Abstract
Inhibitor 2 of protein phosphatase 2A (I2PP2A), a biological inhibitor of the cellular serine/threonine protein phosphatase PP2A, is associated with numerous cellular processes that often lead to the formation and progression of cancer. In this study we hypothesized that targeting the inhibition of I2PP2A’s multiple functions in prostate cancer cells might prevent cancer progression. We have investigated the effect of the small chain C6-ceramide, known to be a bioactive tumor suppressor lipid, on I2PP2A function, thereby affecting c-Myc signaling and histone acetylation in cells. Our data indicated that C6-ceramide treatment of prostate cancer cells induces cell death in PC-3, DU145, and LNCaP cells, but not normal prostate epithelial cells. C6-ceramide was able to disrupt the association between PP2A and I2PP2A. C6-ceramide inhibits I2PP2A’s upregulation of c-Myc and downregulation of histone acetylation in prostate cancer cells. Our data indicated that targeting cancer related signaling pathways through I2PP2A using ceramide as an anti-I2PP2A agent could have beneficial effects as a therapeutic approach to prevent prostate cancer.
Introduction
Prostate cancer (PC) remains the most common malignancy and second leading cause of cancer-related death among males worldwide. Almost 30% of PC patients still suffer in the advanced stage of the disease due to non-responsiveness to hormonal androgen therapy.Citation1-Citation3 Current available drugs for the treatment of advanced PC may increase the survival rate for a few months, but the toxicity and side effects of these drugs are of great concern. The pathogenesis of androgen-resistant PC is poorly understood.Citation4-Citation7 Therefore, a thorough understanding of the molecular mechanisms involved in PC formation, recurrence, and progression is crucial, and alternative chemotherapeutic strategies to treat such patients are also needed.
We focused our study on understanding the role of an oncogenic protein, inhibitor 2 of protein phosphatase 2A (I2PP2A), which is overexpressed in many cancer cells, including brain tumors,Citation8 lung tumors,Citation9 ovarian cancer,Citation10 head and neck tumors,Citation11 Wilm tumors,Citation12 prostate cancer,Citation6,Citation13 and leukemia.Citation14,Citation15 I2PP2A is a biological inhibitor of protein phosphatase 2A (PP2A).Citation16-Citation18 PP2A is a major tumor suppressor serine/threonine phosphatase in mammalian cells and negatively regulates many pro-growth/pro-survival signaling pathways through dephosphorylation of many endogenous substrates, which include Akt, c-Myc, Bcl2, and MAPK. Dysfunction or dysregulation of these pathways often leads to cancer initiation, progression and maintenance. In most cancer cells, including PC, the tumor suppressor activity of PP2A is relatively low, which has been well correlated with the overexpression of I2PP2A.Citation16-Citation20 I2PP2A is a multifunctional protein and regulates a wide variety of cellular functions including cell cycle control,Citation21,Citation22 gene transcription,Citation23,Citation24 epigenetic regulation,Citation25 chromatin remodeling,Citation26 and cell migration,Citation27,Citation28 as well as inhibition of PP2A’s function.Citation15-Citation20,Citation27,Citation29 Several studies have reported that I2PP2A binds with NM23H1, a metastasis suppressor, and forms an inhibitory complex to prevent the DNA exonuclease function of NM23H1Citation30-Citation32 to repair the damaged DNA. The 3′-5′ exonuclease activity is a key function of NM23H1 to suppress metastasis. Therefore, by inhibiting NM23H1 I2PP2A promotes metastasis in cancer cells. Thus, evidence suggests that I2PP2A interacts with numerous signaling pathways, including PP2A-regulated signaling pathways, which in turn promote cancer formation, progression and metastasis.Citation9,Citation15,Citation19-Citation25,Citation27,Citation29
Ceramide, a bioactive tumor suppressor sphingolipid, induces apoptosis in many cancer cells by regulating various cellular signaling pathways/proteins.Citation33-Citation35 In cancer cells, various stress stimuli can induce the generation of endogenous ceramides that mediate anti-proliferative responses by regulating downstream targets or signaling events including activation of PP2A to dephosphorylate c-Myc, Bcl2, c-Fos, and other proteins.Citation36 It has been shown that treatment of various human cancer cells with chemotherapeutic agents, such as daunorubicin, vincristine, or gemcitabine, results in the accumulation of ceramides in these cancer cells and induces apoptosis.Citation33,Citation36,Citation37 Clinical studies have shown decreased levels of ceramides in malignant tumor samples as compared with normal cells, which support the tumor suppressor role of ceramides in cancer pathogenesis.Citation38-Citation41
In this study, we examined the expression of I2PP2A in prostate cancer cells and normal prostate epithelial cells. We explored whether ceramide treatment can block the I2PP2A function in androgen-resistant (PC-3, DU145) and androgen-dependent (LNCaP) PC cells. We selected the short chain, cell-permeable C6-ceramide to test whether it can decrease c-Myc accumulation in PC cells through inhibition of I2PP2A and activation of PP2A function. Since I2PP2A is known as an inhibitor of histone acetylation (INHAT), we also examined if ceramide treatment can block the epigenetic function of I2PP2A through increasing histone acetylation. Overexpression or knockdown of I2PP2A was performed to confirm that I2PP2A could be a pharmacological target for PC prevention. A ceramide can be used as a clinical inhibitor of I2PP2A to block its cellular functions in cancer cells.
