Abstract
Janus kinase (JAK) and Src kinase are the two major tyrosine kinase families upstream of signal transducer and activator of transcription (STAT). Among the seven STAT family proteins, STAT3 is constitutively activated in many diverse cancers. Upon activation, JAK and Src kinases phosphorylate STAT3, and thereby promote cell growth and survival. MLS-2384 is a novel 6-bromoindirubin derivative with a bromo-group at the 6-position on one indole ring and a hydrophilic group at the 3′-position on the other indole ring. In this study, we investigated the kinase inhibitory activity and anticancer activity of MLS-2384. Our data from in vitro kinase assays, cell viability analyses, western blotting analyses, and animal model studies, demonstrate that MLS-2384 is a dual JAK/Src kinase inhibitor, and suppresses growth of various human cancer cells, such as prostate, breast, skin, ovarian, lung, and liver. Consistent with the inactivation of JAK and Src kinases, phosphorylation of STAT3 was inhibited in a dose-dependent manner in the cancer cells treated with MLS-2384. STAT3 downstream proteins involved in cell proliferation and survival, such as c-Myc and Mcl-1, are downregulated by MLS-2384 in prostate cancer cells, whereas survivin is downregulated in A2058 cells. In these two cancer cell lines, PARP is cleaved, indicating that MLS-2384 induces apoptosis in human melanoma and prostate cancer cells. Importantly, MLS-2384 suppresses tumor growth with low toxicity in a mouse xenograft model of human melanoma. Taken together, MLS-2384 demonstrates dual JAK/Src inhibitory activity and suppresses tumor cell growth both in vitro and in vivo. Our findings support further development of MLS-2384 as a potential small-molecule therapeutic agent that targets JAK, Src, and STAT3 signaling in multiple human cancer cells.
Introduction
JAK and Src are the two major tyrosine kinases upstream of STAT3. STAT3 is persistently activated in cancer cells due to aberrant activation of JAK, Src, and/or other tyrosine kinases.Citation1-Citation7 Persistent activation of STAT3 signaling contributes to the malignancy of tumors by promoting tumor cell proliferation and survival, angiogenesis, and immune evasion.Citation8-Citation11 Inhibitors of Src and JAK have been intensively investigated for targeted cancer therapeutics. Several small molecule Src inhibitors are in clinical investigations for cancer therapy.Citation12-Citation14 The recent discovery of JAK V617F mutation in myeloproliferative disease leads to several JAK inhibitors in current clinical trials for myeloproliferative disorder, myelofibrosis, and leukemia.Citation15-Citation22 Ruxolitinib, an inhibitor of JAK1 and JAK2, has been approved recently by the FDA as the first JAK inhibitor to treat myelofibrosis.Citation23 The role of constitutive JAK2 kinase activity in myeloproliferative neoplastic growth provides a rationale for investigating inhibition of JAK/STAT3 in solid tumors.Citation23,Citation24 Several small molecule inhibitors of JAK have been found to have potent anticancer activities in solid tumors.Citation25-Citation33 Inhibitors of Src, JAK, and STAT3 signaling are promising targeted therapeutic agents for cancers.
Indirubin, a bis-indole alkaloid, and its analogs can be isolated from natural sources and chemically synthesized. Initially indirubin was found as the active ingredient of a traditional Chinese herbal medicine for treatment of chronic myelocytic leukemia (CML) in China.Citation34,Citation35 Indirubin and its analogs have been identified as inhibitors of GSK-3β and CDKs.Citation34,Citation36,Citation37 Synthetic bromoindirubins have shown enhanced binding affinity toward kinases such as GSK-3β, CDKs, JAK, and Src.Citation32,Citation38,Citation39 Potent anticancer activities of bromoindirubins have been reported in human cancer cells.Citation32,Citation39-Citation42
In this study, we investigated molecular targets and anticancer activity of a novel synthetic 6-bromoindirubin derivative, MLS-2384 in diverse cancer cells. We have found that MLS-2384 demonstrates potent dual JAK/Src inhibitory activity and anticancer activity in multiple cancer cell lines. Our previous studies showed that a 6-bromoindirubin derivative, 6-bromoindirubin-3′-oxime (6BIO), as a JAK inhibitor, inhibited JAK/STAT3 signaling in human melanoma cells.Citation32 A 7-bromoindirubin derivative, MLS-2438 has been identified as a Src inhibitor.Citation39 Both bromoindirubin derivatives induced apoptosis of human melanoma cells and suppressed tumor growth in a mouse xenograft model of human melanoma. Interestingly, in this study, MLS-2384, a novel 6-bromonindirubin derivative, is identified as a dual JAK/Src inhibitor. JAK and Src demonstrated compensatory effect when cancer cells were treated with a Src inhibitor alone.Citation43,Citation44 Therefore, inhibition of both Src and JAK is more desirable to effectively suppress cancer cell growth.Citation45 Our findings indicate that MLS-2384 is an excellent agent to suppress tumor growth by inhibiting both JAK and Src kinases. MLS-2384 as a dual JAK/Src inhibitor suppresses STAT3 signaling and cancer cell growth, represents a promising lead compound for development of new targeted anticancer therapeutics.
