Abstract
Glioblastoma multiforme (GBM) is the most common and devastating primary brain tumor among adults. Despite recent treatment progress, most patients succumb to their disease within 2 years of diagnosis. Current research has highlighted the importance of a subpopulation of cells, assigned brain cancer stem-like cells (bCSC), to play a pivotal role in GBM malignancy. bCSC are identified by their resemblance to normal neural stem cells (NSC), and it is speculated that the bCSC have to be targeted in order to improve treatment outcome for GBM patients. One hallmark of GBM is aberrant expression and activation of the epidermal growth factor receptor (EGFR) and expression of a deletion variant EGFRvIII. In the normal brain, EGFR is expressed in neurogenic areas where also NSC are located and it has been shown that EGFR is involved in regulation of NSC proliferation, migration, and differentiation. This led us to speculate if EGFR and EGFRvIII are involved in the regulation of bCSC. In this study we use GBM neurosphere cultures, known to preserve bCSC features. We demonstrate that EGFR and EGFRvIII are downregulated upon differentiation and moreover that when EGFR signaling is abrogated, differentiation is induced. Furthermore, we show that differentiation leads to decreased tumorigenic and stem cell-like potential of the neurosphere cultures and that by specifically inhibiting EGFR signaling it is possible to target the bCSC population. Our results suggest that differentiation therapy, possibly along with anti-EGFR treatment would be a feasible treatment option for patients with GBM, by targeting the bCSC population.
Introduction
Glioblastoma multiforme is the most common and aggressive solid tumor occurring in the brain of adults. Despite progress in recent years, the median survival after diagnosis remains around 15 mo.Citation1 There are some molecular hallmarks of GBM, of which amplification and/or mutation of the epidermal growth factor receptor (EGFR) belong to the most common. The most frequent mutation is the vIII variant (EGFRvIII) which arises from deletion of exons 2–7, rendering the receptor constitutively active and unable of ligand binding.Citation2 Both overexpression of wild-type EGFR and expression of EGFRvIII have been linked to a more aggressive phenotype and dismal prognosis of GBM.Citation3-Citation6 However, even though EGFR and variants thereof are believed to play a role in GBM tumorigenicity, clinical trials with EGFR inhibitors have shown inconsistent results.Citation7-Citation9 Therefore, a deeper knowledge regarding the functional role(s) of EGFR and EGFRvIII in GBM is needed. Until recently, a major obstacle in the EGFR/EGFRvIII research has been that endogenous EGFR overexpression and EGFRvIII expression are lost when GBM cells are grown during traditional serum-containing in vitro conditions.Citation10,Citation11 However, in a previous publication from our laboratory we showed that GBM cell cultures established during serum-free stem cell conditions, traditionally used for in vitro growth of neural stem cells (NSC), maintained endogenous expression of EGFR and EGFRvIII.Citation12 This is in line with other reports showing that GBM cultures grown during stem cell conditions maintain the geno- and phenotypes of the original tumor better than GBM cells cultured during serum-containing conditions.Citation13 These stem cell conditions are believed to preserve and promote growth of so-called brain cancer stem-like cells (bCSC), a population of cancer cells found in GBM which share characteristics with normal NSC. The bCSC are defined by their self-renewing potential, their expression of stem cell markers and their capacity to give rise to cells of the three neural lineages, namely astrocytes, oligodendrocytes, and neurons, upon differentiation.Citation14-Citation18 The bCSC have been assigned a role in tumor angiogenesis and treatment resistance, and upon intracranial transplantation onto immunocompromised mice, it has been shown that is the bCSC that are responsible of forming tumors in vivo.Citation19-Citation22 Thus it is likely that the bCSC are involved in the initiation and progression of brain tumors such as GBM and that treatment directed against the bulk of the tumor cells fails to give long-term responses because the bCSC are unaffected and able to recapitulate the tumor. There is thus a rationale for using cultures enriched in bCSC in experimental GBM research, in order to better understand factors that are important for the bCSC and GBM maintenance.
As mentioned above, bCSC are defined by their self-renewing capacity and their ability to differentiate. These are features that potentially could be used in differentiation based cancer therapy targeting bCSC, as differentiation might lead to hampered self-renewing capacity and reduced production of progenies that are able to populate the tumor. One example of this is acute promyelocytic leukemia (APL) in which poorly differentiated leukemia cells populate the bone marrow and blood, and compete with the production of normal blood cells. Upon induced differentiation therapy with all-trans-retinoic acid (RA), these immature cells are forced to differentiate and thereby lose their malignant potential.Citation23,Citation24
In this study, we investigated the impact of induced differentiation on the endogenous expression of EGFR and EGFRvIII in human derived GBM bCSC neurosphere cultures in order to elucidate their roles in GBM tumorigenicity.