Results
Determination of I2PP2A expression levels in prostate cancer cells and normal prostate epithelial cells
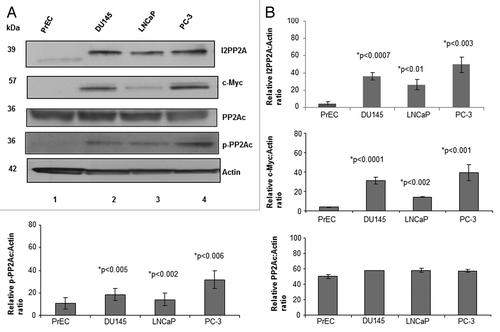
First we examined the relative expression levels of I2PP2A in PC-3, DU145, and LNCaP prostate cancer cells compared to normal prostate epithelial cells (PrEC) by western blot analysis. Results indicated that I2PP2A is overexpressed by 7- to 12-fold in all of the cancer cell lines, whereas expression of I2PP2A was either not found or very minimal in normal epithelial cells (). It was observed () that I2PP2A is more highly expressed in PC-3 cells (12-fold higher than in PrEC) than DU145 (9-fold higher) or LNCaP cells (7-fold higher). We also examined the expression level of another oncoprotein, c-Myc, in PC cells and normal cells (PrEC). c-Myc is a transcription factor that is a well-known endogenous substrate of PP2A, and accumulation of c-Myc enhances cancer cell growth.Citation9,Citation19,Citation29 Our results showed that c-Myc is highly expressed in all of the PC cell lines, whereas the presence of c-Myc was not evident in normal prostate epithelial cells. We also examined the expression level of both PP2Ac (catalytic subunit C, total cellular level) and phospho-PP2Ac (phosphorylated on the Y307 residue) in all of the PC cell lines as well as in normal prostate cells (PrEC). Studies showed that when PP2Ac is phosphorylated on the C-terminal 307 tyrosine residue, it becomes inactive for dephosphorylation and its tumor suppressor activity.Citation14 Our data indicated that the total cellular level of PP2Ac in prostate cancer cells was not altered noticeably compared with PrEC, while p-PP2Ac (inactive PP2A) levels are higher in PC cells than PrEC (). Signal densities of protein bands () were analyzed using ImageJ software and normalized to the loading control β-actin.
Figure 1. Detection and quantification of I2PP2A, c-Myc, and PP2AC in prostate cancer cells and epithelial cells. (A) Cells were grown to 80% confluency and then western blot analysis was performed to determine the expression levels of I2PP2A, c-Myc, PP2Ac (total), and phospho-PP2Ac. Each lane contains 50 µg of total protein. (B) Densitometry analysis of the protein band for the respective protein compared to actin. ImageJ software was used for this analysis. Each experiment was performed in triplicate. Data represent the mean value (error bar represents ± SD) of the relative ratio of the protein to actin; P < 0.05 was considered as significant (calculated using two tailed, paired t test using Prism software).
Determination of ceramide levels in PC-3 and LNCaP cells compared with PrEC
Next, we measured the endogenous ceramide levels in androgen resistant PC-3 cells and androgen sensitive LNCaP cells compared to the ceramide levels in PrEC. Our results indicated that the endogenous levels of specific ceramide species are noticeably different in these two cancer cell lines and in normal prostate epithelial cells; in LNCaP cells, C26-ceramide is very low (60-fold lower) whereas C18- (3-fold higher) and C20-ceramide (6-fold higher) are high compared with PrEC. On the other hand, in PC-3 cells ceramide levels are low throughout from C14- to C26-ceramide except for C20-ceramide (2-fold higher) compared with PrEC (). Our data supports the literature evidence that endogenous ceramide (C14- to C26-ceramide) levels in cancer cells become significantly altered, either higher or lower, compared with normal cells.Citation38-Citation41
Figure 2. Determination of the endogenous ceramide levels in prostate cancer and normal epithelial cells. PrEC, LNCaP, and PC-3 cells were grown until their logarithmic phase; following that cells were harvested, and pellets were collected for ceramide measurement. Ceramide levels were normalized with cellular inorganic phosphate. (A) Total endogenous ceramide levels in LNCaP cells compared with normal epithelial cells. (B) Total endogenous ceramide levels in PC-3 cells compared with PrEC cells. (C) PC-3 cells were treated with 10 µM C6-ceramide for 48 h and then total endogenous ceramide levels were measured and compared with untreated PC-3 cells.
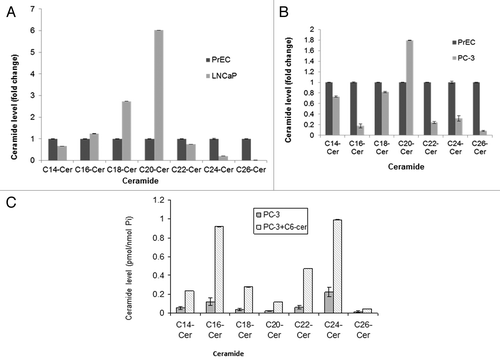
To examine if ceramide treatment of PC3 cells can alter the cellular ceramide levels, we treated the PC3 cells with 10 µM C6-ceramide for 48 h and then analyzed the ceramide levels. Results showed that in PC3 cells ceramide treatment increased the cellular ceramide levels 5- to 8-fold over untreated PC3 cells (). Our data supports the previously reported studies of the role of ceramide in cancer regulation.Citation38-Citation41
Cell viability of prostate cancer and epithelial cells after treatment with C6-ceramide
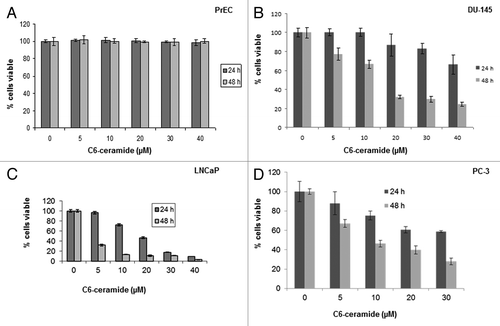
In this study, we used a short chain C6-ceramide to examine if this bioactive lipid can induce cytotoxicity in a dose- and time-dependent manner, especially in prostate cancer cells over PrEC. The data presented in indicates that ceramide was clearly able to induce cell death in all prostate cancer cell lines, and had little or no induced cytotoxicity in PrEC. The concentrations of ceramide resulting in 50% growth inhibition (IC50) following 24 h and 48 h treatment in LNCaP cells were 20 µM (24 h) and 5 µM (48 h); for PC-3 cell lines these values were 40 µM (24 h) and 10 µM (48 h); and for DU145 cells they were >40 µM (24 h) and <20 µM (48 h).
Figure 3. Cell survival of PrEC, DU145, LNCaP and PC3 cells after treatment with ceramide. Cells were plated in 6 well plates at a density of 10 × 104 cells/well, and the next day 0 to 40 µM C6-ceramide was added to the cells, which were then incubated for 24 and 48 h. Cell viability was determined using Trypan blue. (A) PrEC cells, (B) DU145 cells, (C) LNCaP cells, and (D) PC3 cells.
