Abstract
Studies of the decades-long latent stages of breast carcinogenesis have been limited to when hyperplastic lesions are already present. Investigations of earlier stages of breast cancer (BC) latency have been stymied by the lack of fiducial biomarkers needed to identify where in histologically normal tissues progression toward a BC might be taking place. Recent evidence suggests that a marker of chronic oxidative stress (OxS), protein adducts of 4-hydroxy-2-nonenal (4HNE), can meet this need. Specifically: (1) 4HNE immunopositive (4HNE+) mammary epithelial (ME) cells were found to be prevalent in normal (reduction mammoplasty) tissues of most women (including many teenagers) studied, representative of those living in the United States’ high risk-posing environment and: (2) marked (>1.5-fold) differences were identified between tissues of healthy young women with many vs. few 4HNE+ ME cells in the relative levels of transcripts for 42 of the 84 OxS-associated genes represented in SABioscience Oxidative-Stress/Oxidative-Defense PCR array. Herein we used synchrotron radiation-based Fourier-transform infrared (SR-FTIR) microspectroscopy to identify molecular changes associated with 4HNE adducts in basal and luminal ME cells in terminal ductal units (TDLU), which are the cells of origin of BC, and associated intralobular and interlobular stroma, known contributors to carcinogenesis. Multivariate analysis-derived wavenumbers differentiated 4HNE+ and 4HNE− cells in each of the anatomical compartments. Specifically, principal component and linear discriminant analyses of mid-infrared spectra obtained from these cells revealed unambiguous, statistically highly significant differences in the “biochemical fingerprint” of 4HNE+ vs. 4HNE− luminal and basal ME cells, as well as between associated intralobular and interlobular stroma. These findings demonstrate further SR-FTIR microspectroscopy’s ability to identify molecular changes associated with altered physiological and/or pathophysiological states, in this case with a state of chronic OxS that provides a pro-carcinogenic microenvironment.
Introduction
Epidemiological evidence implicates environment/lifestyle factors in the high incidence of breast and prostate cancer in the US and other developed western industrialized societies, as well as in their rising incidence in societies/countries as they become “westernized”.Citation1,Citation2 A further important insight provided by epidemiological studies is the phenomenon of cancer latency, namely, that excess cancers attributable to exogenous carcinogenic agent(s) appear in humans first only after about two decades following initiating exposures and continue to surface during several decades thereafter.Citation3-Citation10 Current knowledge of molecular changes during the decades-long period of cancer latency is limited to its penultimate stages when histological changes are already present. Moreover, tissues available for study are mostly from older subjects and the amounts that can be spared for research is limited by the need for pathological diagnosis.
An opportunity to study earlier stages of cancer-latency in a cancer-prone tissue is offered by the availability of reduction mammoplasty tissues from healthy young women. As simple macromastia, the indication for surgery, is not a risk factor for breast cancer (BC) these tissues represent the norm for subjects sharing a particular physical and cultural environment; the incidence of occult pre-neoplastic and neoplastic lesions in reduction mammoplasties is no higher than would be expected in the population and BC incidence in subjects post-mammoplasty might even be reduced.Citation11-Citation16 Since 1 in 8 women living in the USA can be expected to develop BC in their lifetime, a high proportion of histologically normal tissues from individuals representative of this population is likely to encompass loci that are conducive to the evolution of cells that can initiate a cancer and where some cells are in the latent stages of carcinogenesis. The ability to take advantage of this resource, however, has been limited by the lack of biomarker(s) needed to identify in these tissues loci where progression toward BC might be taking place. Recently we presented evidence suggesting that this obstacle might be overcome by using the presence of protein adducts of 4-hydroxy-2-nonenal (4HNE) as a fiducial, topographic marker; 4HNE is one of the major end products of free radical-initiated lipid peroxidation,Citation17 which when generated at a rate that exceeds the rate at which it can be inactivated forms protein adducts that are identifiable by immunocytochemistry.Citation17-Citation19 Hence, protein adducts of 4HNE have been used extensively in studies of diverse diseases in which chronic oxidative stress (OxS) plays an etiological role.Citation20-Citation30