Results
MLS-2384 inhibits JAK/Src kinase activity in vitro and phosphorylation of JAK2, Src, and STAT3 in cancer cells
MLS-2384 is a new derivative of 6-bromoindirubin-3′-oxime (6BIO). Our previous studies have shown that 6BIO is a JAK inhibitor,Citation32 and a 7-bromoindirubin derivative (MLS-2438) is a Src inhibitor.Citation39 MLS-2384 is a new 6-bromoindurubin derivative with a bromine group at the 6-position on one indole ring as in 6BIO, and a hydrophilic group at the 3′-position on the other indole ring as in MLS-2438. As shown in , MLS-2384 inhibited kinase activity of Src and JAK family proteins, with an IC50 value of 0.2 nmol/L against Src, 28 nmol/L against JAK2, 50 nmol/L agianst TYK2, and 0.6 µmol/L against JAK1. MLS-2384 demonstrated strong inhibition to Src kinase activity, and differential inhibition of kinase activity among the three JAK family proteins, JAK1, JAK2, and TYK2. These data of in vitro kinase assays provide a piece of evidence to show that MLS-2384 is a dual JAK/Src inhibitor.
Table 1. MLS-2384 inhibits kinase activities of Src and JAK family proteins in vitro
To further identify that MLS-2384 is a dual JAK/Src kinase inhibitor, we investigated phosphorylation levels of JAK2 and Src in cancer cells treated with MLS-2384 at different concentrations for 4 h. As shown in , we conducted western blot analyses for proteins isolated from five human cancer cell lines treated with MLS-2384 at concentrations of 1 and 2.5 µmol/L for 4 h. Phosphorylation levels of JAK2 and Src were reduced dramatically at 2.5 µmol/L in DU145, MDA-MB-468, A2058, A2780, and A549 cancer cells, indicating that MLS-2384 inhibited phosphorylation of both JAK2 and Src effectively in the five human cancer cell lines in culture. These data suggest that MLS-2384 is a dual JAK/Src inhibitor both in vitro and in cell culture.
Figure 1. MLS-2384 inhibits phosphorylation of JAK2, Src, and STAT3 in various human cancer cells. Human DU145 prostate, MDA-MB-468 breast, A2058 skin, A2780 ovarian, and A 549 lung cancer cells were treated with MLS-2384 at various concentrations for 4 h. Cells were lysed for western blot analysis using antibodies specific to p-JAK2, JAK2, p-Src, Src, p-STAT3, STAT3, and β-Actin.
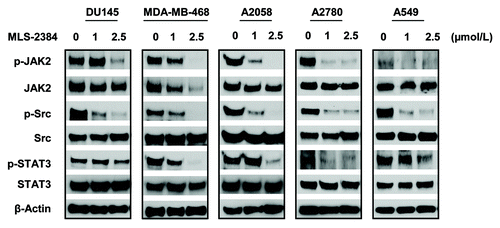
Upon activation, JAK2 and Src phosphorylate STAT3, and thereby changes of phosphorylation levels of JAK2 and Src affect phosphorylation levels of STAT3. In consistent with the reduced levels of phosphorylated JAK2 and Src, phosphorylation of STAT3 was inhibited at the concentration of 2.5 µmol/L. The phosphorylation levels of JAK2, Src, and STAT3 were reduced in the five cancer cell lines in a dose-dependent manner by MLS-2384 (). These findings suggest that MLS-2384 is a dual JAK/Src inhibitor by inhibiting phosphorylation of JAK2, Src, and STAT3 in cancer cells effectively.