Results
Serum and RA induce differentiation of human GBM cells
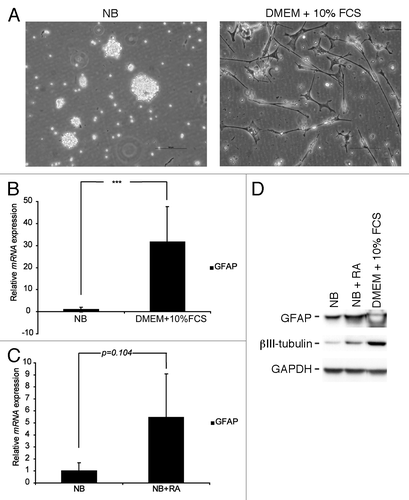
In this study we have used a GBM cell culture established during stem cell conditions in the absence of serum. In this culture system, which enrich for bCSC, the cells grow as neurospheres and share characteristics with normal NSC.Citation25 We have recently shown that such cultures maintain important features of GBM such as expression of EGFR and EGFRvIII,Citation12 whereas other studies have shown that GBM cells grown during traditional cell culture conditions in the presence of serum fail to maintain EGFR and EGFRvIII expression.Citation10,Citation11 In addition, it has been shown that bCSC are able to differentiate in vitro upon serum exposure.Citation17 As a first experiment we therefore wished to study the effect of serum on our GBM neurosphere culture with endogenous EGFR and EGFRvIII expression. Upon serum exposure, some, but not all, neurospheres became adherent and cells started to migrate out from the spheres. The adherent cells grew with a differentiated phenotype, with neurite-like extensions (). Q-RT-PCR analysis confirmed upregulation of the astrocytic marker glial fibrillary acidic protein (GFAP) (), verifying that serum exposure indeed induces differentiation of GBM neurosphere cells. As an additional differentiation agent, RA has in previous studies been shown to induce differentiation of GBM neurosphere cells in vitro.Citation26,Citation27 Indeed, RA induced both morphological differentiation and upregulation of GFAP mRNA in our cells, although not as effectively as serum (data not shown and ). At the protein level, both GFAP and the neuronal marker β-III-tubulin were upregulated in response to either serum or RA ()
Figure 1. GBM neurosphere cells can be induced to differentiate in vitro. (A) GBM neurosphere cells grow with a differentiated morphology upon serum exposure. Scale bar shows 100 µm. (B) GFAP mRNA is upregulated in GBM neurosphere cells when grown in serum containing media. (C) RA induces upregulation of GFAP mRNA in GBM neurosphere cells. Q-RT-PCR reactions are presented as mean mRNA expression ± SD. Statistical significance was calculated using the Student two-sided t test. ***P < 0.005. (D) GFAP and β-III-tubulin protein are upregulated after exposure to serum or RA.
EGFR and EGFRvIII expression are lost upon induced differentiation
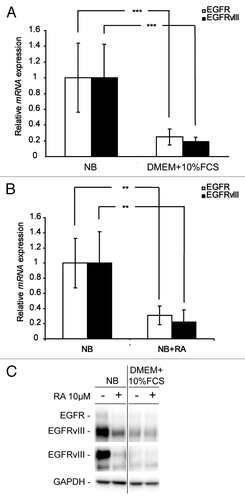
Having verified that serum and RA induce differentiation of our neurosphere cells, we investigated the impact of differentiation on EGFR and EGFRvIII expression. For this we grew the cells in serum containing media or treated them with RA in NB media. We first examined the mRNA level of both EGFR and EGFRvIII. As shown in , both EGFR and EGFRvIII mRNA were downregulated upon induced differentiation with either serum or RA. Furthermore, differentiation led to downregulation of both EGFR and EGFRvIII protein expression (). Although RA was not as effective in inducing GFAP and β-III-tubulin upregulation as serum (), there was no major difference in EGFR and EGFRvIII downregulation ().
Figure 2. EGFR and EGFRvIII are downregulated in GBM neurosphere cells upon differentiation. Q-RT-PCR analyses showing downregulation of EGFR and EGFRvIII mRNA in GBM neurosphere cells upon (A) serum exposure and (B) RA treatment. Q-RT-PCR reactions are presented as mean mRNA expression ± SD. Statistical significance was calculated using the Student two-sided t test. ***P < 0.005 and **P < 0.01. (C) WB showing downregulation of EGFR and EGFRvIII protein after exposure to serum or RA.
Inhibition of EGFR signaling leads to differentiation of GBM neurosphere cells and reduced cell viability
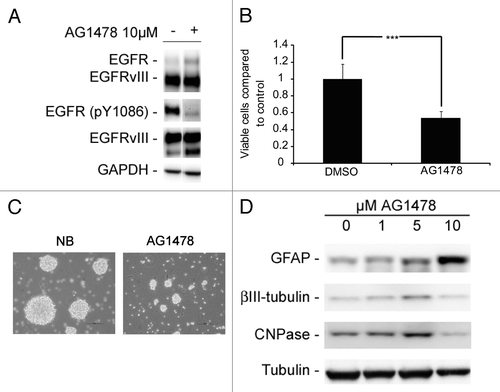
The above results led us to speculate if EGFR and EGFRvIII signaling were coupled to a less differentiated cell phenotype and that these receptors could be involved in maintaining an undifferentiated pool of neurosphere cells. Therefore, we inhibited EGFR/EGFRvIII signaling with the tyrosine kinase inhibitor (TKI) AG1478. Exposure to AG1478 led to decreased phosphorylation of EGFR (pY1086) () along with reduced number of viable cells (), smaller neurospheres in culture and in some cases also adherent and morphologically differentiated cells ( and data not shown). In addition, treatment of GBM neurosphere cells with AG1478 led to upregulation of GFAP, β-III-tubulin, and CNPase in a concentration dependent manner (), indicating that active EGFR/EGFRvIII signaling is involved in maintaining a less differentiated cell phenotype.