The postulate that chronic OxS underlies the high BC incidence in women living in the US, and by inference in other developed western industrialized societies, was first proposed by Malins and coworkers two decades ago. It was based on their Fourier-transform infrared (FTIR) spectroscopic findings of extensive oxidatively-damaged DNA in reduction mammoplasty tissue of healthy women living in the northwest US, a prototypic high BC risk-posing “westernized” environment.Citation31-Citation35 Importantly, in breast tissues of many of the women studied the findings suggested progressive hydroxyl radical-induced changes in DNA that led to a premalignant, cancer-like phenotype.Citation32,Citation33,Citation36 These findings paralleled their finding of similar oxidatively-damaged DNA isolated from livers of English sole with a high incidence of hepatic carcinomas attributable to their living in waters polluted with industrial chemicals.Citation37-Citation42
Our recently reported findings support the proposed role of chronic OxS in the high BC incidence in women living in the US.Citation43 Specifically, first we found 4HNE immunopositive (4HNE+) ME cells to be prevalent in normal (breast reduction) tissues of healthy women and teenagers drawn from a population with a BC incidence comparable to that of the population studied by Malins et al. We then provided evidence suggesting that 4HNE adducts might serve as a fiducial marker of where under the influence of chronic OxS molecular changes of potential relevance to breast carcinogenesis might be taking place. Specifically, we identified molecular changes associated with 4HNE immunostaining. Briefly, we documented >1.5-fold difference between breast tissues of healthy young women (ages 17–27 y) with few vs. many 4HNE+ ME cells in the relative levels of transcripts for 42 of the 84 genes represented in the SABioscience Oxidative Stress/Oxidative Defense PCR array. Remarkably, of the 42 transcripts that differentiated 4HNE+ from 4HNE− tissues, the relative levels of 35 were decreased and only seven were increased >1.5-fold. This is in contrast to the increased levels of transcripts of many of these genes that would be expected under conditions of acute OxS. The predominance of attenuated gene expression identified in 4HNE+ breast tissues, however, corresponds to what has been reported in the few studies in which tissues were subjected to sustained low levels of OxS.Citation44-Citation46 Since our first exploratory study was performed using RNA isolated from whole cryosections of breast parenchyma, the findings represent an average of the mRNA levels derived from diverse cell populations. Here we used synchrotron radiation-based (SR) FTIR (SR-FTIR) microspectroscopy in order to identify molecular changes associated with 4HNE adducts in cells most directly relevant to breast carcinogenesis, namely, ME cells within terminal lobular ductal units (TDLUs), which are the cells of origin of the majority of BC, and the stroma associated with them that participates in process of carcinogenesis.Citation47-Citation53
Infrared (IR) spectroscopy is based on movements exhibited by chemical bonds that link atoms within molecules. These movements occur at specific frequencies corresponding to vibrational modes of energy levels that absorb different regions of the IR spectrum. One region of biological interest is the mid-IR region of 4000–400 cm−1 (2.5–25 μm). Within this region, IR is known to be absorbed by a number of classes of biochemicals that generate identifiable peak absorption frequencies in resultant absorbance spectra. These include: lipids (≈1750 cm−1), amide I (≈1650 cm−1), amide II (≈1550 cm−1), amide III (≈1260 cm−1), asymmetric phosphate stretching vibrations (νasPO2−; ≈1225 cm−1), carbohydrates (≈1155 cm−1), symmetric phosphate stretching vibrations (νsPO2−; ≈1080 cm−1), glycogen (≈1030 cm−1), and protein phosphorylation (≈970 cm−1).Citation54 Computational analysis of the large data sets generated using principal component analysis (PCA) and linear discriminant analysis (LDA) have proven to be highly effective toward extracting differences in biochemical composition of cells and endogenously- or chemically-induced alterations in cellular chemistry.Citation54-Citation56