Overexpression of I2PP2A in prostate epithelial cells induces c-Myc expression
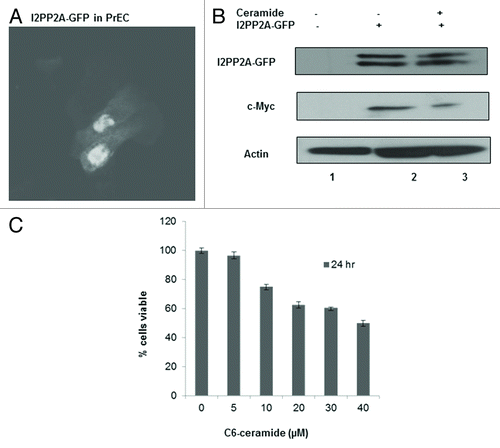
We examined if I2PP2A has any positive role in inducing cellular c-Myc expression. GFP-tagged I2PP2A was overexpressed in PrEC () and following that cells were treated with or without 10 µM ceramide for 48 h. Western blot analysis indicated that cellular c-Myc expression was increased over the control (without overexpression of I2PP2A-GFP) and decreased after treatment with ceramide (), indicating that overexpression of I2PP2A induces c-Myc expression in PrEC and that ceramide was able to block the I2PP2A effect on c-Myc accumulation. We also determined the cell viability of PrEC after overexpression of I2PP2A-GFP and following 10 µM ceramide treatment for 24 h. We observed that the cell viability of I2PP2A-overexpressing PrEC cells was decreased with increasing concentration of ceramide (), although no effect of ceramide treatment was observed in normal PrEC ().
Figure 4. I2PP2A induces c-Myc expression in normal prostate epithelial cells, and ceramide inhibits I2PP2A function. (A) I2PP2A-GFP was overexpressed in PrEC cells; following that cells were treated with or without 10 µM ceramide for 48 h. (B) c-Myc induction and the effect on c-Myc expression of ceramide treatment was examined by western blot analysis. (C) I2PP2A-GFP was overexpressed in PrEC cells; following that cells were treated with 0 to 40 µM ceramide for 24 h. Cell viability was determined using Trypan blue.
Ceramide inhibits I2PP2A regulation of c-Myc in prostate cancer cells
Our data indicated that prostate cancer cells express higher levels of I2PP2A than normal prostate epithelial cells (). Short chain C6-ceramide was also able to induce cytotoxicity in cancer cells, but not in PrEC (). Accumulating evidence suggested that c-Myc accumulation in cancer cells enhances cell growth.Citation9,Citation19 Therefore, in this study we examined if ceramide treatment can block the inhibitory effect of I2PP2A on endogenous PP2Ac for dephosphorylation of c-Myc and decrease the c-Myc accumulation in PC cells. PC cells were incubated with C6-ceramide for 48 h, and the c-Myc expression level was assessed by western blot analysis. The results suggested that ceramide was able to inhibit I2PP2A and decrease c-Myc accumulation in all PC cell lines ().
Figure 5. Ceramide treatment decreases expression of c-Myc in prostate cancer cells. (A) LNCaP, DU145, and PC-3 cells were treated with or without 10 µM ceramide for 48 h; following that the expression levels of I2PP2A and c-Myc were determined by western blot analysis. PrEC was not used in this experiment because PrEC has very low level or no I2PP2A or c-Myc (). (B) Cells were treated with 10 µM ceramide for 24 and 48 h. Immunoprecipitation was performed as described in the text using anti-PP2Ac and anti-I2PP2A. The association of PP2A/I2PP2A was determined by western blot analysis using the respective antibodies. (C and D) Cells were seeded at a density of 100 000 cells/well in 6-well plates, and the cells treated with C6-ceramide for different times (C) and with different concentrations (D) as indicated. The control cells were treated with vehicle alone (0.2% of DMSO). After treatment the cells were harvested and western blot was performed to detect c-Myc, I2PP2A. Actin was used as the loading control for the samples.
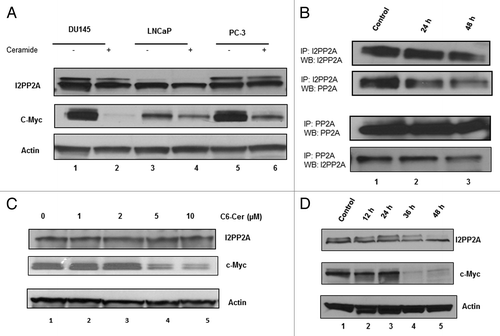
Our data indicated that PC-3 cells express a higher level of I2PP2A compared to the other cancer cell lines (). Since PC-3 cells are highly aggressive, androgen resistant and metastatic, we used only this cell line for the rest of our experiments to examine if ceramide can be used as an anti-I2PP2A agent to inhibit I2PP2A function. We checked the association of PP2A and I2PP2A in PC-3 cells with or without ceramide treatment. Cells were treated with 10 µM ceramide for 24 and 48 h; following that immunoprecipitation was performed with PP2A or I2PP2A antibody as described in Materials and Methods. Results indicated that after immunoprecipitation with either PP2A or I2PP2A, both I2PP2A and PP2A levels, respectively, decreased with increased time of ceramide treatment compared with control (). This data suggested that ceramide disrupts the association between PP2A and I2PP2A in PC-3 cells. Next, we examined if ceramide treatment of PC-3 cells can block endogenous I2PP2A inhibition of PP2Ac activity and its dephosphorylation of c-Myc. Western blot analysis indicated that ceramide treatment significantly decreased c-Myc accumulation compared with control in a time- and dose-dependent manner ().
Overexpression or knockdown of I2PP2A regulates c-Myc accumulation in PC-3 cells, and ceramide treatment enhances the degradation of c-Myc
Next, we overexpressed GFP tagged I2PP2A in PC-3 cells (), and following that cells were treated with 10 µM C6-ceramide for 48 h. Cellular c-Myc levels were examined by western blot in both the treated and untreated cells and compared with the control cells without overexpression of I2PP2A. Results indicated that overexpression of I2PP2A increased the c-Myc expression compared with control (, lanes 3 and 1, respectively). Ceramide treatment decreased the c-Myc level in I2PP2A-GFP expressing cells, but it was still higher compared with control (without overexpression and treated with ceramide) as seen in , lanes 4 and 2, respectively. This data suggested a positive role for I2PP2A to inhibit PP2A function and prevent c-Myc dephosphorylation, increasing c-Myc accumulation in cells.