Figure 3. Abrogation of EGFR signaling with the TKI AG1478 reduces cell viability and leads to induced differentiation. (A) WB showing reduced phosphorylation of EGFR upon AG1478 treatment. (B) AG1478 (10 µM) exposure leads to a decrease in the number of viable cells as measured by MTT. MTT results are presented as mean ± SD . Statistical significance was calculated using the Student two-sided t test. ***P < 0.005 and **P < 0.01. (C) Photographs of GBM neurospheres showing reduced sphere size upon AG1478 treatment. Scale bar shows 100 µm. (D) GFAP, β-III-tubulin, and CNPase protein is upregulated in a concentration-dependent manner upon AG1478 exposure.
Induced differentiation or inhibition of EGFR signaling leads to decreased tumorigenicity and reduced stem cell potential
Having verified that differentiation leads to downregulation of EGFR/EGFRvIII and that inhibition of EGFR/EGFRvIII signaling with AG1478 leads to differentiation, we wished to investigate which impact EGFR/EGFRvIII had on the tumorigenic capacity and stem-like cell potential of our GBM cells. Therefore, we performed a soft agar assay, reflecting the in vitro tumorigenic potential of the cells. Here, it was evident that both induced differentiation with RA and inhibition of EGFR/EGFRvIII with AG1478 led to decreased ability to grow without anchorage as measured by reduced colony formation (). Although serum alone did not reduce colony formation, the colonies were clearly smaller than colonies formed in NB media alone (). In addition, when serum was combined with RA, colony formation was clearly reduced, both in comparison to colonies formed in NB media alone and in comparison to colonies formed in the presence of RA in NB media (). In a sub-sphere assay, reflecting the number of cells with self-renewing potential, i.e., an indirect measurement of the number of stem cells present, the capacity to form spheres was markedly hampered after induced differentiation with serum or inhibition of EGFR/EGFRvIII signaling with AG1478 (). However, sub-sphere formation after pre-treatment with RA in NB media was increased as compared with NB media alone and spheres formed were also larger ( and data not shown). The reduced ability to form sub-spheres upon induced differentiation with serum and AG1478 was accompanied by a decrease in the stem cell marker Nestin ().
Figure 4. GBM neurosphere tumorigenicity and stem cell-like potential is reduced upon induced differentiation and after EGFR inhibition. (A) Soft agar assay showing reduced colony formation after induced differentiation or AG1478 treatment. Data are presented as mean ± SD of two independent experiments performed in duplicates. (B) Representative pictures of soft agar assay using either NB media or DMEM + 10% FCS. (C) Sub-sphere assay showing reduced sphere forming potential after pre-treatment with serum (DMEM + 10% FCS), RA, or AG1478 as compared with spheres formed in NB media only. Data presented are from one out of two independent experiments performed in sextuplicates and are presented as mean number of spheres ± SD. (D) WB showing downregulation of Nestin expression after exposure to serum (DMEM+10%FCS) or inhibition of EGFR signaling with AG1478. (E) Primary sphere formation is reduced upon abrogation of EGFR signaling with AG1478. Data are presented as mean number of spheres ± SD. Statistical significance was calculated between treated and corresponding control, if not indicated otherwise, using the Student two-sided t test. ***P < 0.005, **P < 0.01, and *P < 0.05.
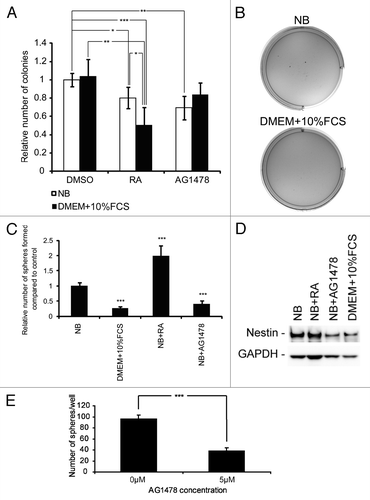
It is considered that the number of bCSCs in a primary culture, i.e., the first passage when a tumor is established as an in vitro culture, can be identified by their ability to form neurospheres during clonogenic dilution.Citation28,Citation29 Therefore the fraction of GBM cells forming spheres in the primary culture can be interpreted as a semi-quantification of the bCSC population in the tumor. To investigate the impact of EGFR/EGFRvIII on primary neurosphere formation and thus indirect the effect on the bCSC population we therefore performed a primary sphere assay in the presence of AG1478. As shown in , our results indicate that upon AG1478 exposure, primary sphere formation was significantly reduced.
In conclusion, our data together imply that functional EGFR/EGFRvIII signaling is important for maintaining an undifferentiated cell phenotype and bCSC potential in GBM neurosphere cultures.
Discussion
Here we show that induced differentiation with serum or RA led to downregulation of EGFR and EGFRvIII expression in human GBM neurosphere cells with endogenous expression of EGFR and EGFRvIII. Furthermore, when inhibiting EGFR and EGFRvIII signaling with an EGFR-specific TKI, differentiation was induced. In addition, downregulation of EGFR and EGFRvIII, as a result of induced differentiation or inhibition of their signaling, led to decreased in vitro tumorigenic capacity and reduced bCSC potential of the GBM cells.
