Abstract
Sorafenib is an oral multikinase inhibitor targeting Raf and other kinases. The anti-tumor effect of sorafenib is thought to be mediated through its inhibition of the RAS–Raf–Erk pathway, as well as its inhibition of VEGFR and PDGFR. Sorafenib has been effective at treating patients with renal cell carcinoma (RCC). Ovarian clear cell carcinoma (OCCC) is a chemoresistant subtype of ovarian cancer. OCCC is represented by cells with clear cytoplasm that resemble those observed in RCC. Using a microarray database, the gene expression profile of OCCC was similar to that of RCC. The effects of sorafenib against human OCCC are unknown. Therefore, we used sorafenib to treat two patients with recurrent chemoresistant OCCC, and observed good effect in both of them without severe side effects. We believe that sorafenib is an effective agent against OCCC. Given the chemoresistant nature of this tumor, this drug appears to be very valuable.
Introduction
Sorafenib is an oral multikinase inhibitor targeting Raf and other kinases (i.e., vascular endothelial growth factor receptor [VEGFR], platelet-derived growth factor receptor [PDGFR], Flt3, and c-KIT). The anti-tumor effect of sorafenib is thought to be mediated through its inhibition of the RAS–Raf–Erk pathway,Citation1 which is involved in cell proliferation, as well as its inhibition of VEGFR and PDGFR, which play a role in angiogenesis.Citation2,Citation3 Sorafenib has been effective at treating patients with renal cell carcinoma (RCC)Citation4 and hepatocellular carcinoma (HCC).Citation5
Ovarian clear cell carcinoma (OCCC) has a specific gene expression profile,Citation6 and is a chemoresistant histological subtype of ovarian cancer. OCCC is represented by cells with clear cytoplasm that resemble those observed in RCC. Using a microarray database, we previously reported that the gene expression profile of OCCC was similar to that of RCC, and we showed good effects of sorafenib in a mouse model of OCCC.Citation7
The effects of sorafenib against human OCCC are unknown. Therefore, we attempted to use sorafenib to treat two patients with recurrent chemoresistant OCCC. This is the first case report to evaluate sorafenib efficacy against OCCC.
Case Description
We used sorafenib monotherapy (400 mg/day, orally) in two patients with recurrent and chemoresistant OCCC after written informed consents were obtained. The efficacy of the treatment was judged based on progression-free survival for at least 6 months (RECIST 1.1). These clinical studies had been approved by the institutional review board (IRB) of Kyoto University in advance.
Clinical case 1
A 63-y-old Japanese postmenopausal female, with 1 gestation and 0 parturitions, who reported dull lower abdominal pain and distension, visited our hospital. A 15 cm cystic tumor containing solid parts was detected in the lower abdomen. Total abdominal hysterectomy (TAH) with a bilateral salpingo-oophorectomy (BSO), pelvic/para-aortic lymphadenectomies (PELA/PALAs) and omentectomy were performed. After the surgery, an OCCC of stage Ia (PT1a, N0, M0) was diagnosed. Due to the early stage of the disease and her complication of IgA nephropathy, adjuvant chemotherapy was omitted. Thirteen months after the primary surgery, recurrent nodes 2 cm in diameter appeared in the ascending colon and the omentum. After resections of these recurrent nodes, 6 courses of irinotecan (90 mg/m2 × 2)/cisplatin (60 mg/m2) combination chemotherapy were administered.
After one year, however, disseminated nodes and swelling of the lymph nodes were widely observed in the abdomen. Six courses of paclitaxel (175 mg/m2)/carboplatin (AUC 6) combination chemotherapy were added. After 6 mo, the recurrent peritoneal tumors had grown vigorously.