Figure 6. Effect of ceramide on I2PP2A function and c-Myc accumulation after overexpression or knockdown of I2PP2A. (A) I2PP2A-GFP was overexpressed in PC-3 cells; following that cells were treated with or without 10 µM C6-ceramide for 48 h and the expression level of c-Myc was determined. (B) Knockdown of I2PP2A was performed using siRNA of I2PP2. Next cells were treated with or without 10 µM C6-ceramide for 48 h, and the expression level of c-Myc was determined. Non-targeting siRNA was used as a control. (C) After overexpression of PP2Ac, PC-3 cells were treated with or without 10 µM ceramide for 48 h and western blot was performed to examine the cellular c-Myc level. (D) Cells were pre-incubated with 10 nM okadaic acid overnight; okadaic acid was left in the media for the next 48 h while treating the cells with 10 µM ceramide, and subsequently the c-Myc level was examined.
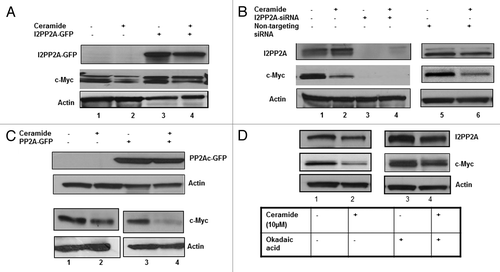
To further examine the role of I2PP2A in c-Myc regulation, we performed the reciprocal experiment, knockdown of I2PP2A in PC-3 cells using siRNA of I2PP2A (). After knocking down I2PP2A, cells were treated with 10 µM C6-ceramide for 48 h and following that western blot analysis was performed to determine c-Myc expression level. Results indicated that siRNA treatment completely abolished c-Myc expression (, lanes 3 and 4). We used non-targeting siRNA as a second control to examine if this siRNA has any effect on I2PP2A knockdown, and found the cellular levels of I2PP2A and c-Myc remained similar to controls (, lanes 5 and 6 vs. 1 and 2, respectively).
We further examined c-Myc expression level after overexpression of PP2Ac in PC-3 cells to determine if the I2PP2A inhibitory effect on c-Myc degradation can be overcome by PP2A overexpression. Our data suggested that PP2Ac overexpression decreased the c-Myc accumulation compared to control (, lanes 3 and 1, respectively), and that C6-ceramide treatment of the cells (10 μM, 48 h) after overexpression of PP2Ac produced an additional synergistic effect on c-Myc degradation in cells (lanes 4 and 2, respectively). Next, we treated the cells with the potent inhibitor of PP2A okadaic acid (10 nM) to check if inhibition of PP2A can increase I2PP2A function resulting in c-Myc accumulation in cells and if ceramide treatment can block the okadaic acid effect on I2PP2A function. PC-3 cells were pretreated overnight with okadaic acid (10 nM) and following that cells were treated with 10 μM C6-ceramide for 48 h. We showed that okadaic acid treatment resulted in higher c-Myc expression levels that were unaffected by ceramide (, lanes 3 and 4), than the control (lanes 1 and 2). Our data indicated that the okadaic acid inhibition of PP2A results in an additional inhibitory effect on PP2A function, which in turn results in increased levels of c-Myc in treated cells compared to the controls.
I2PP2A inhibits histone 4 acetylation in PC-3 cells
I2PP2A is known as an inhibitor of histone acetyl transferase (INHAT) and is involved in the regulation of gene transcription and chromatin remodeling.Citation25,Citation26 In prostate cancer histone acetylation has a significant role in gene transcription and cancer progression.Citation42 Evidence suggested that global histone modification patterns could predict independently the tumor stage, preoperative prostate-specific antigen levels, and capsule invasion.Citation42,Citation43 Hyperacetylation of histones induces cell cycle aberrations, chromatin reorganization and altered gene expression profiles in prostate cancer.Citation44 Ceramide has emerged as a potential treatment strategy in cancer cells to induce apoptotic cell death. Studies have reported that the short chain C2-ceramide mediated inhibition of the pro-metastatic enzyme MM2, and that the invasiveness of broncho-carcinoma cells involves decreases in histone acetylation.Citation45 Other studies showed that C6-ceramide synergistically potentiates the anti-tumor effect of the histone deacetylase inhibitor trichostatin A in pancreatic and ovarian carcinoma cells.Citation46
In this study, we examined the level of histone acetylation (H4-acetylation) in prostate cancer and epithelial cells () as we did for c-Myc (). Results indicated that histone acetylation is decreased in PC3 cancer cells almost 50% compared with normal epithelial cells (). No detectable change of histone acetylation was observed in DU145 or LNCaP cells compared with PrEC (). Hereafter we used only PC-3 cells to examine the cellular levels of H4-actylation (H4-Ac) in the rest of the experiments. Next, we checked if ceramide treatment can increase the H4-acetylation in PC-3 cells. Our data showed that with increases in time and concentration of ceramide the cellular level of H4-acetylation increased ().
Figure 7. The role of I2PP2A in histone acetylation in PC3 cells. (A) Detection and quantification of histone 4 acetylation in PC and PrEC cells. Cells were grown to 80% confluency and then western blot analysis was performed to determine the level of acetylated histone 4. (B) Densitometry analysis of the protein bands of H4-Ac and actin were determined using ImageJ software, and the results for H4-Ac normalized to the loading control actin; P < 0.05 was considered as significant (calculated using two tailed, paired t test). (C) C6-ceramide inhibits I2PP2A function and increases H4-Ac with time. Cells were treated for 0 to 48 h with 10 µM C6-ceramide, and then H4-AC levels were determined. (D) Increasing the concentration of C6-ceramide increases H4-Ac through inhibition of I2PP2A. Cells were treated with the indicated concentration of C6-ceramide, and H4-Ac levels were evaluated. (E) Overexpression of I2PP2A in PC3 cells enhances the decrease in H4-Ac levels. (F) Knockdown of I2PP2A in PC3 cells increases H4-Ac levels, and 10 μM ceramide treatment for 48 h increases H4-Ac levels.