A major limitation to the application of IR microscopy toward analysis of the chemical composition of cells in tissue sections has been its beam-width; this has limited spatial resolution and degraded signal-to-noise ratio (SNR). This limitation has now been overcome by using IR sources based on synchrotron radiation, which can generate a highly collimated beam of photons of high enough brilliance to enable delivery of a beam through the sampling aperture that is small enough to generate spectra at a spatial resolution of 10 μm × 10 μm and a SNR 1000-times greater than conventional FTIR microspectroscopy.Citation57-Citation61 Hence, SR-FTIR microspectroscopy can provide information about the chemical composition and biomolecular phenotype of individual cells within their native environment within tissue sections. It has demonstrated ability to reveal differences between the spectral signature of cells associated with different physiological or pathophysiological states in formalin-fixed paraffin-embedded (FFPE) tissue sections, and to be highly sensitive toward detecting alterations in cellular chemistry.Citation60,Citation62,Citation63 Herein we present evidence of significant differences identified by this technology between the spectral signature of ME cells within 4HNE+ vs. 4HNE− TDLUs in breast tissues of healthy young women, as well as between the stroma within and surrounding the TDLUs. The findings support the hypothesis that the state of chronic OxS implied by the presence of 4HNE adducts is associated with molecular changes in ME cells from which a cancer might ultimately arise, as well as in neighboring stroma that define their microenvironment. The findings also provide evidence that a fiducial immunocytochemical marker of chronic OxS combined with a biomolecular technique with “omics” capability, such as SR-FTIR microspectroscopy, can open up for study early latent/promotion stages of carcinogenesis prior to the onset of any histological changes.
Results
Terminal lobular ductal units (TDLUs) and associated intralobular and interlobular stroma in unstained, de-waxed 10-μm-thick tissue sections were point mapped using the SR-FTIR microspectroscopy system. The tissue sections analyzed were selected from subjects at either end of the spectrum of immunostaining for 4HNE adducts, designated 4HNE+ and 4HNE− tissues, respectively, specifically, three subjects with at most few minimally 4HNE immunopositive (4HNE+) ME cells in TDLUs (ages 20, 21, and 24 y) and from two subjects with many strongly 4HNE+ ME cells in TDLUs (ages 23 and 25 y). The criteria for categorizing tissues as either 4HNE+ or 4HNE− have been described previously.Citation43 IR spectra were obtained from each of nine TDLUs in sections from each subject; 60 IR spectra per luminal and basal epithelial cells (n = 540 total), 10 spectra per intralobular stroma (n = 90 spectra total) and 20 spectra per interlobular stroma (n = 180 total). In order to compare IR spectra of the luminal and basal (myoepithelial) epithelial cells lining the ductules in TDLUs, independent point IR spectra were acquired via a 10 μm × 10 μm beam aperture with 10 μm step-wise movement from individual cells from each of the two cell types (n = 195 luminal and n = 90 basal (myoepithelial) spectra). The data were subjected to multivariate computational analysis using PCA and LDA.
shows brightfield photomicrograph of a representative TDLUs in an unstained breast tissue section from which IR spectra were acquired from epithelial cells (Epi), intralobular stroma (LS), and interlobular stroma (ILS). The IR spectra were obtained in a randomized fashion from nine TDLUs with n = 540 spectra derived from epithelia lining ductules, n = 90 spectra from intralobular stroma, and n = 180 spectra interlobular stroma. Via the 10 μm × 10 μm beam aperture employed, a good SNR was obtained; however, discrimination of spectra derived from TDLUs of 4HNE+ vs. 4HNE− tissues was not readily discernible (). This observation was associated with spectra derived from all the three tissue compartments (epithelial [Epi] vs. intralobular stromal [LS] vs. interlobular stromal [ILS]). From derived point maps acquired via the 10 μm × 10 μm beam aperture, IR spectra were extracted and assigned to their designated location (Epi, LS, or ILS) ().