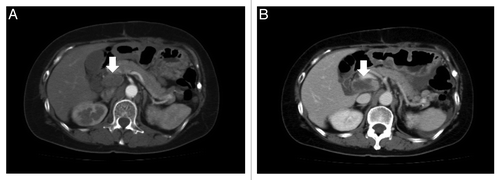
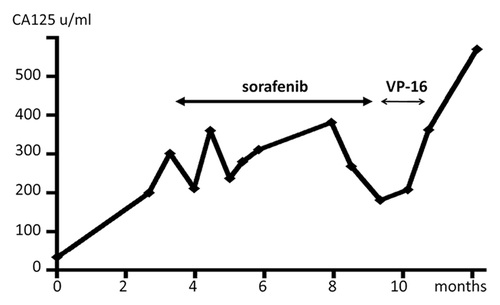
Accordingly, the patient was administered 800 mg/day of sorafenib. The patient experienced a hand–foot skin reaction (grade 2, CTCAE v4.0) 2 weeks later, and subsequently, the dose was decreased to 400 mg/day, which was continued for 6 mo. The sizes of the lymph nodes in the hepatic portal region were not significantly different before and after sorafenib monotherapy (). The patient’s serum levels of CA125 were 200.2 U/ml and 181.2 U/ml before and after treatment, respectively (). The effect of sorafenib was judged to be stable disease (progression-free survival) for 6 mo. The side effects were moderate hand–foot syndrome (grade 2, CTCAE v4.0) and hypertension (160–170 mmHg, grade 2). After sorafenib therapy, the tumor grew again. In spite of oral VP-16 therapy (50 mg/day), the tumor grew vigorously. As a result of DIC, the patient finally died 4.5 y after the primary surgery.
Clinical case 2
A 63-y-old Japanese postmenopausal female, with 3 gestations and 3 parturitions, who visited her neighborhood hospital due to lower abdominal pain, was diagnosed with an ovarian tumor. She was referred to our hospital and a TAH with a BSO, omentectomy, appendectomy, and sampling of the peritoneal nodes/right external iliac lymph nodes, were performed. The main tumor was demonstrated to be 10 cm in diameter, and extended widely into the peritoneal cavity. An OCCC of stage IIIc (PT3c, Nx, M0) was diagnosed. Three courses of intraperitoneal cisplatin (60 mg/m2), three courses of intravenous irinotecan (70 mg/m2 × 2)/mitomycin (10 mg/body) chemotherapy, and three courses of weekly paclitaxel (50 mg/m2)/carboplatin (90 mg/m2) combination chemotherapy were administered. The residual tumors were markedly reduced, and thus, optimum PELA/PALAs were performed. Six rounds of low-dose cyclophosphamide (230 mg/m2)/adriamycin (20 mg/m2)/cisplatin (15 mg/m2) combination chemotherapy were administered monthly.
However, recurrence appeared in the neck lymph nodes. Neck lymphadenectomies were performed, and then three courses of VP16 (60 mg/m2)/nedaplatin (60 mg/m2) chemotherapy and irradiation (total 50 Gy) of the left neck were administered. For the next 4 y, 5 courses of monthly docetaxel (50 mg/m2)/carboplatin (200 mg/m2) chemotherapy, 8 courses of irinotecan (60 mg/m2 × 2)/cisplatin (50 mg/m2) chemotherapy, 6 courses of gemcitabine hydrochloride (1000 mg/m2 × 2/month), and 7 courses of nogitecan hydrochloride (0.5 mg/m2 × 5/month) were administered. By 8 y after the primary surgery, the mediastinal, axillary, and neck lymph nodes had increased by degrees.
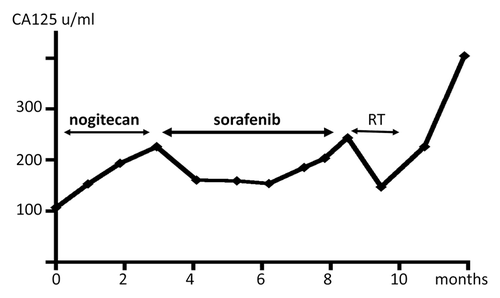
The patient then was prescribed 400 mg/day of sorafenib for 5 mo. The sizes of the neck lymph nodes were not significantly different before and after treatment (), which was judged to indicate “stable disease” for 6 mo. The serum levels of CA125 were 193.2 U/ml and 203.5 U/ml before and after the treatment, respectively (). The only side effect was mild hand–foot syndrome (grade 1).
Figure 3. The size of the left neck lymph node was not significantly different before (A) and after (B) treatment (14.3, 10.3 mm vs. 14.3, 10.8 mm, respectively [−30%~+20%; shortest diameter rule, RECIST 1.1]).
![Figure 3. The size of the left neck lymph node was not significantly different before (A) and after (B) treatment (14.3, 10.3 mm vs. 14.3, 10.8 mm, respectively [−30%~+20%; shortest diameter rule, RECIST 1.1]).](/cms/asset/257ce3ea-6d9f-432a-a847-7a6119af5b61/kcbt_a_10926608_f0003.gif)
Figure 4. Clinical course of the patient and changes of CA125 (case 2). Sorafenib monotherapy decreased CA125 level temporarily.