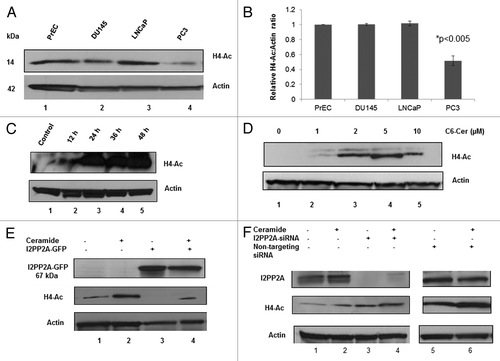
Next, we examined the effect of H4-acetylation level after overexpression or knockdown of I2PP2A in PC-3 cells and also checked if ceramide treatment (10 µM, 48 h) after knockdown/overexpression can alter the H4-acetylation level, as we did for c-Myc (). We observed that overexpression of I2PP2A abolished acetylation of histone 4 (, lane 3). Ceramide treatment was able to increase H4-acetylation level, but to a lower level than the ceramide-treated control (without overexpression of I2PP2A, , lanes 4 and 2, respectively). Our complementary experiment of knocking down I2PP2A in PC-3 cells showed that lower expression or absence of I2PP2A in cells increased H4-acetylation over the control (, lanes 3 and 1, respectively) and that ceramide treatment further increased H4-acetylation in cells (, lanes 4 and 2, respectively). Our results suggested that I2PP2A negatively regulates histone 4 acetylation and that ceramide was able to block the INHAT activity of I2PP2A in cells.
Discussion
This study attempts to elucidate the role of I2PP2A in prostate cancer progression in PC cell culture models by focusing on two I2PP2A-dependent pathways: (1) inhibition of PP2A resulting in c-Myc accumulation, and (2) inhibition of histone acetylation affecting gene suppression in prostate cancer cells (). We studied the role of ceramide in blocking I2PP2A inhibition of PP2A function and histone acetylation to prevent cancer progression ().
Figure 8. A schematic diagram of a possible mechanism for prostate cancer cell proliferation through modulation of I2PP2A function. (A) High expression of I2PP2A inhibits PP2A and HAT activity and stimulates cancer cell proliferation. (B) Ceramide binds and inhibits I2PP2A, which frees PP2A and HAT for c-Myc dephosphorylation and histone acetylation, respectively, and inhibits cancer cell proliferation.
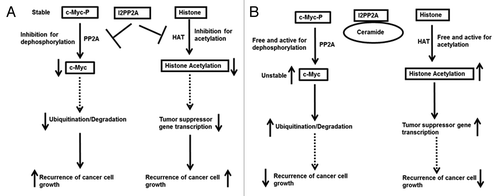
Our data showed that I2PP2A was overexpressed in all prostate cancer cell lines but not in normal prostate epithelial cells, suggesting that I2PP2A has a positive role in prostate cancer formation. Overexpression of I2PP2A or PP2Ac and knockdown of I2PP2A or okadaic acid treatment in PC-3 cells further supported that I2PP2A has a positive role in c-Myc upregulation and PC progression. I2PP2A is also known to be an inhibitor of histone acetyl transferase (HAT) by masking the N-terminal tails of histones and preventing acetylation of the various lysine residues, which in turn suppresses gene expression. Our results indicated that in PC-3 cells the level of histone acetylation was noticeably decreased compared to PrEC, suggesting that overexpression of I2PP2A might be the reason for this masking of histones from acetylation by HAT. I2PP2A’s ability to inhibit histone acetylation was further confirmed by overexpression or knocking down of I2PP2A in PC-3 cells.
We have chosen the short chain, cell-permeable C6-ceramide for our studies as an anti-I2PP2A agent to block I2PP2A regulation of c-Myc and histone acetylation in PC cells. Ceramides have emerged as tumor suppressor lipids because of their capability to induce apoptosis in many cancer cells.Citation33-Citation37,Citation40 It has been shown that following androgen ablation in LNCaP cells, endogenous C16-ceramide accumulates in cells and induces apoptosis in vitro.Citation41 Exogenous C2-ceramide also induces cell death in irradiated LNCaP cells.Citation38 Clinical studies reported that the total content of ceramide was decreased in ovarian tumors compared with normal tissue.Citation33,Citation34 We also measured the endogenous ceramide levels in androgen resistant PC-3 cells and androgen sensitive LNCaP cells compared to the ceramide levels in PrEC. Our results indicated that the endogenous levels of specific ceramide species are noticeably different in these two cancer cell lines compared with normal prostate epithelial cells, which supports the literature evidence that endogenous ceramide (C14- to C26-ceramide) levels in cancer cells are changed significantly compared with normal cells.Citation38-Citation41
A large number of inhibitors of PI3K/Akt/mTOR signal transduction pathways and also antagonists for the androgen receptor are currently under clinical investigation for advanced prostate cancer treatment.Citation2,Citation47,Citation48 Some of them may have pronounced cytotoxicity, but the side effects of these agents are of concern, as some of them may lead to cardiac arrest and patient death.Citation2,Citation4,Citation5,Citation47,Citation48 PP2A signaling regulation is pharmacologically desirable for cancer prevention since PP2A is a major tumor suppressor phosphatase in cells and is negatively associated with numerous cancer related cellular signaling pathways. Our approach is to find a cellular target for cancer prevention which is involved in multiple pathways for cancer progression, recurrence, and metastasis. Therefore targeting I2PP2A, which is a multifunctional protein associated with numerous cancer related pathways including cell migration for metastasis, could be a beneficial strategy for PC prevention. It has also been reported that I2PP2A regulates androgen production by cytochrome P450c17; I2PP2A is found in adrenals and promotes 17,20-lyase activity to produce sex steroids.Citation49 Ceramide has been well studied for inducing cell death in various cancer cells. Ceramide is not a specific anti-cancer agent for cancer cells, but it is a natural bioactive tumor suppressor lipid associated with numerous cell signaling pathways, including PP2A/I2PP2A signaling, and regulates various cellular functions such as cellular growth, proliferation, senescence and others. Treatment of mice with lung cancerCitation9 and breast cancerCitation50-Citation52 xenografts with the exogenous small chain C6-ceramide resulted in significant decreases in tumor growth. Treatment with C6-ceramide in combination with the histone deacetylase inhibitor trichostatin A has also shown anti-tumor effects in vitro and in vivo in mouse xenograft models of pancreatic and ovarian cancer.Citation46 Our data indicated significant cell death in all PC cell lines with ceramide treatment (), but no toxicity toward normal prostate epithelial cells, indicating that unlike conventional chemotherapeutics, ceramide has no or minimal effects on normal cells. Thus, application of ceramide as an anti-I2PP2A agent to block I2PP2A function in PC cells is a strength of this study.