Figure 1. (A) Brightfield photomicrograph of representative terminal lobular ductal unit (TDLUs) in unstained breast tissue section from which infrared (IR) spectra were acquired from epithelial cells (E), intralobular stroma (LS), and interlobular stroma (ILS). Tissue sections used were selected from subjects at either end of the spectrum of immunostaining for 4-hydroxy2-nonenal (4HNE adducts) adducts.Citation43 Specifically, sections in the 4HNE+ category were from subjects whose breast tissue sections showed consistently a preponderance of TDLUs occupied by many 4HNE+ ME cells, while those in the 4HNE− category were from subjects whose tissue sections showed consistently at most a few TDLUs occupied by a small number of 4HNE+ ME cells.Citation43 4HNE− and 4HNE+ TDLUs were interrogated by synchrotron radiation-based Fourier-transform IR (SR-FTIR) microspectroscopy via a 10 μm × 10 μm beam aperture either to acquire point IR spectra or in a 10 μm step-wise fashion to derive image maps The red crosses identify locations from which point spectra were acquired one after another. The software used enables tracking along the brightfield image of the tissue section and pinpoint the exact locations from which spectra were derive spectra, i.e., along the epithelial cells. Once the points of interest are identified, the spectral acquisition program is activated, a motorized stage in chronological order moves each point of interest to the focus of the synchrotron IR beam to allow for spectral acquisition. (B) Average of all the IR spectra acquired from point IR spectra from 4HNE+ and 4HNE− TDLUs associated with E, LS, or ILS showing a typical wavenumber-absorbance intensity profile.
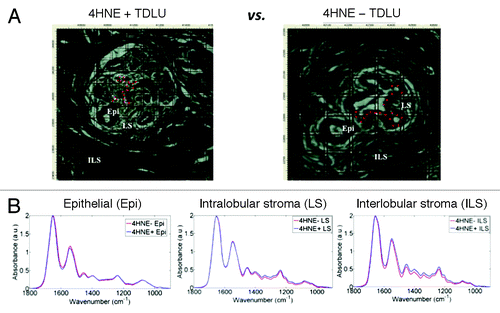
Figure 2. Principal component analysis-linear discriminant analysis (PCA-LDA) of infrared (IR) spectra of three anatomical compartments of 4-hydroxy-2-nonenal (4HNE) immunopositive (4HNE+) and 4HNE immunonegative (4HNE−) breast terminal ductal lobular units (TDLUs). Tissue sections in the 4HNE+ category were from subjects whose tissue sections showed consistently a preponderance of TDLUs occupied by many 4HNE+ ME cells, while those in the 4HNE− category were from subjects whose breast tissue sections showed consistently at most a few TDLUs occupied by a small number of 4HNE+ ME cells.Citation43 (A) PCA-LDA scores plot of point-map IR spectra (n = 540 total) of epithelial cells (EPI). Each spectral point is derived from the average of 10 IR spectra. A total of four 4HNE+ TDLUs were interrogated from two 4HNE+ sections, whereas five 4HNE− TDLUs were interrogated from three tissue sections. Discriminating wavenumbers were determined from consequent cluster vectors plots. (B) PCA-LDA scores plot of point-map IR spectra of intralobular stroma (LS; n = 90). Each spectral point is derived from the average of two IR spectra. 4HNE+ and 4HNE− TDLUs were compared as independent categories. Discriminating wavenumbers were determined from consequent cluster vectors plots. (C) PCA-LDA scores plot of point-map IR spectra of interlobular stroma (ILS; n = 180). Each spectral point is derived from the average of two IR spectra. Designated 4HNE+ and 4HNE− TDLUs were compared as independent categories. Discriminating wavenumbers were determined from consequent cluster vectors plots. Significance of category segregation was determined using an unpaired t test. Three wavenumbers contributed most to segregation of 4HNE+ vs. 4HNE− categories for a particular tissue component are listed (see each corresponding table per column).
Given the large number of IR spectra, and to facilitate subsequent visual comparison within scores plots, the average of every 10 (for Epi-derived IR spectra) and 2 (for LS- or ILS-derived IR spectra) were taken; this decision was based on the much higher number of IR spectra taken from ME cells than from the stroma, and that the minimum input value for PCA-LDA is n = 50. The averaging process was derived in chronological order from epithelia and stroma. This process generated the data set for subsequent computational analyses. PCA-LDA was used to reduce each spectrum to a single point and in order to minimize within-category heterogeneity while maximizing between-category differences. In PCA-LDA scores plots, clear segregation between categories (4HNE+ vs. 4HNE−) representing TDLU epithelial spectral points was noted (). This segregation was highly significant (P < 0.0001), and was most associated with νsPO2− (DNA/RNA) and amide I. Spectra from 4HNE− TDLUs formed a single cluster of points in the PCA scores plot. In contrast, the PCA-LDA scores plots for the stromal elements, although still significantly segregated (P < 0.0001), showed marked overlap (), and discriminating wavenumbers associated with 4HNE+ and 4HNE− categories in these two anatomical compartments were more associated with amide II, protein, and lipid alterations.