After sorafenib therapy, the metastatic neck lymph nodes increased suddenly. External irradiation against the bilateral neck regions (60 Gy), the right axillary lymph nodes (28 Gy), and the para-aortic lymph nodes (30 Gy) was performed. However, airway narrowing occurred due to swelling of the neck lymph nodes. After a tracheotomy, the patient died due to ileus and bleeding from the bronchi. This was approximately 9 y after the primary surgery.
Discussion
Epithelial ovarian carcinoma is histologically divided into serous (OS), endometrioid (OE), mucinous (OM), and OCCC subtypes. OM and OCCC are both commonly considered to be chemoresistant tumors. OCCCs are rare tumors in Europe and the United StatesCitation8; however, they occur often in Japan (20%).Citation9 Therefore, we have searched for agents that are effective against OCCC.
We previously showed that the gene expression profile of OCCC was similar to that of RCC using microarray data sets of ovarian cancer cell lines and human cancer tissues specimens.Citation7 We also showed the genes exclusively expressed in OCCC, termed OCCC signature, contained a large gene network consisting of 66 genes, including hepatocyte nuclear factor 1-β (HNF1B) and hypoxia inducible factor 1-α subunit (HIF1A).Citation6 HNF1B is functionally associated with renal morphogenesis.Citation10 In addition, pathway analysis indicated the RAS–Raf–Erk signaling is activated in OCCC.Citation7
RCC is also a chemoresistant tumor. Sorafenib has recently been approved for RCC, and is considered to be a reasonable molecular targeting drug against RCC. All sporadic and inherited forms of RCC are associated with mutations in the VHL gene and loss of heterozygosity,Citation11,Citation12 resulting in the stabilization of the HIF1A protein. HIF1A activates multiple growth factor receptors, such as the EGFR, PDGFR, and VEGFR. In addition, increased RAS pathway signaling is observed in RCC.Citation13 The anti-tumor effect of sorafenib is thought to be mediated through its inhibition of the RAS–Raf–Erk pathway as well as its inhibition of VEGFR and PDGFR.Citation1-Citation3 We showed that orally administered sorafenib significantly inhibited the growth of RMG-2, a representative OCCC cell line, in nude mice.Citation7 We therefore attempted to use sorafenib for human patients with recurrent chemoresistant OCCC.
Both of our patients with OCCC were judged to have “stable disease” following the use of sorafenib monotherapy and exhibited progression-free survival of at least 6 mo. This suggests that sorafenib is an effective agent against recurrent OCCC. Our 2 patients were able to continue sorafenib monotherapy (400 mg/day) without severe side effects. Both patients had mild/moderate hand–foot syndrome (grades 1 and 2) and one also had hypertension (grade 2). In fact, we used sorafenib monotherapy for another patient with OCCC for 2 mo. However, therapy had to be stopped due to Trousseau syndrome. The disease was stable for two months, and the side effect was a mild rash (grade 1).
Recently, the Gynecologic Oncology Group reported that sorafenib had modest antitumor activity, but did not provide a sufficient effect as a treatment for ovarian cancer.Citation14 Fifty-nine patients with measurable diseases of recurrent ovarian or peritoneal carcinoma were enrolled in this study, but only 14 patients (24%) survived progression-free for 6 mo. This study was conducted mostly for the OS subtype (90%), whereas only one patient (1.4%) had OCCC. Interestingly, in this study, one of the two patients with partial responses was the patient with OCCC. The data from this study appear to support our result suggesting promising effects of sorafenib against OCCC.
The conventional dose of sorafenib is 800 mg/day.Citation14,Citation15 For case 1, we initiated sorafenib treatment with a dose of 800 mg/day, but the dose was soon decreased to 400 mg/day due to hand–foot skin reaction. In a clinical trial conducted for Japanese patients with RCC, dose reduction or therapy interruption due to adverse events was required for as many as 81% of the patients, which is a much higher percentage than for American or European patients.Citation16 We therefore initiated treatment with a dose of 400 mg/day for case 2. Regardless of the decreased dose, sorafenib showed significant anti-tumor activity in the two OCCC patients.
We believe that sorafenib is an effective agent against OCCC. Given the chemoresistant nature of this tumor, this drug appears to be very valuable. A clinical trial should be performed using sorafenib to treat OCCC. In addition, a further combination regimenCitation17 using sorafenib may be more effective against OCCC.
Disclosure of Potential Conflicts of Interest
The authors have no financial conflicts of interest relevant to the present work to declare.