Our results indicated that inhibition of I2PP2A, a multifunctional protein that is involved in numerous cancer-associated signaling pathways, via ceramide treatment decreases PC cell growth. Therefore we conclude that targeting this protein clinically could be a beneficial approach to prevent prostate cancer growth and metastasis. The approach of I2PP2A inhibition by ceramide treatment in PC cells provides us new insight into the molecular mechanisms of invasive PC formation and will help in the design of better drugs to inhibit I2PP2A in cancer cells.
Materials and Methods
Cell lines and culture conditions
Human prostate cancer cell lines PC-3, DU145, and LNCaP were obtained from American Type Culture Collection (ATCC). Cell lines were cultured and maintained in EMEM (ATCC) for PC-3 and DU145, and RPMI 1640 (ATCC) for LNCaP with 10% fetal bovine serum (Atlanta Biologicals) and 1% penicillin/streptomycin (Invitrogen). Cells were maintained in incubators containing humidified air with 5% CO2 at 37 °C and passaged every fourth to fifth day by trypsinization with trypsin/EDTA (Invitrogen). The prostate epithelial cell line PrEC was purchased from Lonza and maintained according to the manufacturer’s protocol.
Transient transfection of I2PP2A and PP2Ac
I2PP2A-GFP, cloned in the pEGFP-C3 (BD Biosciences) vector, was kindly donated by Dr Besim Ogretmen from the Medical University of South Carolina.Citation9 The catalytic subunit of PP2A, PP2Ac, with GFP and similarly cloned in the pEGFP-C3 vector, was kindly donated by Dr Yusuf Hannun from the Medical University of South Carolina. For transient transfection of PC-3 cells, we used the Xfect transfection reagent (Clontech) according to the manufacturer’s protocol.
Immunoprecipitation of PP2A and I2PP2A
PC-3 cells were grown until 80% confluency, and following that cells were treated with 10 µM C6-ceramide for 24 and 48 h to examine PP2A/I2PP2A association. Cells were lysed with RIPA buffer, and the total protein concentration was adjusted to 250 µg in 1 mL lysis buffer. Protein A/G agarose beads (Santa Cruz, 50 µL slurry) were washed with chilled lysis buffer three times and then added to 250 µg total protein. After incubation for 2 h at room temperature, beads were collected by centrifugation as a control (pre-cleared beads). The supernatant was incubated with 2 µg of PP2A or I2PP2A antibody overnight at 4 °C. The next day beads (50 µL) were added to the mixture, which was then incubated for 2 h at room temperature. Beads were collected by centrifugation and washed three times with cold lysis buffer. SDS loading buffer (1×, 40 µL) was added to the beads, the mixture heated at 95 °C for 5 min and then briefly centrifuged, and the supernatant analyzed by SDS-PAGE.
Western blot analysis
After harvesting the cells, cell pellets were lysed in RIPA buffer consisting of 10 mM TRIS-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Triton-X-100, 0.1% SDS, 1% sodium deoxycholate, and protease inhibitor cocktail (Sigma). Cell lysates were centrifuged for 5 min at 5000× g and the supernatants were collected. Total protein concentration was measured from the cell lysates using the standard Bradford assay. Western blot analysis was performed using the following antibodies: I2PP2A (rabbit polyclonal, 1:5000 dilution, Abcam); c-Myc (rabbit monoclonal, 1:2000 dilution, Cell Signaling Technologies); histone 4 acetylation (rabbit polyclonal, 1:2000 dilution); actin (mouse monoclonal, 1:5000 dilution, Sigma), PP2Ac (mouse monoclonal, 1:5000 dilution, BD Biosciences), p-PP2Ac (rabbit polyclonal, 1:5000 dilution, Epitomics). Signal densities were determined using ImageJ software (NIH, Bethesda) and normalized with actin as the loading control.
Ceramide treatment and cell viability assay
Prostate cancer cells and PrEC were plated in 6 well plates at a density of 10 × 104 cells/well in 2 mL of media. Following that, cells were treated with 0 to 40 µM C6-ceramide (Sigma) for 24 h and 48 h as indicated. Viable cells were counted using a hematocytometer in the presence of Trypan blue (Sigma) as described by the manufacturer.
Endogenous ceramide level measurement
Endogenous ceramides were measured by high performance liquid chromatography/mass spectroscopy (LC/MS) as described in reference Citation53. In brief, after the cells were collected by centrifugation, lipids were extracted directly from cell pellets, and the cellular levels of ceramides and inorganic phosphate from the same extracts were measured as described previously.Citation53 Ceramide levels were normalized to total cellular inorganic phosphate levels.
| Abbreviations: | ||
| GFP | = | green fluorescence protein |
| H4-Ac | = | histone 4 acetylation |
| HAT | = | histone acetyltransferase |
| I2PP2A | = | inhibitor 2 of protein phosphatase 2A |
| PC | = | prostate cancer |
| PP2A | = | protein phosphatase 2A |
| PrEC | = | prostate epithelial cells |
Acknowledgments
We would like to acknowledge Dr Besim Ogretmen from the Medical University of South Carolina for providing the I2PP2A-GFP plasmid, Dr Yusuf Hannun from the Medical University of South Carolina for his kind donation of the PP2A-GFP plasmid, and Dr Elias Michelis from Higuchi Biosciences Center, the University of Kansas, for providing support of this study.
Submitted
05/03/2013
Revised
07/03/2013
Accepted
07/29/2013
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Authors’ Contributions
AM performed the experiments, analyzed the data, and wrote the manuscript, KT contributed to the experiments, and RC and JVA provided expert comments on the experiments and edited the manuscript. All authors read and approved the final manuscript.
