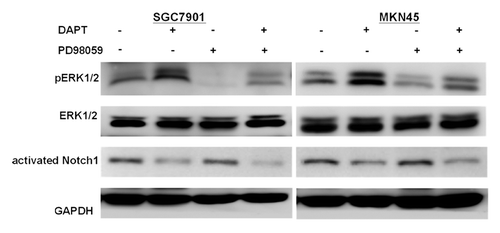
Using western blot analysis, both the SGC7901 and MKN45 cell lines exhibited basal expression of phosphorylated ERK1/2, indicating constitutive activation of the ERK1/2 signaling and the expression of phosphorylated ERK1/2 increased in response to the treatment with DAPT (). Treatment with the ERK inhibitor, PD98059, significantly inhibited both basal and DAPT-increased expression of phosphorylated ERK1/2.
Figure 2. ERK1/2 activity in gastric cancer cell lines treated with DAPT in the presence or absence of the ERK inhibitor PD98059. SGC7901 and MKN45 cells treated with DAPT at 10 μM or PD98059 at 10 μM or DAPT-PD98059 combination for 24 h and analyzed by immunoblot with antibody for phosphorylated ERK1/2 (p-ERK1/2), total ERK1/2 (ERK1/2), activated Notch1, or GAPDH.
ERK1/2 inhibition enhanced the anti-proliferative effect of DAPT in GC cells
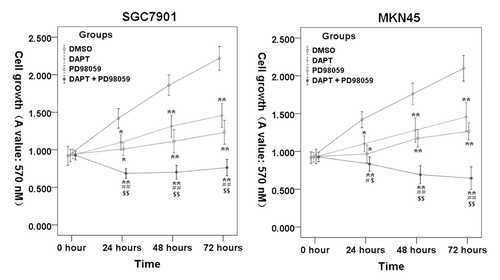
To examine whether blockade of ERK1/2 signaling with PD98059 potentiated the ability of DAPT to inhibit the cell proliferation of gastric cancer cell, SGC7901 and MKN45 cells were cultured in the presence of either DAPT (10 μM) or/and PD98059 (10 μM) for 24−72 h. As shown in , treatments with DAPT or PD98059 alone resulted in moderate growth-inhibitory effects in the SGC7901 and MKN45 cell lines. In contrast, the DAPT-PD98059 combination treatment resulted in a greater reduction in cell growth than single-agent therapy. These data suggest that the DAPT-PD98059 combination may be truly cytotoxic rather than simply cytostatic.
Figure 3. Inhibition of DAPT and PD98059 on SGC7901 and MKN45 cells proliferation. SGC7901 and MKN45 cells were cultured with 10 μM PD98059 and/or 10 μM DAPT for 24–72 h, cell proliferation was measured by MTS. Data represent the mean ± SE of three independent experiments and each experiment was performed in triplicate. 0.1% DMSO was used as control (*P < 0.05, **P < 0.01 vs. DMSO control cells; #P < 0.05, # #P < 0.01 vs. DAPT alone; $P < 0.05, $$P < 0.01 vs. PD98059 alone).
Effect of PD98059 on DAPT-induced apoptosis
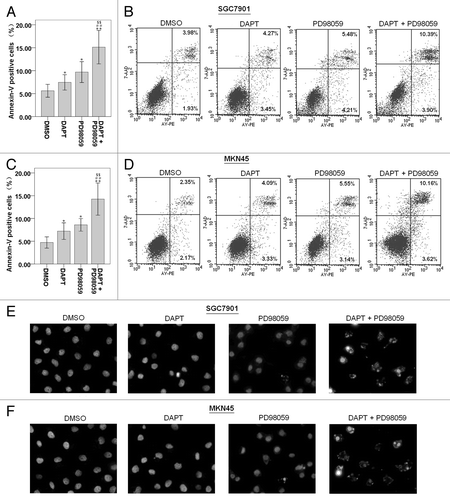
Here, we demonstrated that 10 μM PD98059 could significantly inhibit phosphorylation of ERK1/2 () without showing severe toxicity () to the SGC7901 and MKN45 cells. Therefore, we treated the cells with the concentration of 10 μM for further studies. Compared with DMSO control, exposure to 10 μM PD98059 alone caused a 2-fold induction of apoptosis in SGC7901 and MKN45 cells, as measured by the Annexin V-FITC/PI assays for apoptosis analysis (). While treatment with DAPT alone induced apoptosis in 5–9% of the SGC7901 and MKN45 cells, combined treatment with PD98059 markedly augmented the cytotoxic effect of DAPT, leading to the apoptotic cell death of approximately 13–28% (). Apoptotic cells show the characteristic morphological changes that include chromatin condensation, nuclear fragmentation, so the apoptosis was also determined using DAPI nuclear staining. Consistently, we found that the SGC7901 and MKN45 cells exposed to both PD98059 and DAPT exhibited increased chromatin condensation and nuclear fragmentation compared with either single agent alone ().