MLS-2384 suppresses viability of diverse human cancer cells in culture
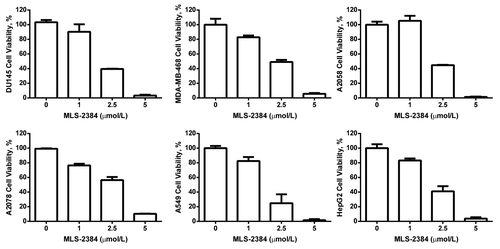
We investigated anticancer activity of MLS-2384 by cell viability assays in human DU145 prostate, MDA-MB-468 breast, A2058 skin, A2780 ovarian, A549 lung, and HepG2 liver human cancer cells. As shown in , MLS-2384 inhibited cell viability of the six human cancer cell lines. The IC50 values are approximately 2 µmol/L in the six cancer cell lines. The reduction of cell viability () is consistent with the inhibition of phosphorylation of JAK2, Src, and STAT3 (). At concentration of 2.5 µmol/L, MLS-2384 significantly inhibited cancer cell viability and phosphorylation of JAK2, Src, and STAT3 in cell culture. MLS-2384 inhibited cell viability of diverse cancers in a dose-dependent manner.
Figure 2. MLS-2384 suppresses viability of diverse human cancer cells. Prostate (DU145), breast (MDA-MB-468), skin (A2058), ovarian (A2780), lung (A549), and liver (HepG2) cancer cells were treated with MLS-2384 at various concentrations for 24 h. Viable cells were counted by using a cell viability analyzer. The values of cell viability were calculated as percentages of viable cell numbers from bromoindirubin-treated cells to viable cell numbers from the DMSO-treated cells.
MLS-2384 induces cleavage of PARP in A2058 human melanoma and DU145 prostate cancer cells
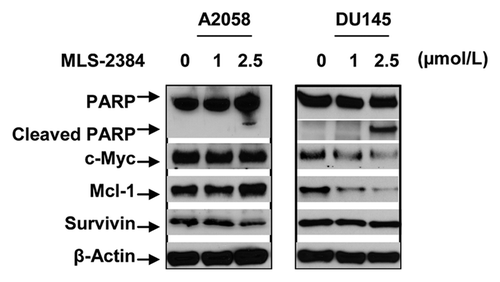
To characterize the cell death, we analyzed a protein marker of apoptosis, cleaved PARP in A2058 and DU145 cells by using western blotting analysis. As shown in , cleavage of PARP was detected in both A2058 and DU145 cells at 2.5 µmol/L. This observation is consistent with inhibition of cell viability () and decreased phosphorylation levels of JAK2, Src, and STAT3 () at the same concentration (2.5 µmol/L). The cleavage of PARP indicates that MLS-2384 induces apoptosis in A2058 and DU145 cancer cells.
Figure 3. Cleavage of PARP and expression levels of STAT3 downstream proteins in human A2058 melanoma and DU145 prostate cancer cells. A2058 and DU145 cells were treated with MLS-2384 at various concentrations for 24 h. Cells were lysed for western blot analysis using antibodies specific to PARP/cleaved PARP, c-Myc, Mcl-1, survivin, and β-Actin.
MLS-2384 downregulates cell survival and proliferation proteins in A2058 and DU145 cancer cells
Furthermore, we investigated STAT3 downstream proteins such as c-Myc, Mcl-1, and survivin, which are involved in cell proliferation and survival. A2058 and DU145 cells were treated with MLS-2384 at concentrations of 1 and 2.5 µmol/L for 24 h. Proteins were isolated from the treated cells. As shown in , in A2058 cells, MLS-2384 reduced the protein level of survivin at 2.5 µmol/L, but levels of c-Myc and Mcl-1 were not changed at the concentration of 2.5 µmol/L. In DU145 cells, the reduction of c-Myc, Mcl-1 protein levels was observed at 1 and 2.5 µmol/L, whereas survivin protein level was not changed at 1 and 2.5 µmol/L, indicating survivin is not a major protein responsible for the inhibition of cell growth, or the induction of apoptosis in DU145 cells. In sum, c-Myc and Mcl-1 are involved in the MLS-2384 mediated cell death in DU145 cells, whereas survivin is involved in A2058 cells.
MLS-2384 demonstrates low toxicity in normal human cells and normal mice
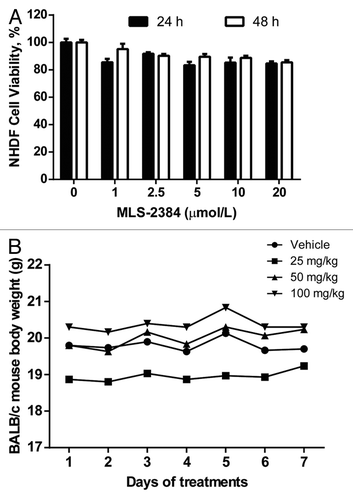
MLS-2384 inhibits approximately 50% viability of cancer cells at a concentration of 2.5 µmol/L (). We further tested MLS-2384 in normal human dermal fibroblast (NHDF) cells and normal mice. As shown in , NHDF cell viability was stable to treatments from 1 up to 20 µmol/L for 24 and 48 h, indicating low toxicity of MLS-2384 in normal human cells. We also investigated toxicity of MLS-2384 in BALB/c normal mice (). BALB/c mice were treated with vehicle and MLS-2384 at doses of 25, 50, and 100 mg/kg once daily through oral gavage for 7 d. Mouse body weight was measured every day. As shown in , there was no weight loss in the treated mice, suggesting low toxicity of MLS-2384 in normal mice.