References
- Amaral TM, Macedo D, Fernandes I, Costa L. Castration-resistant prostate cancer: mechanisms, targets, and treatment. Prostate Cancer 2012; 2012:327253; http://dx.doi.org/10.1155/2012/327253; PMID: 22530130
- Hoffman-Censits J, Kelly WK. Enzalutamide: a novel antiandrogen for patients with castrate-resistant prostate cancer. Clin Cancer Res 2013; 19:1335 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-12-2910; PMID: 23300275
- Michielsen DP, Braeckman JG, Denis L. Cabazitaxel for the treatment of prostate cancer. Expert Opin Pharmacother 2011; 12:977 - 82; http://dx.doi.org/10.1517/14656566.2011.567268; PMID: 21406025
- El-Amm J, Aragon-Ching JB. The changing landscape in the treatment of metastatic castration-resistant prostate cancer. Ther Adv Med Oncol 2013; 5:25 - 40; http://dx.doi.org/10.1177/1758834012458137; PMID: 23323145
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 2001; 1:34 - 45; http://dx.doi.org/10.1038/35094009; PMID: 11900250
- Anazawa Y, Nakagawa H, Furihara M, Ashida S, Tamura K, Yoshioka H, Shuin T, Fujioka T, Katagiri T, Nakamura Y. PCOTH, a novel gene overexpressed in prostate cancers, promotes prostate cancer cell growth through phosphorylation of oncoprotein TAF-Ibeta/SET. Cancer Res 2005; 65:4578 - 86; http://dx.doi.org/10.1158/0008-5472.CAN-04-4564; PMID: 15930275
- Isaacs W, De Marzo A, Nelson WG. Focus on prostate cancer. Cancer Cell 2002; 2:113 - 6; http://dx.doi.org/10.1016/S1535-6108(02)00103-4; PMID: 12204531
- Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006; 9:287 - 300; http://dx.doi.org/10.1016/j.ccr.2006.03.003; PMID: 16616334
- Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J 2009; 23:751 - 63; http://dx.doi.org/10.1096/fj.08-120550; PMID: 19028839
- Ouellet V, Le Page C, Guyot MC, Lussier C, Tonin PN, Provencher DM, Mes-Masson AM. SET complex in serous epithelial ovarian cancer. Int J Cancer 2006; 119:2119 - 26; http://dx.doi.org/10.1002/ijc.22054; PMID: 16823850
- Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, Ondrey FG, Adams GL, Gaffney PM. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res 2004; 64:55 - 63; http://dx.doi.org/10.1158/0008-5472.CAN-03-2144; PMID: 14729608
- Carlson SG, Eng E, Kim EG, Perlman EJ, Copeland TD, Ballermann BJ. Expression of SET, an inhibitor of protein phosphatase 2A, in renal development and Wilms’ tumor. J Am Soc Nephrol 1998; 9:1873 - 80; PMID: 9773788
- Korkola JE, Houldsworth J, Chadalavada RS, Olshen AB, Dobrzynski D, Reuter VE, Bosl GJ, Chaganti RS. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res 2006; 66:820 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-05-2445; PMID: 16424014
- Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, Mao H, Chang JS, Galietta A, Uttam A, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 2005; 8:355 - 68; http://dx.doi.org/10.1016/j.ccr.2005.10.015; PMID: 16286244
- Perrotti D, Neviani P. Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer Metastasis Rev 2008; 27:159 - 68; http://dx.doi.org/10.1007/s10555-008-9119-x; PMID: 18213449
- Adachi Y, Pavlakis GN, Copeland TD. Identification and characterization of SET, a nuclear phosphoprotein encoded by the translocation break point in acute undifferentiated leukemia. J Biol Chem 1994; 269:2258 - 62; PMID: 8294483
- Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry 1995; 34:1988 - 96; http://dx.doi.org/10.1021/bi00006a020; PMID: 7531497
- Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem 1996; 271:11059 - 62; http://dx.doi.org/10.1074/jbc.271.19.11059; PMID: 8626647
- Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol 2006; 26:2832 - 44; http://dx.doi.org/10.1128/MCB.26.7.2832-2844.2006; PMID: 16537924
- Westermarck J, Hahn WC. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med 2008; 14:152 - 60; http://dx.doi.org/10.1016/j.molmed.2008.02.001; PMID: 18329957
- Canela N, Rodriguez-Vilarrupla A, Estanyol JM, Diaz C, Pujol MJ, Agell N, Bachs O. The SET protein regulates G2/M transition by modulating cyclin B-cyclin-dependent kinase 1 activity. J Biol Chem 2003; 278:1158 - 64; http://dx.doi.org/10.1074/jbc.M207497200; PMID: 12407107
- Carujo S, Estanyol JM, Ejarque A, Agell N, Bachs O, Pujol MJ. Glyceraldehyde 3-phosphate dehydrogenase is a SET-binding protein and regulates cyclin B-cdk1 activity. Oncogene 2006; 25:4033 - 42; http://dx.doi.org/10.1038/sj.onc.1209433; PMID: 16474839
- Compagnone NA, Zhang P, Vigne JL, Mellon SH. Novel role for the nuclear phosphoprotein SET in transcriptional activation of P450c17 and initiation of neurosteroidogenesis. Mol Endocrinol 2000; 14:875 - 88; http://dx.doi.org/10.1210/me.14.6.875; PMID: 10847589
- Nagata K, Kawase H, Handa H, Yano K, Yamasaki M, Ishimi Y, Okuda A, Kikuchi A, Matsumoto K. Replication factor encoded by a putative oncogene, set, associated with myeloid leukemogenesis. Proc Natl Acad Sci U S A 1995; 92:4279 - 83; http://dx.doi.org/10.1073/pnas.92.10.4279; PMID: 7753797
- Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell 2001; 104:119 - 30; http://dx.doi.org/10.1016/S0092-8674(01)00196-9; PMID: 11163245
- Gamble MJ, Erdjument-Bromage H, Tempst P, Freedman LP, Fisher RP. The histone chaperone TAF-I/SET/INHAT is required for transcription in vitro of chromatin templates. Mol Cell Biol 2005; 25:797 - 807; http://dx.doi.org/10.1128/MCB.25.2.797-807.2005; PMID: 15632079
- ten Klooster JP, Leeuwen Iv, Scheres N, Anthony EC, Hordijk PL. Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET. EMBO J 2007; 26:336 - 45; http://dx.doi.org/10.1038/sj.emboj.7601518; PMID: 17245428