Figure 4. Effects of DAPT and/or PD98059 on apoptosis of SGC7901 and MKN45 cells. After cultured with DAPT (10 μM) and/or PD98059 (10 μM) for 24 h, cells were stained with Annexin V-PE/ 7-AAD and analyzed by flow cytometry. Lower left quadrants, viable cells. The numerical results represent the mean of triplicate plates (A and C) and a representative experiment is shown (B and D). Data represent the mean ± SE of three independent experiments and each experiment was performed in triplicate (*P < 0.05, **P < 0.01 vs. DMSO control cells; #P < 0.05, ##P < 0.01 vs. DAPT alone; $P < 0.05, $$P < 0.01 vs. PD98059 alone). Cells were treated with the appropriate drug combinations for 24 h, stained with DAPI and photographed (E and F).
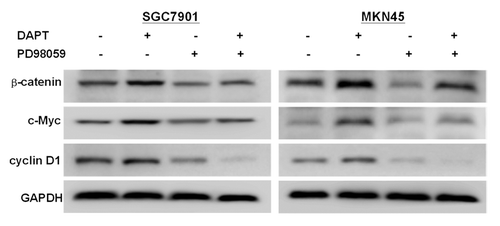
Effect of PD98059 on DAPT-induced change in related signaling proteins
To gain further into the molecular mechanisms underlying the effects on growth inhibition and apoptosis, we assayed the expression of two target genes of Notch signaling, c-Myc and cyclin D1,Citation10,Citation11 by western blot. However, our experiments found that the expressions of c-Myc and cyclin D1 were significantly upregulated after treatment with DAPT (). Interestingly, when DAPT was combined with PD98059, DAPT-induced alteration of c-Myc and cyclin D1 was significantly decreased (). Since c-Myc and cyclin D1 are also both target genes of WNT/β-catenin signaling, we tested the expression levels of β-catenin, a key component of WNT/β-catenin signaling. Interestingly, treatment with 10 μM DAPT increased β-catenin levels and treatment with 10 μM PD98059 markedly decreased the DAPT-induced alteration of β-catenin levels in the SGC7901 and MKN45 cells ().
Antitumor effect of combination therapy in vivo
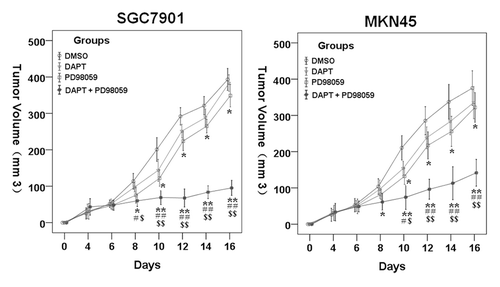
To test whether ERK inhibitor would enhance antitumor activity of DAPT in vivo, we treated SGC7901 and MKN45 xenografts in animals with the vehicle control DMSO, DAPT, PD98059, or combination of both drugs. The inhibition of tumor growth by treatments was determined over time. Ten milligrams per kilogram DAPT or 10 mg/kg PD98059 alone had only minor antitumor activity (). However, combination therapy with DAPT and PD98059 led to significant growth inhibition (), indicating the combination of PD98059 and DAPT may serve as a novel antitumor treatment against gastric cancer in vivo.