Figure 4. Toxicity studies in NHDF cells and BALB/c mice. (A) NHDF cells were treated with MLS-2384 at various concentrations for 24 and 48 h. MTS cell proliferation assays were performed according manufacturer’s instruction. (B) Vehicle and MLS-2384 at doses of 25, 50, 75, and 100 mg/kg were administrated through oral gavage once daily to normal BALB/c mice for 7 d. Mouse body weight was measured every day. Points, mean (n = 4).
MLS-2384 demonstrates anticancer activity in a mouse xenograft model of human melanoma
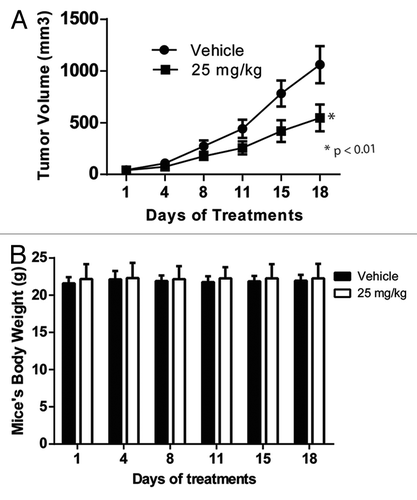
The low toxicity of MLS-2384 in normal human cells and normal mice supported in vivo study of MLS-2384 for anticancer activity. We tested the anticancer activity of MLS-2384 in vivo by using a human melanoma A2058 xenograft mouse model. A dose of 25 mg/kg of MLS-2384 was administrated by oral gavage and twice daily for 18 d in a NSG mouse xenograft model. As shown in , the tumor growth was significantly suppressed. No side effects were observed in the MLS-2384-treated mice. As shown in , mouse body weight remained stable. These findings show the antitumor activity of MLS-2384 in vivo against human melanoma cells in a mouse xenograft model with low toxicity.
Figure 5. MLS-2384 suppresses tumor growth of A2058 human melanoma xenografts in NSG mice. MLS-2384 was administrated to NSG immunodeficient mice through oral gavage twice daily at a dose of 25 mg/kg for 18 d. Mouse tumor volume and body weight were measured for every 3 or 4 d for MLS-2384-or vehicle- treated mice. (A) Tumor volume vs. days of treatments. Points, mean (n = 8); bars, SE; *P < 0.01 vs. control. (B) Body weight vs. days of treatments. Points, mean (n = 8); bars, SE.
Discussion
In previous studies, we showed that 6BIO, a 6-bromoindirubin derivative, targeted JAK/STAT3 signaling as a pan-JAK inhibitor, as well as targeting CDKs and GSK-3. We also demonstrated that a 7-bromoindirubin derivative, MLS-2438, targeted Src/STAT3 signaling as a Src inhibitor. 6BIO and MLS-2438 are different in substitution groups and positions. In an indirubin molecule, 6BIO has a bromo-group at the 6-position, whereas MLS-2438 has a bromo-group at the 7-position. In addition, MLS-2438 and 6BIO have different hydrophilic groups at the 3′-position. The compound in this study, MLS-2384, is a new 6-bromoindirubin derivative. It has a bromo-group at the 6-position same as 6BIO, and the same hydrophilic group at the 3′-position as MLS-2438. Based on the structural features and kinase inhibitory profiles of these compounds, we proposed that MLS-2384 may be a dual JAK/Src inhibitor and more potent in suppression of cancer cell growth. Our data have provided multiple lines of evidence to support that MLS-2384 is a dual JAK/Src inhibitor and suppresses growth of diverse human cancer cells effectively, both in vitro and in vivo. These findings suggest that substitutions and their positions on the indirubin molecule may modify the molecular binding affinity to different targets. In this study, the bromine substitution at the 6-position of indirubin molecule is important for molecular binding affinity to a target such as JAK, and the hydrophilic group at the 3′-position is important for binding a molecular target such as Src.