- Goc A, Abdalla M, Al-Azayzih A, Somanath PR. Rac1 activation driven by 14-3-3ζ dimerization promotes prostate cancer cell-matrix interactions, motility and transendothelial migration. PLoS One 2012; 7:e40594; http://dx.doi.org/10.1371/journal.pone.0040594; PMID: 22808202
- Arnold HK, Sears RC. A tumor suppressor role for PP2A-B56alpha through negative regulation of c-Myc and other key oncoproteins. Cancer Metastasis Rev 2008; 27:147 - 58; http://dx.doi.org/10.1007/s10555-008-9128-9; PMID: 18246411
- Fan Z, Beresford PJ, Oh DY, Zhang D, Lieberman J. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell 2003; 112:659 - 72; http://dx.doi.org/10.1016/S0092-8674(03)00150-8; PMID: 12628186
- Jensen SL, Wood DP Jr., Banks ER, Veron M, Lascu I, McRoberts JW, Rangnekar VM. Increased levels of nm23 H1/nucleoside diphosphate kinase A mRNA associated with adenocarcinoma of the prostate. World J Urol 1996; 14:Suppl 1 S21 - 5; http://dx.doi.org/10.1007/BF00182060; PMID: 8738406
- Switzer CH, Cheng RY, Vitek TM, Christensen DJ, Wink DA, Vitek MP. Targeting SET/I(2)PP2A oncoprotein functions as a multi-pathway strategy for cancer therapy. Oncogene 2011; 30:2504 - 13; http://dx.doi.org/10.1038/onc.2010.622; PMID: 21297667
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008; 9:139 - 50; http://dx.doi.org/10.1038/nrm2329; PMID: 18216770
- Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett 2006; 580:5467 - 76; http://dx.doi.org/10.1016/j.febslet.2006.08.052; PMID: 16970943
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 2004; 4:604 - 16; http://dx.doi.org/10.1038/nrc1411; PMID: 15286740
- Modrak DE, Gold DV, Goldenberg DM. Sphingolipid targets in cancer therapy. Mol Cancer Ther 2006; 5:200 - 8; http://dx.doi.org/10.1158/1535-7163.MCT-05-0420; PMID: 16505092
- Riboni L, Campanella R, Bassi R, Villani R, Gaini SM, Martinelli-Boneschi F, Viani P, Tettamanti G. Ceramide levels are inversely associated with malignant progression of human glial tumors. Glia 2002; 39:105 - 13; http://dx.doi.org/10.1002/glia.10087; PMID: 12112362
- Kimura K, Markowski M, Edsall LC, Spiegel S, Gelmann EP. Role of ceramide in mediating apoptosis of irradiated LNCaP prostate cancer cells. Cell Death Differ 2003; 10:240 - 8; http://dx.doi.org/10.1038/sj.cdd.4401145; PMID: 12700652
- Koyanagi S, Kuga M, Soeda S, Hosoda Y, Yokomatsu T, Takechi H, Akiyama T, Shibuya S, Shimeno H. Elevation of de novo ceramide synthesis in tumor masses and the role of microsomal dihydroceramide synthase. Int J Cancer 2003; 105:1 - 6; http://dx.doi.org/10.1002/ijc.11024; PMID: 12672022
- Rylova SN, Somova OG, Dyatlovitskaya EV. Comparative investigation of sphingoid bases and fatty acids in ceramides and sphingomyelins from human ovarian malignant tumors and normal ovary. Biochemistry (Mosc) 1998; 63:1057 - 60; PMID: 9795275
- Eto M, Bennouna J, Hunter OC, Hershberger PA, Kanto T, Johnson CS, Lotze MT, Amoscato AA. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate 2003; 57:66 - 79; http://dx.doi.org/10.1002/pros.10275; PMID: 12886525
- Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature 2005; 435:1262 - 6; http://dx.doi.org/10.1038/nature03672; PMID: 15988529
- Watson JA, McKenna DJ, Maxwell P, Diamond J, Arthur K, McKelvey-Martin VJ, Hamilton PW. Hyperacetylation in prostate cancer induces cell cycle aberrations, chromatin reorganization and altered gene expression profiles. J Cell Mol Med 2010; 14:6B 1668 - 82; http://dx.doi.org/10.1111/j.1582-4934.2009.00835.x; PMID: 19583812
- Dart DA, Brooke GN, Sita-Lumsden A, Waxman J, Bevan CL. Reducing prohibitin increases histone acetylation, and promotes androgen independence in prostate tumours by increasing androgen receptor activation by adrenal androgens. Oncogene 2012; 31:4588 - 98; http://dx.doi.org/10.1038/onc.2011.591; PMID: 22179832
- Debret R, Brassart-Pasco S, Lorin J, Martoriati A, Deshorgue A, Maquart FX, Hornebeck W, Rahman I, Antonicelli F. Ceramide inhibition of MMP-2 expression and human cancer bronchial cell invasiveness involve decreased histone acetylation. Biochim Biophys Acta 2008; 1783:1718 - 27; http://dx.doi.org/10.1016/j.bbamcr.2008.06.001; PMID: 18598724
- Zhu QY, Wang Z, Ji C, Cheng L, Yang YL, Ren J, Jin YH, Wang QJ, Gu XJ, Bi ZG, et al. C6-ceramide synergistically potentiates the anti-tumor effects of histone deacetylase inhibitors via AKT dephosphorylation and α-tubulin hyperacetylation both in vitro and in vivo. Cell Death Dis 2011; 2:e117; http://dx.doi.org/10.1038/cddis.2010.96; PMID: 21368888
- Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets 2009; 9:237 - 49; http://dx.doi.org/10.2174/156800909787580999; PMID: 19275762
- Rudner J, Ruiner CE, Handrick R, Eibl HJ, Belka C, Jendrossek V. The Akt-inhibitor Erufosine induces apoptotic cell death in prostate cancer cells and increases the short term effects of ionizing radiation. Radiat Oncol 2010; 5:108; http://dx.doi.org/10.1186/1748-717X-5-108; PMID: 21080918
- Pandey AV, Mellon SH, Miller WL. Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 2003; 278:2837 - 44; http://dx.doi.org/10.1074/jbc.M209527200; PMID: 12444089
- Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 2013; 13:51 - 65; http://dx.doi.org/10.1038/nrc3398; PMID: 23235911
- Morad SA, Levin JC, Shanmugavelandy SS, Kester M, Fabrias G, Bedia C, Cabot MC. Ceramide--antiestrogen nanoliposomal combinations--novel impact of hormonal therapy in hormone-insensitive breast cancer. Mol Cancer Ther 2012; 11:2352 - 61; http://dx.doi.org/10.1158/1535-7163.MCT-12-0594; PMID: 22962326
- Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res 2005; 11:3465 - 74; http://dx.doi.org/10.1158/1078-0432.CCR-04-1770; PMID: 15867249
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 2006; 39:82 - 91; http://dx.doi.org/10.1016/j.ymeth.2006.05.004; PMID: 16828308