Figure 6. Therapeutic effects of treatments on the growth of tumor. Tumor sizes of all mice were assessed every 3 d according to the following formula: volume (mm3) = (width2 × length)/2. Compared with the control group and the 10 mg/kg DAPT or 10 mg/kg PD98059 group, the DAPT-PD98059 combining regimen significantly inhibits tumor growth. Data are mean ± SE of at least two independent experiments (*P < 0.05, **P < 0.01 vs. DMSO control cells; #P < 0.05, # #P < 0.01vs. DAPT alone; $P < 0.05 vs. PD98059 alone).
Discussion
In vertebrates and mammals, Notch1 is one of the receptors in Notch signaling, and dysfunctions of this pathway has been linked to many human cancers.Citation12 As different results of Notch1 expression in GC were obtained by different researchers,Citation6,Citation7 in order to investigate the therapeutic role of DAPT on GC, we first clarified Notch1 activation in GC tissues and cell lines with various degrees of differentiation as well as a human gastric mucosa epithelial cell line, GES-1. The GC tissue array showed that compared with their paired adjacent normal gastric mucosa, majority of GC tissue expressed higher levels of activated Notch1. Activated Notch1 expressions were also determined in all four gastric cancer cell lines and GES-1. Therefore, we chose SGC7901 and MKN45 cells as the study objects to investigate the antitumor role of DAPT because of their high expression of activated Notch1. The results demonstrated that DAPT induced cell proliferation inhibition significantly and also mediated visible cell apoptosis in SGC7901 and MKN45 cells, even though its in vivo tumor inhibition effect was not significant.
However, several different studies demonstrated different results about Notch1 expression in GC. Some researchers showed that normal gastric mucosal and several human GC cell lines expressed total Notch1 even though the expression level varied with the cell line,Citation7,Citation13 but others detected activated Notch1 in 34 GC samples (34/74) and no expression of Notch1 in 4 GC cell lines as well as 10 normal gastric specimen.Citation6 It's worth noting that a research group recently reported that DAPT didn't bring about cell death by trypan blue staining in AGS and BGC823 GC cells which were absent of activated Notch1.Citation6 The results of this study were not consistent with ours. The reason for this phenomenon may be due to Notch signaling controlling the cell fates in a context- and cell type-dependent manner.Citation2
ERK signaling is one member of the mitogen-activated protein kinase (MAPK) superfamily. The ERK pathway is known to be activated by growth stimuli and generally acts as a prosurvival mediator.Citation14 Some studies have shown that ERK pathway can also be activated by some drugs, such as chemotherapeutic drugs.Citation15 And even more interestingly, chemotherapy reagent-mediated ERK activation contributes to chemoresistance in gastric cancer cells.Citation16,Citation17 In our previous study, we have also found that activation of ERK1/2 protects gastric carcinoma SGC7901 cells from histone deacetylase inhibitor TSA-mediated apoptosis.Citation18 Blockade of the ERK pathway sensitized tumor xenografts to the cytotoxicity of histone deacetylase inhibitor MS-275 in vivo.Citation19 However, some studies have shown that ERK1/2 inactivation by ERK1/2 inhibitor markedly blocked cisplatin-induced apoptosis in human malignant testicular germ cells.Citation20 In view of these facts, we further investigate the possible potential links between Notch pathway and ERK phosphorylation in GC cells. The results demonstrated that phosphorylated ERK1/2 increased in response to γ-secretase inhibitor DAPT, which means ERK signaling was activated by DAPT. Importantly, inhibition of DAPT-induced ERK1/2 activity with PD98059 markedly enhanced the DAPT-induced apoptosis in two human gastric cancer cell lines, SGC7901 and MKN45. Moreover, ERK activity was determined via phosphorylation of the ERK substrate myelin basic protein (MBP), so the phosphorylation status of MBP directly refects ERK activity. Therefore, further studies need to be conducted to determine ERK activity by testing phosphorylation of MBPs.