STAT3 has a central role in tumor cell growth and survival in many various cancer cells, and thereby, we focused on JAK and Src kinases because they are the two major tyrosine kinases upstream of STAT3. Indirubin and its derivatives have been documented as inhibitors of kinases such as CDK and GSK-3β. Here, we demonstrate that MLS-2384 is a dual JAK/Src inhibitor in multiple human cancer cell lines. However, we cannot exclude other molecular targets of MLS-2384 involved in cancer cells.
MLS-2384 displays anticancer activity as a dual JAK/Src inhibitor in various cancer cells. Previously we reported molecular targets and anticancer activities in human melanoma cell lines for two other bromoindirubin derivatives, 6BIO and MLS-2438. In this study, we have extended our investigation of anticancer activities in diverse human cancer cell lines for this new 6-bromoindiribin derivative, MLS-2384. We have found that MLS-2384 effectively suppresses growth of multiple human cancer cells such as prostate, breast, skin, ovarian, lung, and liver, at a concentration of 2.5 µmol/L in cell culture medium.
JAK has been reported for its compensatory effect in response to a Src inhibitor in cancer cells. When the cancer cells were treated with a Src inhibitor, phosphorylation of Src was decreased, but phosphorylation of JAK2 was maintained to compensate the inhibition of Src. Therefore, phosphorylation of STAT3 was less effectively inhibited by the treatment of a Src inhibitor alone.Citation44 In this study, we present a dual JAK/Src inhibitor, MLS-2384 with potent anticancer activity in diverse cancer cell lines and in a mouse xenograft model. It may be more effective than a JAK or Src inhibitor to inhibit STAT3 signaling pathway in cancer cells. Furthermore, MLS-2384 is promising to be combined with other inhibitors to inhibit multiple cell signaling pathways, or combined with chemotherapeutics to overcome drug resistance. Thus, MLS-2384 is promising for development as a novel therapeutic agent targeting JAK/Src and STAT3 signaling in various human cancers. Future studies will be directed toward in vivo pre-clinical evaluation of MLS-2384.
Materials and Methods
Reagents
The preparation of MLS-2384 has been described previously.Citation38 The compound MLS-2384 was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 20 mmol/L and stocked at –20 °C before use for in vitro experiments and treatments in cells. For in vivo experiments, MLS-2384 was freshly prepared in 30% Solutol (Basf) at a concentration of 10 mg/mL. Anti-survivin was from Novus. Anti-β-Actin was from Sigma. Horeseradish peroxidase (HRP)-labeled anti-mouse and anti-rabbit secondary antibodies were from GE Healthcare. All other antibodies were from Cell Signaling.
Cell lines and culture
The human DU145 (prostate), MDA-MB-468 (breast), A2058 (skin), A2780 (ovarian), A549 (lung), and HepG2 (liver) cancer cell lines were obtained from American Type Culture Collection. Normal Human Dermal Fibroblast (NHDF) cells were purchased from PromoCell. DU145, A2058, A2780 Cells were maintained in RPMI 1640 medium. MDA-MB-468, A549, HepG2, and NHDF cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM). The cell culture medium was supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin (P/S).
Cell viability analysis
Human cancer cells were seeded at 12-well plates with 25 000 cells per well. After 24-h incubation, cells were treated with MLS-2384 or DMSO as the vehicle control for 24 h. Dead cells were removed by washing with PBS buffer solution. Then viable cells were collected by trypsinization. Viable cells were counted by Vi-CELLTM XR Cell Viability Analyzer. The values of cell viability were calculated as percentages of viable cell numbers from bromoindirubin-treated cells to viable cell numbers from the DMSO-treated cells.
MTS cell proliferation assay
MTS reagent (CellTiter 96 AQueousOne Solution Cell proliferation Assay) was purchased from Promega. According to the manufacturer’s instruction, human cancer cells were seeded at 96-well plates with 5000 cells per well. After 24 h incubation, cells were treated with MLS-2384 or DMSO as vehicle control for 24 or 48 h. MTS reagent was added to the cell culture medium and absorbance was measured at 490 nm within 4 h by using a micro-plate reader (BIO-RAD). The values of cell viability were calculated as percentages of absorbance from treated samples to absorbance from the vehicle control.