Over the last decade, several studies have identified a population of cells in GBM, with resemblance to normal NSC regarding self-renewal and multi differentiation potential and therefore designated bCSC.Citation14-Citation18 These bCSC have been suggested to be responsible for treatment resistance and recapitulating tumor growth in GBM patients.Citation13,Citation14,Citation16,Citation18-Citation22 However, the question regarding how to identify and target the bCSC still remains unanswered. The CD133 marker has gained much attention as a suitable bCSC marker, yet several studies have now shown that also cells without CD133 expression have stem cell potential and are able to form tumors in vivo and also that there are several distinct pools of bCSC that coexist within the same tumor.Citation30,Citation31 Nevertheless, even if the exact molecular profile of bCSC in GBM is presently unknown, it is likely the bCSC that have to be targeted in order to inhibit tumor growth, and especially remission. Therefore it is of importance to identify factors that are involved in bCSC maintenance and as such could be used for bCSC-directed cancer therapy. The cell of origin for bCSCs still needs to be elucidated. Clinically, most GBM are located in association to neurogenic areas of the brain such as the subventricular zone (SVZ) lining the lateral ventricles and in the dentate gyrus of the hippocampus. It is also in these areas where normal NSC are most abundant,Citation32,Citation33 and it is therefore not farfetched to speculate that these cells could be involved the genesis of bCSC and tumor initiation.Citation32,Citation34 Furthermore, EGFR is expressed in neurogenic regions of the brain such as the SVZ,Citation35 and it has been shown that EGFR is involved in the regulation of NSC proliferation, differentiation and migration.Citation36-Citation38 It is thus likely that EGFR also plays a role in bCSC. We have in a recent study shown that expression of EGFR and especially EGFRvIII is maintained in GBM neurosphere cultures in the presence of EGF and bFGF,Citation12 and others have shown that such cultures promote and preserve growth of bCSC.Citation13 It is, furthermore, well known that GBM cells lose the expression of EGFRvIII and overexpression of EGFR in cultures established under in vitro growth conditions which contain serum.Citation11 Our results here show that when GBM neurosphere cultures are grown in the presence of serum, they are induced to differentiate, as shown by prominent upregulation of GFAP and β-III-tubulin. This is also in line with other studies showing that serum cultured neurosphere cells express more GFAP and Tuj1 as compared with their NB cultured counterparts.Citation13 Along with differentiation, we observed a downregulation of EGFR and EGFRvIII, both at mRNA and protein levels. This led us to the conclusion that when GBM cells are grown in the presence of serum, EGFR and EGFRvIII expression are lost due to induced differentiation, and that this might explain the previous lack of success of establishing GBM cultures with endogenous EGFR and EGFRvIII expression, as the majority of studies have used serum containing in vitro conditions.Citation11
RA has been shown to induce GBM neurosphere cell differentiation in vitro as shown by changes in morphology and upregulation of differentiation markers.Citation26,Citation27 Treatment with RA in our study also resulted in morphological differentiation and upregulation of GFAP and β-III-tubulin. However, the increase was not as prominent as after serum exposure. This is in contrast to what was shown by Campos et al., who showed that RA was a better inducer of astrocytic differentiation than serum.Citation26 Despite these differences, we concluded that RA induces differentiation of GBM neurosphere cells. As with serum induced differentiation, exposure of GBM neurosphere cells to RA resulted in downregulation of both EGFR and EGFRvIII expression.
EGFR and EGFRvIII have been coupled to a cancer stem-like cell (CSC) phenotype in previous studies. For example, EGFR knockdown in EGFR-positive GBM neurosphere cultures led to differentiation and less malignant tumors in vivoCitation39 and in another study EGFR inhibition resulted in reduced sphere formation in the presence of EGF in EGFR positive neurosphere cultures.Citation40 In breast cancer, EGFRvIII has been shown to contribute to the CSC phenotype as it was correlated with increased expression of stem cell-related genes in primary breast cancer samples and led to increased sphere formation in vitro and tumor formation in vivo.Citation41 Furthermore, in GBM it has been demonstrated that EGFRvIII is co-expressed with CD133 and that these EGFRvIII/CD133 expressing cells have elevated sphere forming capacity.Citation42,Citation43 Previous studies have shown that differentiation induced by RA targets bCSC in GBM as shown by downregulation of stem cell markers, decreased colony formation in soft agar and a decreased number of CD133 positive cells.Citation26,Citation27 Our results here show that induced differentiation of GBM cells in vitro led to downregulation of EGFR and EGFRvIII accompanied by a downregulation of the stem cell marker Nestin, decreased ability of anchorage independent growth, reduced colony formation in soft agar, and diminished sub-sphere formation, indicating that both EGFR and EGFRvIII are involved in bCSC maintenance. However, in these assays there were some differences regarding the effect of serum- and RA-induced differentiation. In the soft agar assay, RA was a potent inhibitor of colony formation, whereas it actually increased both the number of spheres formed and their size in the sub-sphere assay. On the contrary, serum failed to inhibit colony formation in the soft agar assay, although colonies formed were clearly smaller than colonies formed in the control. Furthermore, in the sub-sphere assay, serum exposure led to a decrease in the sphere forming potential. One major difference between the two assays performed in this study is that in the sub-sphere assay, differentiation was induced prior to formation of spheres whereas in the soft agar assay the cells were treated while forming colonies. As such, the outcome on sphere- and colony formation cannot be entirely compared. Still, the differences might be explained by the magnitude of differentiation, and as such it seems as if RA is a weaker inducer of differentiation than serum (). If RA targets bCSC to differentiate into progenitor cells that are able of limited self-renewal but proliferate fasterCitation44 this would explain the increased sphere number and size in the sub-sphere assay when RA treatment is relieved and also the low GFAP and β-III-tubulin expression observed after induced differentiation. On the other hand serum might induce a close-to terminal differentiation of bCSC or progenitor cells, which explains the higher GFAP and β-III-tubublin expression and decreased sphere number in the sub-sphere assay after dissociation. Still, these cells might retain some tumorigenic potential, although not stem cell characteristics, and as such can form colonies in soft agar. However, one could speculate that upon dissociation these cells would not be able to form new colonies or spheres, as was the result in the sub-sphere assay. The difference in magnitude between serum- and RA-induced differentiation was also supported by their effect on expression of the stem cell marker Nestin. Here we observed that RA treatment did not have a major impact on Nestin expression whereas it was prominently downregulated after serum exposure, indicating that less stem cells were present.