PD98059 increased apoptotic potential of DAPT () suggested that DAPT-induced ERK1/2 activation might be a way to evade DAPT-induced apoptosis rather than a death-inducing stimulus in GC cells. However, a previous study has found that another GSI LSN-411575 downregulated ERK1/2 activity in KrasG12V-driven non-small cell lung carcinoma cells,Citation21 which is different from our results. These studies suggest GSIs may induce or inhibit ERK1/2 activity in different cell context. Furthermore, DAPT combination with PD98059 markedly suppressed the growth of xenografts in nude mice, while DAPT alone showed only moderate tumor inhibitory effect on these nude mice bearing SGC7901 and MKN45 xenografts. These results suggest that the therapeutic efficacy of the drug combinations is synergistic. Although the number of cell lines used remains small and further in-depth studies are needed, these findings highlighted the cytoprotective role of the ERK1/2 pathway in DAPT-induced apoptosis and suggested that a combined treatment with GSIs and ERK inhibitors may provide an attractive therapeutic strategy for safer anticancer chemotherapies with enhanced efficacy in cancers in which the ERK pathway is constitutively activated.
On the other hand, there are reciprocal interactions between WNT/β-catenin and Notch signals in the process of cell fate decision,Citation22 therefore, to understand the relationship of these two signaling pathways in gastric cancer is important for us to clarify the pathogenesis of GC. β-catenin is a key component of WNT/β-catenin signaling. In the present study, we found that inhibition of Notch1 with DAPT led to upregulation of β-catenin protein expression; however, a previous study demonstrated that downregulation of Notch1 resulted in accumulation of active β-catenin protein without changes in β-catenin protein.Citation23 Since c-Myc and cyclin D1 are biologically relevant to Notch1 inhibition-induced apoptosis and cell cycle arrest in cancer cells,Citation10,Citation11 and c-Myc and cyclin D1 are also both target genes of WNT/β-catenin signaling,Citation24 we focused on the effect of DAPT on their expressions in SGC7901 and MKN45 cells. However, our experiments found that c-Myc and cyclin D1 were significantly upregulated after treatment with DAPT, and the trends of c-Myc and cyclin D1 were not completely consistent with in vitro and in vivo antitumor efficacy in GC cells, suggesting c-Myc and cyclin D1 might not be directly regulated by Notch1 in GC, and some other downstream genes or signaling pathways might participate in the regulation. We also found that inhibition of ERK1/2 with PD98059 inhibited the upregulation of β-catenin protein expression as well as c-Myc and cyclin D1 expressions. Interestingly, the change trend of c-Myc and cyclin D1 was almost consistent with β-catenin, so we speculate that ERK1/2 inhibition might suppress c-Myc and cyclin D1 expressions, at least in part, by downregulating β-catenin, and ERK1/2 might act as upstream of β-catenin signaling in GC cells. However, the exact relationships between β-catenin and ERK1/2 need further investigation by downregulation of β-catenin with specific inhibitors or RNAi.
Collectively, our present demonstration that inhibitors of the ERK1/2 pathway can enhance the cell killing capacity by GSI DAPT suggests that intervention of ERK1/2 pathway may have added advantages for GSI cancer chemotherapy. We envision that inhibitors of ERK1/2, such as PD98059 in this study, would also enhance cell killing effects of DAPT both in vitro and in vivo. Although we have demonstrated the roles of β-catenin, c-Myc, and cyclin D1 in the apoptosis induced by DAPT and PD98059, the exact mechanism by which these two drugs act together to regulate β-catenin, c-Myc, and cyclin D1 remains unknown. These findings do not only highlight the importance of the crosstalk of Notch with ERK1/2 pathways, but they may also suggest a new avenue of combination therapy for some GCs. Further studies will shed light on details of their regulation. Regardless, out results suggest that the strategy of combining GSIs with ERK inhibitors in GC may be viable therapeutic approach.