Following the observation that DNA/RNA alterations might be most responsible for distinguishing 4HNE+ vs. 4HNE− TDLUs, IR spectra (epithelial–cell spectra, n = 540 averaged every 10 spectra, from point maps only) were examined using unsupervised PCA using only this portion of the IR spectrum (1450 cm−1–900 cm−1) (). Interestingly, while spectral points from different 4HNE− TDLUs co-clustered, spectral points from 4HNE+ TDLUs were segregated into two distinct clusters in the resultant scores plot. One could surmise that this might be a consequence of inter-individual differences in factors such as exposures, damage induction or repair.
Figure 3. Unsupervised exploratory analyses by principal component analysis (PCA) of infrared (IR) spectra (1450 cm−1–900 cm−1) derived from 4-hydroxy-2-nonenal (4HNE) immunopositive (4HNE+) (n = 4) vs. 4HNE− immunonegative (4HNE−) (n = 5) terminal lobular ductal units (TDLUs). Tissue sections were categorized as 4HNE+ and 4HNE− as described in Methods and in legends to and . IR spectra were obtained from 4HNE+ and 4HNE− ME cells within the two classes of TDLUs (n = 540 total), giving n = 240 for 4HNE+ and n = 300 for 4HNE−. Spectra from 4HNE− TDLUs formed a single cluster of points in the PCA scores plot (red symbols), whereas those derived from 4HNE+ TDLUs formed two distinct clusters (blue symbols).
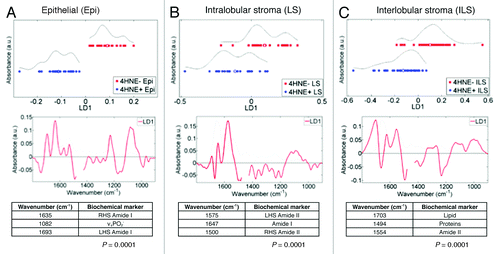
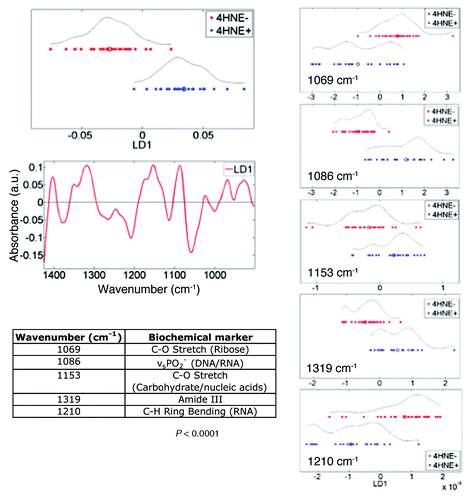
To explore further the between-category heterogeneity from 4HNE+ or 4HNE− TDLUs, IR spectra cut to the 1450 cm−1–900 cm−1 region were examined using PCA-LDA (epithelial-cell spectra, n = 540 averaged every 10 spectra, from point maps only) (). This allowed identification in consequent cluster vectors plot the most important discriminating wavenumbers and to rank them according to weight of importance. Five major discriminating wavenumbers were noted in this spectral region and ranked in order of importance; from each, a PCA-LDA scores plot was derived in order to identify which might best segregate the 4HNE+ vs. 4HNE− TDLUs (). Of the five spectral regions, νsPO2− (1086 cm−1) resulted in the least apparent overlap between the points representing the two categories. This discriminating wavenumber has been found in previous studies to be associated with stem cells in a variety of tissues.Citation59-Citation61,Citation64
Figure 4. Segregating wavenumbers in the DNA/RNA region (1450 cm−1–900 cm−1) of the infrared (IR) spectrum discriminating 4-hydroxy-2-nonenal (4HNE) immunopositive (4HNE+) (n = 4) vs. 4HNE immunonegative (4HNE−) (n = 5) mammary epithelial (ME) cells in breast terminal ductal lobular units (TDLUs). Tissue sections were categorized as 4HNE+ and 4HNE− as described in Methods and in legends to and . The PCA-LDA scores plot showed good segregation between IR spectra (n = 540) derived from epithelial cells of 4HNE+ vs. 4HNE− TDLUs. From the consequent cluster vectors plot, the five wavenumbers contributing most to segregation were prioritized. To identify the most segregating wavenumbers, these isolated spectral regions were then inputted into a PCA-LDA scores plot. Significance of category segregation was determined using an unpaired t test.