Figure 5. Schematic view of proposed serum- and RA-induced differentiation of bCSC. Serum induces a close-to terminal differentiation of immature cancer cells such as bCSC and progenitor cells. The resulting cells lose EGFR and EGFRvIII expression, and downregulates Nestin while upregulating GFAP expression. However, the cells still retain some tumorigenic potential and proliferate, although they are not able to self-renew. RA induces less differentiation than serum as visible by less upregulation of GFAP and almost unchanged Nestin expression. In addition, the cells retain some capacity of self-renewal and proliferation and as such they could represent some stage of progenitor cell differentiation. However, both EGFR and EGFRvIII expression are lost upon differentiation, indicating that these receptors are expressed in an immature and undifferentiated cell type such as the bCSC.
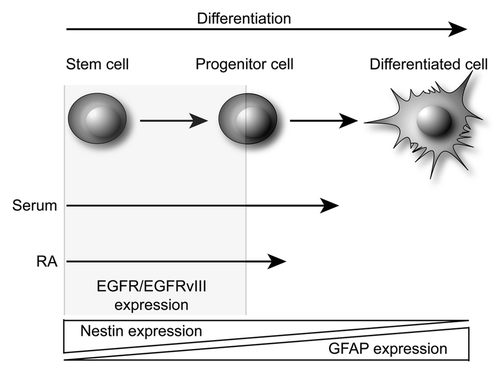
If EGFR and EGFRvIII are important for bCSC maintenance in GBM, one could speculate that it would be possible to target bCSC by inhibiting EGFR and downstream signaling, and that such inhibition would result in differentiation of the bCSC and thus reduced bCSC and tumorigenic potential. Soeda et al. showed that EGF addition increased the CD133-positive population in bCSC cultures and that this increase could be inhibited by blockage of EGFR signaling.Citation45 In addition, in a recent study it was shown that when inhibiting EGFR/EGFRvIII signaling with AG1478 in breast cancer cell lines transfected with EGFRvIII, the fraction of aldefluor-positive cells, as a read out for CSC, was decreased.Citation41 Indeed, when we inhibited EGFR/EGFRvIII signaling with AG1478, a concentration dependent upregulation of GFAP, β-III-tubulin, and CNPase was observed indicating increased differentiation. Again, the upregulation was smaller than with RA and serum. There are, however, several explanations to this. First, the cell cultures we investigate most probably only contain a minority population of bCSC. Second, it is most likely that there are several groups of different types of bCSC and they might not all be equal regarding their molecular profiles.Citation31 This has also been shown in breast cancer where EGFRvIII was expressed in a subset of the CSC.Citation41 As such, EGFR and EGFRvIII might not be expressed on all bCSC present. Therefore, when targeting the bCSC through EGFR and EGFRvIII, the molecular effect might not be as substantial as when differentiation is induced by serum or RA, which targets all the cells in the culture. Even though, we still observed an effect on cell viability and in vitro tumorigenic potential from AG1478 treatment. Furthermore, as an indirect quantification of the bCSC population, inhibition of EGFR/EGFRvIII with AG1478 in both the sub-sphere and primary sphere assays resulted in a significantly reduced sphere forming capacity. In addition, treatment with AG1478 led to downregulation of Nestin expression. This indicates that EGFR/EGFRvIII are involved in bCSC maintenance and that it would be possible to target bCSC that express EGFR/EGFRvIII through inhibition of EGFR signaling. However, as discussed above, bCSC only constitute a fraction of the total number of tumor cells, and it is likely that not all bCSC express EGFR or EGFRvIII. Indeed, in a recent study by Liu et al., it was shown that CD133 positive cells exclusively expressed EGFRvIII and not EGFR.Citation43 In addition, these CD133+/EGFRvIII+ cells were resistant toward the EGFR inhibitor gefitinib.Citation43 Based on our own data, differentiation therapy, resulting in downregulation of EGFRvIII, would be a promising strategy to target these cells.
It has recently been shown that GBM tumors can be divided into several sub groups depending on gene expression, possibly reflecting tumor origin.Citation46,Citation47 Therefore we believe that it is of importance to identify other potential therapeutic targets, specific for each tumor sub group and other bCSC subsets, and use these in combination with anti-EGFR therapy, along with differentiation inducing agents, in the treatment of GBM.
In conclusion, based the presented results vi suggest that active EGFR/EGFRvIII signaling plays an important role for maintaining the stem cell-like features of a subset of bCSC in EGFR/EGFRvIII positive GBM and for these cells to uphold their tumorigenic potential.