Materials and Methods
Reagents and cell cultures
DAPT and PD98059 (1,4-diamino-2,3-dicyano-1,4-bis[phenylthio] butadiene) was purchased from Calbiochem. Human gastric cancer cell line AGS and MKN45 (poorly differentiated, Type Culture Collection of Chinese Academy of Sciences, TCCCAS), SGC7901 (moderately differentiated, TCCCAS) and MKN28 (well differentiated, American Type Culture Collection, ATCC) were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich) and penicillin (100 U/ml) at 37 °C in a humidified incubator containing 5% CO2. Human gastric epithelial cell line GES-1Citation25 (TCCCAS) were cultured in Dulbecco's modified Eagle medium (DMEM) medium supplemented with 10% FBS.
Cell proliferation assay (MTS)
Cell proliferation was detected with Non-Radioactive Cell Proliferation Assay with MTS reagents (Promega). The treated or control cells (1 × 104/well) were plated into 96 well for 24, 48, and 72 h respectively. After incubation with Cell Titer 96® AQueous One Solution reagent for 1 h, the absorbance was measured at 490 nm according to the manufacture instruction.
DAPI staining
Changes in the cell morphology characteristic of apoptosis were examined by fluorescence microscopy of DAPI-stained cells. The cells were harvested and washed with PBS. Formaldehyde (3.7%) was added to fix the cells for 10 min at the room temperature. The fixed cells were permeabilized with 0.2% Triton X-100 in PBS for 3 min, stained with 1 μg/ml of DNA-specific fluorochrome 4,6-diamidino-2-phenylindol (DAPI) for 30 min and then washed with PBS three times. DAPI-stained cells were counted under a fluorescent microscope.
Flow cytometry analysis for apoptosis
Apoptosis was analyzed by a double-staining method using FITC-conjugated annexin V and PI. This method is based on the translocation of phosphatidylserine from the inner side of the plasma membrane to the o uter layer during the early stages of apoptosis. Phosphotidylserine exposure on the outer layer of the cell membrane was measured using the binding of annexin V-fluorescein isothiocyanate (FITC). Cells were harvested and washed with cold PBS. And then the cells were incubated for 15 min with annexin V-FITC and propidium iodide and were analyzed by flow cytometry (Becton Dickinson).
Immunohistochemistry staining
Evaluation of fascin expression by immunohistochemistry was performed using a tissue array of OD-CT-DgSt m01-014, containing 71 gastric carcinoma tissue samples. Tumor staging was done by two senior pathologists using the TNM staging system according to the WHO protocol. All images (40×) were captured and analyzed with an Aperio scanner (USC). Both the percentage of positive tumor cells and the intensity of staining were assessed in a semiquantitative fashion, and the slide was assigned a total score based on both results. The following system was employed to score the percentage of positive tumor cells: 0–25% = 0; 26–50% = 1; 51–75% = 2; >75% = 3. Intensity of staining was graded as follows: absent or faint blush = 0; weak = 1; moderate = 2; strong = 3. Thus we multiplied the two scores and the final results range from 0 to 9. If the relative score of cancer and corresponding normal adjacent tissue is greater than zero, that means positive expression of activated Notch1.
Western blotting
Whole-cell protein extracts were prepared with RIPA Lysis Buffer (Santa Cruz Biotechnology) according to the manufacturers’ instructions. Protein concentrations were determined using an assay kit (Bio-Rad). Lysates containing 100 μg of protein were mixed with loading-buffer with 5% β-mercaptoethanol and heated for 5 min at 100 °C. Samples were separated by sodium dodecyl sulfate-PAGE (SDS-PAGE) and transferred onto nitrocellulose membranes by semidry blotting. Membranes were incubated in blocking buffer (tris buffered saline [TBS], 0.1% Tween 20 and 5% non-fat dry milk) for 1 h at room temperature, followed by hybridization with anti-activated Notch1 antibody (1:1000 dilution), anti-Hes-1 antibody (1:1500 dilution), anti-β-catenin antibody (1:600 dilution), anti-c-Myc antibody (1:800 dilution), anti-cyclin D1(1:500 dilution) and anti GAPDH antibody (1:100 dilution), at 4 °C overnight. After three washes in TBS/0.1% Tween 20, the membranes underwent hybridization with a horseradish peroxidase-conjugated secondary antibody rabbit IgG (1:5000 dilutions) for 1 h at room temperature. After three washes in TBS/0.1% Tween 20, signals were detected by chemiluminescence using the western blotting luminal reagent (Santa Cruz Biotechnology).