References
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64:7099 - 109; http://dx.doi.org/10.1158/0008-5472.CAN-04-1443; PMID: 15466206
- Chang YS, Henderson A, Xue D, Chen C, McNabola A, Wilkie D, Rowley B, Carter CA, Riedl B, Trail PA, et al. BAY 43-9006 (Sorafenib) inhibits ectopic and orthotopic growth of a murine model of renal adenocarcinoma (Renca) predominantly through inhibition of tumor angiogenesis. Proc Am Assoc Cancer Res 2005; 46 Abstract.
- Levy J, Pauloski N, Braun D, Derome M, Jordan J, Shi H, Weaver D, Chang Y, Bortolon E, Henderson A, et al. Analysis of transcription and protein expression changes in the 786-O human renal cell carcinoma tumor xenograft model in response to treatment with the multi-kinase inhibitor sirafenib (BAY 43-9006). [Abstract] Proc Am Assoc Cancer Res 2006; 47:213 - 4
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al, TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356:125 - 34; http://dx.doi.org/10.1056/NEJMoa060655; PMID: 17215530
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al, SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378 - 90; http://dx.doi.org/10.1056/NEJMoa0708857; PMID: 18650514
- Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, Matsui S, Murphy SK, Konishi I. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic process. Oncogene 2009; 470:1 - 12
- Matsumura N, Mandai M, Okamoto T, Yamaguchi K, Yamamura S, Oura T, Baba T, Hamanishi J, Kang HS, Matsui S, et al. Sorafenib efficacy in ovarian clear cell carcinoma revealed by transcriptome profiling. Cancer Sci 2010; 101:2658 - 63; http://dx.doi.org/10.1111/j.1349-7006.2010.01736.x; PMID: 21040214
- Mink PJ, Sherman ME, Devesa SS. Incidence patterns of invasive and borderline ovarian tumors among white women and black women in the United States. Results from the SEER Program, 1978-1998. Cancer 2002; 95:2380 - 9; http://dx.doi.org/10.1002/cncr.10935; PMID: 12436446
- Skírnisdóttir I, Seidal T, Karlsson MG, Sorbe B. Clinical and biological characteristics of clear cell carcinomas of the ovary in FIGO stages I-II. Int J Oncol 2005; 26:177 - 83; PMID: 15586238
- Kato N, Motoyama T. Hepatocyte nuclear factor-1beta(HNF-1beta) in human urogenital organs: its expression and role in embryogenesis and tumorigenesis. Histol Histopathol 2009; 24:1479 - 86; PMID: 19760597
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 1994; 7:85 - 90; http://dx.doi.org/10.1038/ng0594-85; PMID: 7915601
- Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol 2006; 24:5601 - 8; http://dx.doi.org/10.1200/JCO.2006.08.5415; PMID: 17158546
- Patel PH, Chadalavada RS, Chaganti RS, Motzer RJ. Targeting von Hippel-Lindau pathway in renal cell carcinoma. Clin Cancer Res 2006; 12:7215 - 20; http://dx.doi.org/10.1158/1078-0432.CCR-06-2254; PMID: 17189392
- Matei D, Sill MW, Lankes HA, DeGeest K, Bristow RE, Mutch D, Yamada SD, Cohn D, Calvert V, Farley J, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol 2011; 29:69 - 75; http://dx.doi.org/10.1200/JCO.2009.26.7856; PMID: 21098323
- Bodnar L, Górnas M, Szczylik C. Sorafenib as a third line therapy in patients with epithelial ovarian cancer or primary peritoneal cancer: a phase II study. Gynecol Oncol 2011; 123:33 - 6; http://dx.doi.org/10.1016/j.ygyno.2011.06.019; PMID: 21723597
- Tanigawa G, Kawashima A, Yamaguchi S, Nishimura K, Miyoshi S, Kajikawa J, Meguro N, Yosioka T, Oka T, Hara T, et al, Osaka Renal Cell Carcinoma Clinical Study Collaboration. Clinical outcome and prognostic factors of sorafenib in Japanese patients with advanced renal cell carcinoma in general clinical practice. Jpn J Clin Oncol 2011; 41:1265 - 70; http://dx.doi.org/10.1093/jjco/hyr137; PMID: 21965163
- Ramasubbaiah R, Perkins SM, Schilder J, Whalen C, Johnson CS, Callahan M, Jones T, Sutton G, Matei D. Sorafenib in combination with weekly topotecan in recurrent ovarian cancer, a phase I/II study of the Hoosier Oncology Group. Gynecol Oncol 2011; 123:499 - 504; http://dx.doi.org/10.1016/j.ygyno.2011.08.033; PMID: 21955480