Materials and Methods
Cell culture and reagents
Establishment and characterization of the human derived GBM xenograft (NGBM_CPH047) and the in vitro GBM cell culture (NGBM_CPH047p3m1) used in this study has been previously described and show endogenous EGFR and EGFRvIII expression.Citation12 All cells were maintained as neurosphere cultures in Neurobasal®-A media (NB) (Invitrogen, 10888-022) with the following additives: N2 (17502-048), B27 (12587-010), bFGF (10 ng/ml, 13256-029), EGF (10 ng/ml, PHG0311), l-glutamine (25030-024), penicillin (50 U/ml), and streptomycin (50 µg/ml, 15070-063) (all from Invitrogen), in an atmosphere of 5% CO2 and 21% O2 at 37 °C. Fresh media was added twice a week and spheres were mechanically dissociated at every passage. For experiments, cells were dissociated, counted and plated in NB media plus all additives as above or in DMEM (Invitrogen, 21885-108) with the addition of FCS (10%, Invitrogen, 10106), penicillin (50 U/ml) and streptomycin (50 µg/ml) prior to initiation of treatment with all-trans-retinoic acid (RA) (10 µM, Sigma-Aldrich, R2625–50MG) or AG1478 (1, 5, or 10 µM, Calbiochem, 658548) for 12 to 14 d. In control experiments DMSO was added at the same volume as the drugs, if not stated otherwise.
Primary sphere assay
Single cells from acutely dissociated GBM xenograft tissue (NGBM_CPH047) were plated in 96-well microwell plates at a density of 10 cells/μl and directly treated with 0 or 5 μM AG1478. Zero micromolar AG1478 was used as a control in this assay. At day 14, the number of spheres per well was scored and the primary sphere frequency was calculated.
Western blotting
Whole cell protein lysates were separated on 3–8% NuPAGE TA gels (EA03755BOX) or on 4–12% NuPAGE Bis-Tris gels (NP0336BOX) (Invitrogen) and electroblotted onto nitrocellulose membranes (Invitrogen, LC2000). The membranes were then blocked for 1 h at room temperature (RT) and incubated with primary antibodies in 5% non-fat milk overnight (ON) at 4 °C followed by secondary antibodies for 1 h at RT. Blots were developed using the SuperSignal West Dura Extended Duration Substrate (Pierce Biotechnology, 34076) and the Biospectrum Imaging System (UVP). Primary antibodies used: mouse monoclonal anti-Nestin (Chemicon, MAB5326), mouse monoclonal anti-βIII-tubulin (Millipore, MAB1637), mouse monoclonal anti-CNPase (Abcam, ab6319), goat polyclonal anti-EGFR (Fitzgerald Industries International, 20-ES04), mouse monoclonal anti-EGFRvIII (L8A4, a kind gift from Dr Darell Bigner, Duke University Medical Center), mouse monoclonal anti-GFAP (Cell Signaling, 3670), rabbit polyclonal anti-EGFR [pY1086] (Biosource, Invitrogen, 44–790), rabbit monoclonal anti-α-tubulin (Cell Signaling, 2125), and rabbit anti-GAPDH (Santa Cruz Biotechnology, sc-25778).
Quantitative real-time PCR (Q-RT-PCR)
GBM neurosphere cultures were harvested, quick frozen in liquid N2 and stored at –80 °C until used. Total RNA was purified with the RNeasy Mini kit (74104) and QIAshredder (79654) (both from Qiagen) as described by the manufacturer. All RNA was DNase treated using the RNase-Free DNase Set (79254) from Qiagen according to the manufacturer’s instructions. cDNA was synthesized using the SuperscriptTM III Platinum® Two Step qRT-PCR kit with SYBR® Green (Invitrogen, 11735-032), which was also used for quantitative real-time PCR (Q-RT-PCR) reactions. Relative quantification of gene expression levels was performed according to the comparative Ct method. All data was normalized to the expression of three housekeeping genes (TOP1, EIF4A2, and CYC1) included in the human geNorm house-keeping gene selection kit (Primerdesign ge-SY-12). PCR reactions were optimized to distinguish between EGFR and EGFRvIII expression as previously described.Citation12 Primers used were: EGFR forward, 5′-TCCTTGGGAA TTTGGAAATT-3′; EGFR reverse, 5′-GGCATAGGAA TTTTCGTAGT ACAT-3′; EGFRvIII forward, 5′-ATGCGACCCT CCGGGACG-3′; EGFRvIII reverse, 5′-ATCTGTCACC ACATAATTAC CT-3′; GFAP forward, 5′-CGCTGGTAGA GATGGAGGAG-3′; GFAP reverse, 5′-CTGGGGTTAA GAAGCAGCAG-3′.
MTT
MTT assays were performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay according to the manufacturer’s instructions (Sigma M-5655). Briefly, neurospheres were dissociated and cells were plated in a 96-well cell culture plate at a density of 8 × 104 cells per well and allowed to grow ON. Drugs were added at the indicated concentrations where after the plates were incubated for 12 d at 37 °C, 5% CO2, and 21% O2. At the end of experiment, 20 μl 5 mg/ml MTT solution (dissolved in sterile water) was added to each well and incubated for 4 h before addition of 100 μl solubilization buffer (10% SDS, 0.03 M HCl). Next day, the absorbance was read at 570 nm using a Synergy2 microplate reader with the Gen5, Microplate Data Collection and Analysis Software (Biotek). A reference filter at 690 nm was used to subtract background absorbance. Each experimental condition was tested in sextuplicates and repeated at least two times.
Soft agar
Dissociated neurosphere cells (1 × 105/ml) were casted in semisolid agar with either DMEM + 10% FCS or NB media including all supplements as described above, in the presence of either RA (10 µM) or AG1478 (5 µM or 10 µM). At day 14, the colonies were stained with a 0.005% Crystal Violet solution and the number of colonies was manually counted.