Xenograft tumor model
All animal experiments were approved by the Committee of Animal Ethics of Taizhou University. SGC7901 or MKN45 cells (8 × 106 cells) were injected subcutaneously into the flank of mice 4 weeks old female athymic nude mice (nu/nu) which were purchased from Shanghai Laboratory Animal Co Ltd (SLAC, Shanghai, China). When tumors became palpable (−75 mm3) after implantation of tumor cells, animals were randomly assigned to different treatment groups (n = 7 in each group). Animals were injected intraperitoneally with indicated treatments once every three days for three times in total. For injection, 10 mg/kg DAPT and/or 10 mg/kg PD98059 for each mouse. Tumor volume was assessed every 3 d. Tumor volume was calculated according to the following formula: V = L × W2/2 where V = volume (mm3), L = biggest diameter (mm) and W = smallest diameter (mm).
Statistical analysis
Results are expressed as the mean ± S.E. Mean values were compared using a two-tailed t test. Differences were considered statistically significant at P < 0.05. All analyses were performed using SPSS version 16.0 (SPSS Inc.).
| Abbreviations: | ||
| PD98059 | = | 1,4-diamino-2,3-dicyano-1,4-bis(phenylthio) butadiene |
| DAPT | = | N-[N-(3,5-difluorophenacetyl)-L-ananyl]-S-phenyglycine t-butyl ester |
| GC | = | gastric cancer |
| GSIs | = | γ-secretase inhibitors |
| NICD | = | the Notch1 intracellular domain |
| ERK | = | extracellular signal-regulated kinase |
| MAPK | = | the mitogen-activated protein kinase |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81001113) and Zhejiang Natural Science Foundation (LQ13H190003 and LY12C05002).
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Notes
† These authors contributed equally to this work.
References
- Sethi N, Kang Y. Notch signalling in cancer progression and bone metastasis. Br J Cancer 2011; 105:1805 - 10; http://dx.doi.org/10.1038/bjc.2011.497; PMID: 22075946
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009; 137:216 - 33; http://dx.doi.org/10.1016/j.cell.2009.03.045; PMID: 19379690
- Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S. Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol 2007; 19:166 - 75; http://dx.doi.org/10.1016/j.ceb.2007.02.012; PMID: 17317139
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 2012; 13:654 - 66; http://dx.doi.org/10.1038/nrg3272; PMID: 22868267
- Katoh M, Katoh M. Notch signaling in gastrointestinal tract (review). [review] Int J Oncol 2007; 30:247 - 51; PMID: 17143535
- Sun Y, Gao X, Liu J, Kong QY, Wang XW, Chen XY, et al. Differential Notch1 and Notch2 expression and frequent activation of Notch signaling in gastric cancers. Arch Pathol Lab Med 2011; 135:451 - 8; PMID: 21466361
- Piazzi G, Fini L, Selgrad M, Garcia M, Daoud Y, Wex T, et al. Epigenetic regulation of Delta-Like1 controls Notch1 activation in gastric cancer. Oncotarget 2011; 2:1291 - 301; PMID: 22249198
- Izrailit J, Berman HK, Datti A, Wrana JL, Reedijk M. High throughput kinase inhibitor screens reveal TRB3 and MAPK-ERK/TGFβ pathways as fundamental Notch regulators in breast cancer. Proc Natl Acad Sci U S A 2013; 110:1714 - 9; http://dx.doi.org/10.1073/pnas.1214014110; PMID: 23319603
- Maraver A, Fernandez-Marcos PJ, Herranz D, Cañamero M, Muñoz-Martin M, Gómez-López G, et al. Therapeutic effect of γ-secretase inhibition in KrasG12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell 2012; 22:222 - 34; http://dx.doi.org/10.1016/j.ccr.2012.06.014; PMID: 22897852