Western blot analysis
Cells were treated with DMSO or MLS-2384. After treatment, cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer supplemented with inhibitors of proteases (Roche Diagnostics GmbH) and sodium orthovanadate, an inhibitor of phosphotases (Aldrich). Protein concentrations were determined by BioMate Spectrometer (Thermo) and protein assay (Bio-Rad). A sample of 40 µg or 20 µg of each protein was resolved in 8% or between 8% and 16% gradient SDS-PAGE gels (Pierce). After gel electrophoresis, proteins were transferred to Hybond-C membranes (Amersham). The membranes were blocked in 5% nonfat milk in PBS containing 0.1% Tween 20 (Polysorbate 20; PBST) at room temperature for 1 to 3 h followed by an overnight incubation at 4 °C with primary antibodies in PBST containing 5% nonfat milk. The membranes were then washed with PBST and incubated with HRP-conjugated secondary antibody in 5% nonfat milk/PBST solution for 1 to 3 h at room temperature, or overnight at 4 °C. Specific proteins were detected by exposure to X-ray film by using Super Signal West Pico Chemiluminescent Substrate or Super Signal west Dura Extended Duration Substrate (Pierce).
In vitro kinase assay
Recombinant JAK family (JAK1, JAK2, TYK2) and Src proteins, substrates, and 33P-labeled ATP were used for the in vitro kinase assays. The recombinant protein catalytic domains were tagged with glutathione S-transferase (GST) and purified from insect cells. The substrate is pEY (mg/ml, Glu;Tyr = 4:1, molecular weight = 5000–20 000) for c-Src and JAK family kinases. The substrate was prepared in freshly constituted Base Reaction Buffer (comprising 20 mmol/L HEPES, pH 7.5; 10 mmol/L MgCl2; 1 mmol/L EGTA; 0.02% BRIJ-35; 0.02 mg/ml BSA; 0.1 mmol/L Na3VO4; 2 mmol/L dithiothreitol [DTT]; and 1% DMSO). Then, required cofactors and kinase were added into the substrate solution. MLS-2384 in DMSO was delivered into the kinase reaction mixture, and then 33P-labeled ATP (specific activity: 0.01 μCi/μl final) was added into the reaction mixture to initiate the reaction. The kinase reaction mixture was incubated for 120 min at room temperature. Reactions were spotted onto P81 ion-exchange paper (Whatman) for measurement of radioactivity.
In vivo therapeutic efficacy
BALB/c mice (6–8 weeks old) were purchased from the National Cancer Institute for toxicity study. Immunodeficient NOD/SCID/IL2Rgamma null (NSG) mice (female; 6–8 weeks old) were purchased from The Jackson Laboratory for use as the xenograft model. The experimental protocol for animal experiments was approved by the Institutional Animal Care and Use Committee (IACUC) of the Beckman Research Institute at City of Hope Medical Center. A2058 human melanoma cells at a density of 2.5 × 106 cells in 0.1 mL serum-free medium were inoculated subcutaneously into the dorsal area of NSG mice to create the xenograft model. MLS-2384 was freshly prepared in vehicle, 30% Soluto (Basf). When tumors became palpable, MLS-2384 or vehicle control was administered via oral gavage twice daily at a dose of 25 mg/kg body weight. Tumor growth was monitored every other day. Tumor volumes and mouse body weights were measured every 3 or 4 d. Tumor volumes were calculated by the formula: 0.5 × (larger diameter) × (small diameter)2.
Statistical analysis
A 2-sided t-test was used to evaluate statistical significance of differences between treated and control groups. P < 0.05 was considered statistically significant.
| Abbreviations: | ||
| STAT | = | signal transducer and activator of transcription |
| JAK | = | Janus activated kinase |
| CDK | = | cyclin-dependent kinase |
| GSK-3 | = | glycogen synthase kinase-3 |
| CML | = | chronic myelocytic leukemia |
| 6BIO | = | 6-bromoindirubin-3′-oxime |
| PARP | = | poly (ADP-ribose) polymerase |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by R01-CA115674 from NIH (R Jove).