Sub-sphere analysis
Neurospheres were mechanically dissociated and seeded at a density of 1 × 106 cells in either DMEM + 10% FCS or NB media including all supplements as described above and allowed to grow ON. RA (10 µM) or AG1478 (5 or 10 µM) or the corresponding volume DMSO as a control was added to the cells, which were then incubated for 12 d for pre-treatment. Adherent and suspension cells were then collected by centrifugation, dissociated, and diluted in NB media with all additives. Ten or 100 cells/well were then plated in 96-well plates and allowed to form spheres. The total number of spheres was counted manually after 2 weeks.
| Abbreviations: | ||
| GBM | = | glioblastoma multiforme |
| EGFR | = | epidermal growth factor receptor |
| EGFRvIII | = | epidermal growth factor receptor variant III |
| bCSC | = | brain cancer stem-like cells |
| NSC | = | neural stem cells |
| APL | = | acute promyelocytic leukemia |
| RA | = | all-trans-retinoic acid |
| NB | = | Neurobasal®–A media |
| GFAP | = | glial fibrillary acidic protein |
| TKI | = | tyrosine kinase inhibitor |
| SVZ | = | subventricular zone |
| CSC | = | cancer stem-like cell |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank technicians Mette Villingshøj and Pia Pedersen for skilful technical assistance. Grant support was obtained from Fonden til lægevidenskabens fremme, Rigshospitalets forskningspuljer, University of Copenhagen and Kathrine og Vigo Skovgaards Fond.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352:987 - 96; http://dx.doi.org/10.1056/NEJMoa043330; PMID: 15758009
- Ekstrand AJ, Longo N, Hamid ML, Olson JJ, Liu L, Collins VP, James CD. Functional characterization of an EGF receptor with a truncated extracellular domain expressed in glioblastomas with EGFR gene amplification. Oncogene 1994; 9:2313 - 20; PMID: 8036013
- Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 1997; 272:2927 - 35; http://dx.doi.org/10.1074/jbc.272.5.2927; PMID: 9006938
- Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A 1994; 91:7727 - 31; http://dx.doi.org/10.1073/pnas.91.16.7727; PMID: 8052651
- Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 2003; 63:6962 - 70; PMID: 14583498
- Torp SH, Helseth E, Dalen A, Unsgaard G. Relationships between Ki-67 labelling index, amplification of the epidermal growth factor receptor gene, and prognosis in human glioblastomas. Acta Neurochir (Wien) 1992; 117:182 - 6; http://dx.doi.org/10.1007/BF01400618; PMID: 1414519
- Lassman AB, Rossi MR, Raizer JJ, Abrey LE, Lieberman FS, Grefe CN, Lamborn K, Pao W, Shih AH, Kuhn JG, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res 2005; 11:7841 - 50; http://dx.doi.org/10.1158/1078-0432.CCR-05-0421; PMID: 16278407
- Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 2005; 353:2012 - 24; http://dx.doi.org/10.1056/NEJMoa051918; PMID: 16282176
- Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol 2004; 22:133 - 42; http://dx.doi.org/10.1200/JCO.2004.08.110; PMID: 14638850
- Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, Bigner DD. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res 1990; 50:8017 - 22; PMID: 2253244
- Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer 2004; 39:29 - 36; http://dx.doi.org/10.1002/gcc.10300; PMID: 14603439
- Stockhausen MT, Broholm H, Villingshøj M, Kirchhoff M, Gerdes T, Kristoffersen K, Kosteljanetz M, Spang-Thomsen M, Poulsen HS. Maintenance of EGFR and EGFRvIII expressions in an in vivo and in vitro model of human glioblastoma multiforme. Exp Cell Res 2011; 317:1513 - 26; http://dx.doi.org/10.1016/j.yexcr.2011.04.001; PMID: 21514294
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006; 9:391 - 403; http://dx.doi.org/10.1016/j.ccr.2006.03.030; PMID: 16697959
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 2004; 64:7011 - 21; http://dx.doi.org/10.1158/0008-5472.CAN-04-1364; PMID: 15466194
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 2002; 39:193 - 206; http://dx.doi.org/10.1002/glia.10094; PMID: 12203386
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63:5821 - 8; PMID: 14522905
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature 2004; 432:396 - 401; http://dx.doi.org/10.1038/nature03128; PMID: 15549107
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 2004; 23:9392 - 400; http://dx.doi.org/10.1038/sj.onc.1208311; PMID: 15558011
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444:756 - 60; http://dx.doi.org/10.1038/nature05236; PMID: 17051156
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 2006; 66:7843 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-06-1010; PMID: 16912155
- Lathia JD, Gallagher J, Myers JT, Li M, Vasanji A, McLendon RE, Hjelmeland AB, Huang AY, Rich JN. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One 2011; 6:e24807; http://dx.doi.org/10.1371/journal.pone.0024807; PMID: 21961046
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2006; 5:67; http://dx.doi.org/10.1186/1476-4598-5-67; PMID: 17140455
- Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol 2006; 17:1620 - 4; http://dx.doi.org/10.1093/annonc/mdl074; PMID: 16600978
- Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol 2004; 51:1 - 28; http://dx.doi.org/10.1016/j.critrevonc.2004.04.007; PMID: 15207251
- Kristoffersen K, Villingshøj M, Poulsen HS, Stockhausen MT. Level of Notch activation determines the effect on growth and stem cell-like features in glioblastoma multiforme neurosphere cultures. Cancer Biol Ther 2013; 14:625 - 37; http://dx.doi.org/10.4161/cbt.24595; PMID: 23792644
- Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G, et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res 2010; 16:2715 - 28; http://dx.doi.org/10.1158/1078-0432.CCR-09-1800; PMID: 20442299
- Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, Guerrero-Cazares H, Quinones-Hinojosa A, Laterra J, Xia S. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene 2011; 30:3454 - 67; http://dx.doi.org/10.1038/onc.2011.58; PMID: 21383690
- Engstrom CM, Demers D, Dooner M, McAuliffe C, Benoit BO, Stencel K, Joly M, Hulspas R, Reilly JL, Savarese T, et al. A method for clonal analysis of epidermal growth factor-responsive neural progenitors. J Neurosci Methods 2002; 117:111 - 21; http://dx.doi.org/10.1016/S0165-0270(02)00074-2; PMID: 12100976
- Mori H, Ninomiya K, Kino-oka M, Shofuda T, Islam MO, Yamasaki M, Okano H, Taya M, Kanemura Y. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J Neurosci Res 2006; 84:1682 - 91; http://dx.doi.org/10.1002/jnr.21082; PMID: 17044035
- Joo KM, Kim SY, Jin X, Song SY, Kong DS, Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest 2008; 88:808 - 15; http://dx.doi.org/10.1038/labinvest.2008.57; PMID: 18560366
- Piccirillo SG, Combi R, Cajola L, Patrizi A, Redaelli S, Bentivegna A, Baronchelli S, Maira G, Pollo B, Mangiola A, et al. Distinct pools of cancer stem-like cells coexist within human glioblastomas and display different tumorigenicity and independent genomic evolution. Oncogene 2009; 28:1807 - 11; http://dx.doi.org/10.1038/onc.2009.27; PMID: 19287454
- Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol 1999; 156:333 - 44; http://dx.doi.org/10.1006/exnr.1999.7028; PMID: 10328940
- Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci 2005; 8:723 - 9; http://dx.doi.org/10.1038/nn1473; PMID: 15908947
- Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol 2007; 9:424 - 9; http://dx.doi.org/10.1215/15228517-2007-023; PMID: 17622647
- Weickert CS, Webster MJ, Colvin SM, Herman MM, Hyde TM, Weinberger DR, Kleinman JE. Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J Comp Neurol 2000; 423:359 - 72; http://dx.doi.org/10.1002/1096-9861(20000731)423:3<359::AID-CNE1>3.0.CO;2-0; PMID: 10870078
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 2010; 467:323 - 7; http://dx.doi.org/10.1038/nature09347; PMID: 20844536
- Ayuso-Sacido A, Moliterno JA, Kratovac S, Kapoor GS, O’Rourke DM, Holland EC, García-Verdugo JM, Roy NS, Boockvar JA. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J Neurooncol 2010; 97:323 - 37; http://dx.doi.org/10.1007/s11060-009-0035-x; PMID: 19855928
- Boockvar JA, Kapitonov D, Kapoor G, Schouten J, Counelis GJ, Bogler O, Snyder EY, McIntosh TK, O’Rourke DM. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol Cell Neurosci 2003; 24:1116 - 30; http://dx.doi.org/10.1016/j.mcn.2003.09.011; PMID: 14697673
- Mazzoleni S, Politi LS, Pala M, Cominelli M, Franzin A, Sergi Sergi L, Falini A, De Palma M, Bulfone A, Poliani PL, et al. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res 2010; 70:7500 - 13; http://dx.doi.org/10.1158/0008-5472.CAN-10-2353; PMID: 20858720
- Howard BM, Gursel DB, Bleau AM, Beyene RT, Holland EC, Boockvar JA. EGFR signaling is differentially activated in patient-derived glioblastoma stem cells. J Exp Ther Oncol 2010; 8:247 - 60; PMID: 20734923
- Del Vecchio CA, Jensen KC, Nitta RT, Shain AH, Giacomini CP, Wong AJ. Epidermal growth factor receptor variant III contributes to cancer stem cell phenotypes in invasive breast carcinoma. Cancer Res 2012; 72:2657 - 71; http://dx.doi.org/10.1158/0008-5472.CAN-11-2656; PMID: 22419663
- Wong A, Mitra S, Del Vecchio CA, Shirboll S. Expression of EGFRvIII in brain tumor stem cells. J Clin Oncol 2012; 26:15s
- Liu XJ, Wu WT, Wu WH, Yin F, Ma SH, Qin JZ, Liu XX, Liu YN, Zhang XY, Li P, et al. A minority subpopulation of CD133(+) /EGFRvIII(+) /EGFR(-) cells acquires stemness and contributes to gefitinib resistance. CNS Neurosci Ther 2013; 19:494 - 502; http://dx.doi.org/10.1111/cns.12092; PMID: 23575351
- Dirks PB. Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol 2008; 26:2916 - 24; http://dx.doi.org/10.1200/JCO.2008.17.6792; PMID: 18539973
- Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, Nakashima S, Kunisada T, Iwama T. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J Biol Chem 2008; 283:10958 - 66; http://dx.doi.org/10.1074/jbc.M704205200; PMID: 18292095
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006; 9:157 - 73; http://dx.doi.org/10.1016/j.ccr.2006.02.019; PMID: 16530701
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al, Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17:98 - 110; http://dx.doi.org/10.1016/j.ccr.2009.12.020; PMID: 20129251