- Ling H, Sylvestre JR, Jolicoeur P. Notch1-induced mammary tumor development is cyclin D1-dependent and correlates with expansion of pre-malignant multipotent duct-limited progenitors. Oncogene 2010; 29:4543 - 54; http://dx.doi.org/10.1038/onc.2010.186; PMID: 20562911
- Shepherd C, Banerjee L, Cheung CW, Mansour MR, Jenkinson S, Gale RE, et al. PI3K/mTOR inhibition upregulates NOTCH-MYC signalling leading to an impaired cytotoxic response. Leukemia 2013; 27:650 - 60; http://dx.doi.org/10.1038/leu.2012.285; PMID: 23038273
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006; 7:678 - 89; http://dx.doi.org/10.1038/nrm2009; PMID: 16921404
- Sekine A, Akiyama Y, Yanagihara K, Yuasa Y. Hath1 up-regulates gastric mucin gene expression in gastric cells. Biochem Biophys Res Commun 2006; 344:1166 - 71; http://dx.doi.org/10.1016/j.bbrc.2006.03.238; PMID: 16647036
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010; 1802:396 - 405; http://dx.doi.org/10.1016/j.bbadis.2009.12.009; PMID: 20079433
- Steelman LS, Franklin RA, Abrams SL, Chappell W, Kempf CR, Bäsecke J, et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia 2011; 25:1080 - 94; http://dx.doi.org/10.1038/leu.2011.66; PMID: 21494257
- Liu SQ, Yu JP, Yu HG, Lv P, Chen HL. Activation of Akt and ERK signalling pathways induced by etoposide confer chemoresistance in gastric cancer cells. Dig Liver Dis 2006; 38:310 - 8; http://dx.doi.org/10.1016/j.dld.2006.01.012; PMID: 16527552
- Liu L, Zhang H, Sun L, Gao Y, Jin H, Liang S, et al. ERK/MAPK activation involves hypoxia-induced MGr1-Ag/37LRP expression and contributes to apoptosis resistance in gastric cancer. Int J Cancer 2010; 127:820 - 9; PMID: 19998339
- Yao J, Qian CJ, Ye B, Zhang X, Liang Y. ERK inhibition enhances TSA-induced gastric cancer cell apoptosis via NF-κB-dependent and Notch-independent mechanism. Life Sci 2012; 91:186 - 93; http://dx.doi.org/10.1016/j.lfs.2012.06.034; PMID: 22781708
- Sakamoto T, Ozaki KI, Fujio K, Kajikawa SH, Uesato SI, Watanabe K, et al. Blockade of the ERK pathway enhances the therapeutic efficacy of the histone deacetylase inhibitor MS-275 in human tumor xenograft models. Biochem Biophys Res Commun 2013; 433:456 - 62; http://dx.doi.org/10.1016/j.bbrc.2013.03.009; PMID: 23501104
- Schweyer S, Soruri A, Meschter O, Heintze A, Zschunke F, Miosge N, et al. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. Br J Cancer 2004; 91:589 - 98; http://dx.doi.org/10.1038/sj.bjc.6601919; PMID: 15266324
- Maraver A, Fernandez-Marcos PJ, Herranz D, Cañamero M, Muñoz-Martin M, Gómez-López G, et al. Therapeutic effect of γ-secretase inhibition in KrasG12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell 2012; 22:222 - 34; http://dx.doi.org/10.1016/j.ccr.2012.06.014; PMID: 22897852
- Hayward P, Kalmar T, Arias AM. Wnt/Notch signalling and information processing during development. Development 2008; 135:411 - 24; http://dx.doi.org/10.1242/dev.000505; PMID: 18192283
- Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, et al. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat Cell Biol 2011; 13:1244 - 51; http://dx.doi.org/10.1038/ncb2313; PMID: 21841793
- Thévenod F, Chakraborty PK. The role of Wnt/beta-catenin signaling in renal carcinogenesis: lessons from cadmium toxicity studies. Curr Mol Med 2010; 10:387 - 404; http://dx.doi.org/10.2174/156652410791316986; PMID: 20455852
- Ke Y, Ning T, Wang B. [Establishment and characterization of a SV40 transformed human fetal gastric epithelial cell line-GES-1]. Zhonghua Zhong Liu Za Zhi 1994; 16:7 - 10; PMID: 8033753