References
- Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci 2004; 117:989 - 98; http://dx.doi.org/10.1242/jcs.01111; PMID: 14996930
- Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002; 21:7001 - 10; http://dx.doi.org/10.1038/sj.onc.1205859; PMID: 12370822
- Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 2000; 19:5419 - 27; http://dx.doi.org/10.1038/sj.onc.1203947; PMID: 11114718
- Darnell JE Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264:1415 - 21; http://dx.doi.org/10.1126/science.8197455; PMID: 8197455
- Wojcik EJ, Sharifpoor S, Miller NA, Wright TG, Watering R, Tremblay EA, Swan K, Mueller CR, Elliott BE. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene 2006; 25:2773 - 84; http://dx.doi.org/10.1038/sj.onc.1209306; PMID: 16407846
- Sam MR, Elliott BE, Mueller CR. A novel activating role of SRC and STAT3 on HGF transcription in human breast cancer cells. Mol Cancer 2007; 6:69; http://dx.doi.org/10.1186/1476-4598-6-69; PMID: 17967179
- Hbibi AT, Lagorce C, Wind P, Spano JP, Des Guetz G, Milano G, Benamouzig R, Rixe O, Morere JF, Breau JL, et al. Identification of a functional EGF-R/p60c-src/STAT3 pathway in colorectal carcinoma: analysis of its long-term prognostic value. Cancer Biomark 2008; 4:83 - 91; PMID: 18503159
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer 2004; 4:97 - 105; http://dx.doi.org/10.1038/nrc1275; PMID: 14964307
- Gao SP, Bromberg JF. Touched and moved by STAT3. Sci STKE 2006; 2006:pe30; PMID: 16835434
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798 - 809; http://dx.doi.org/10.1038/nrc2734; PMID: 19851315
- Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev 2005; 24:315 - 27; http://dx.doi.org/10.1007/s10555-005-1580-1; PMID: 15986140
- Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci 2012; 33:122 - 8; http://dx.doi.org/10.1016/j.tips.2011.11.002; PMID: 22153719
- Creedon H, Brunton VG. Src kinase inhibitors: promising cancer therapeutics?. Crit Rev Oncog 2012; 17:145 - 59; http://dx.doi.org/10.1615/CritRevOncog.v17.i2.20; PMID: 22471705
- Chatzizacharias NA, Kouraklis GP, Giaginis CT, Theocharis SE. Clinical significance of Src expression and activity in human neoplasia. Histol Histopathol 2012; 27:677 - 92; PMID: 22473690
- Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer 2007; 7:673 - 83; http://dx.doi.org/10.1038/nrc2210; PMID: 17721432
- Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O’Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev 2008; 223:132 - 42; http://dx.doi.org/10.1111/j.1600-065X.2008.00644.x; PMID: 18613833
- Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE, Gozo M, McDowell EP, Levine RL, Doukas J, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 2008; 13:311 - 20; http://dx.doi.org/10.1016/j.ccr.2008.02.009; PMID: 18394554
- Sayyah J, Sayeski PP. Jak2 inhibitors: rationale and role as therapeutic agents in hematologic malignancies. Curr Oncol Rep 2009; 11:117 - 24; http://dx.doi.org/10.1007/s11912-009-0018-2; PMID: 19216843
- Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology / the Education Program of the American Society of Hematology American Society of Hematology 2009:636-42.
- Lee HJ, Daver N, Kantarjian HM, Verstovsek S, Ravandi F. The role of JAK pathway dysregulation in the pathogenesis and treatment of acute myeloid leukemia. Clin Cancer Res 2013; 19:327 - 35; http://dx.doi.org/10.1158/1078-0432.CCR-12-2087; PMID: 23209034
- Komrokji R, Verstovsek S. Assessing efficacy in myelofibrosis treatment: a focus on JAK inhibition. Expert Rev Hematol 2012; 5:631 - 41; http://dx.doi.org/10.1586/ehm.12.50; PMID: 23216593
- Constantinescu SN, Vainchenker W. Small-molecule inhibitors in myeloproliferative neoplasms: are we aiming for the right targets?. Hematology Am Soc Hematol Educ Program 2012; 2012:553 - 60; PMID: 23233634
- Kontzias A, Kotlyar A, Laurence A, Changelian P, O’Shea JJ. Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease. Curr Opin Pharmacol 2012; 12:464 - 70; http://dx.doi.org/10.1016/j.coph.2012.06.008; PMID: 22819198
- Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist Updat 2010; 13:67 - 78; http://dx.doi.org/10.1016/j.drup.2010.04.001; PMID: 20471303
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 2003; 63:1270 - 9; PMID: 12649187
- Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A 2005; 102:4700 - 5; http://dx.doi.org/10.1073/pnas.0409894102; PMID: 15781862
- Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene 2005; 24:3236 - 45; http://dx.doi.org/10.1038/sj.onc.1208470; PMID: 15735720
- Liby K, Voong N, Williams CR, Risingsong R, Royce DB, Honda T, Gribble GW, Sporn MB, Letterio JJ. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res 2006; 12:4288 - 93; http://dx.doi.org/10.1158/1078-0432.CCR-06-0215; PMID: 16857804
- Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)-->signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res 2008; 68:2920 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-07-3036; PMID: 18413761
- Wang Y, Ma X, Yan S, Shen S, Zhu H, Gu Y, Wang H, Qin G, Yu Q. 17-hydroxy-jolkinolide B inhibits signal transducers and activators of transcription 3 signaling by covalently cross-linking Janus kinases and induces apoptosis of human cancer cells. Cancer Res 2009; 69:7302 - 10; http://dx.doi.org/10.1158/0008-5472.CAN-09-0462; PMID: 19706767
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009; 16:487 - 97; http://dx.doi.org/10.1016/j.ccr.2009.10.015; PMID: 19962667
- Liu L, Nam S, Tian Y, Yang F, Wu J, Wang Y, Scuto A, Polychronopoulos P, Magiatis P, Skaltsounis L, et al. 6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. Cancer Res 2011; 71:3972 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-10-3852; PMID: 21610112
- Tian Y, Nam S, Liu L, Yakushijin F, Yakushijin K, Buettner R, Liang W, Yang F, Ma Y, Horne D, et al. Spirooxindole derivative SOID-8 induces apoptosis associated with inhibition of JAK2/STAT3 signaling in melanoma cells. PLoS One 2012; 7:e49306; http://dx.doi.org/10.1371/journal.pone.0049306; PMID: 23166634
- Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol 1999; 1:60 - 7; PMID: 10559866
- Xiao Z, Hao Y, Liu B, Qian L. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leuk Lymphoma 2002; 43:1763 - 8; http://dx.doi.org/10.1080/1042819021000006295; PMID: 12685829
- Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL, Greengard P, Biernat J, Wu YZ, Mandelkow EM, et al. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors?. J Biol Chem 2001; 276:251 - 60; http://dx.doi.org/10.1074/jbc.M002466200; PMID: 11013232
- Jakobs S, Merz KH, Vatter S, Eisenbrand G. Molecular targets of indirubins. Int J Clin Pharmacol Ther 2005; 43:592 - 4; http://dx.doi.org/10.5414/CPP43592; PMID: 16372530
- Vougogiannopoulou K, Ferandin Y, Bettayeb K, Myrianthopoulos V, Lozach O, Fan Y, Johnson CH, Magiatis P, Skaltsounis AL, Mikros E, et al. Soluble 3′,6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase -3 alter circadian period. J Med Chem 2008; 51:6421 - 31; http://dx.doi.org/10.1021/jm800648y; PMID: 18816110
- Liu L, Kritsanida M, Magiatis P, Gaboriaud N, Wang Y, Wu J, Buettner R, Yang F, Nam S, Skaltsounis L, et al. A novel 7-bromoindirubin with potent anticancer activity suppresses survival of human melanoma cells associated with inhibition of STAT3 and Akt signaling. Cancer Biol Ther 2012; 13:1255 - 61; http://dx.doi.org/10.4161/cbt.21781; PMID: 22895078
- Ferandin Y, Bettayeb K, Kritsanida M, Lozach O, Polychronopoulos P, Magiatis P, Skaltsounis AL, Meijer L. 3′-Substituted 7-halogenoindirubins, a new class of cell death inducing agents. J Med Chem 2006; 49:4638 - 49; http://dx.doi.org/10.1021/jm060314i; PMID: 16854069
- Ribas J, Bettayeb K, Ferandin Y, Knockaert M, Garrofé-Ochoa X, Totzke F, Schächtele C, Mester J, Polychronopoulos P, Magiatis P, et al. 7-Bromoindirubin-3′-oxime induces caspase-independent cell death. Oncogene 2006; 25:6304 - 18; http://dx.doi.org/10.1038/sj.onc.1209648; PMID: 16702956
- Choi SJ, Lee JE, Jeong SY, Im I, Lee SD, Lee EJ, Lee SK, Kwon SM, Ahn SG, Yoon JH, et al. 5,5′-substituted indirubin-3′-oxime derivatives as potent cyclin-dependent kinase inhibitors with anticancer activity. J Med Chem 2010; 53:3696 - 706; http://dx.doi.org/10.1021/jm100080z; PMID: 20361800
- Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, Cascone T, Mills GB, Heymach JV, Johnson FM. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res 2009; 15:6852 - 61; http://dx.doi.org/10.1158/1078-0432.CCR-09-0767; PMID: 19861436
- Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res 2009; 69:1958 - 65; http://dx.doi.org/10.1158/0008-5472.CAN-08-2944; PMID: 19223541
- Johnson FM, Saigal B, Tran H, Donato NJ. Abrogation of signal transducer and activator of transcription 3 reactivation after Src kinase inhibition results in synergistic antitumor effects. Clin Cancer Res 2007; 13:4233 - 44; http://dx.doi.org/10.1158/1078-0432.CCR-06-2981; PMID: 17634553