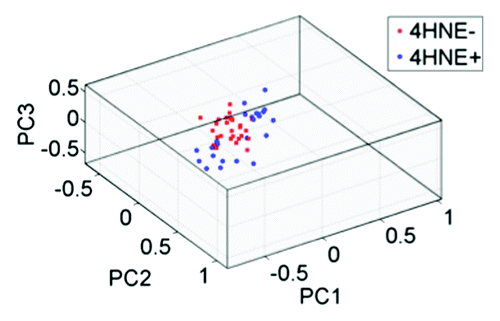
In order to compare IR spectra of the luminal and basal (myoepithelial) epithelial cells lining the ductules in TDLUs, independent point IR spectra were acquired, via a 10 μm × 10 μm beam aperture with 10 μm step-wise movement, from individual cells from each of the two cell types (n = 195 luminal and n = 90 myoepithelial spectra); these spectra had been extracted from the image maps (not averaged). Each point in a resultant PCA scores plot is a single point on a spectral image map. Unsupervised exploratory analysis demonstrated good segregation between the luminal vs. basal, myoepithelial cell clusters (). Both PC2 (P < 0.0001) and PC3 (P < 0.05) significantly segregated the epithelial-cell categories; PC2 was predominantly associated with 1402 cm−1, whereas PC3 was associated with νsPO2− wavenumbers. When luminal epithelial or basal (myoepithelial) cells from 4HNE+ vs. 4HNE− TDLUs were compared using PCA-LDA, good segregation of categories was observed in the scores plots (). Interestingly, different profiles of prioritized discriminating wavenumbers were extracted from the consequent cluster vectors plots for the two epithelial cell types; the major discriminating wavenumber for luminal epithelial cells for 4HNE+ vs. 4HNE− TDLUs was νsPO2−. As noted above, this discriminating wavenumber has in previous studies been found to be associated with stem cells in a variety of tissues.Citation59-Citation61,Citation64
Figure 5. Unsupervised exploratory analyses by principal component analysis (PCA) of infrared (IR) spectra derived from luminal cells vs. basal, myoepithelal of cells in breast terminal ductal lobular units (TDLUs; n = 3) and extracted from image maps. A PCA scores plot shows good segregation between cells in the luminal epithelium (red symbols; n = 195) and in the basal, myoepithelium (blue symbols; n = 90) clusters. Loadings plots along principal components (PCs) were then derived in order to identify discriminating wavenumbers. Significance of category segregation along individual PCs was determined using an unpaired t-test. Distinction between 4HNE+ and 4HNE− is not needed here, as this is proof that one can separate luminal and myoepithelial cell spectra with no a priori knowledge.
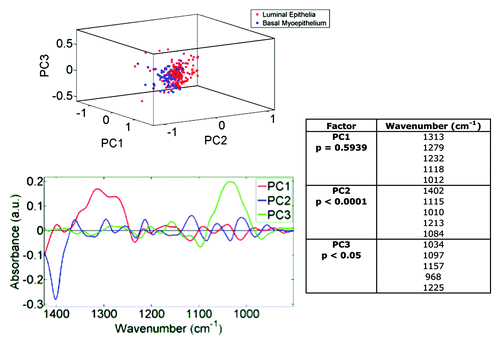
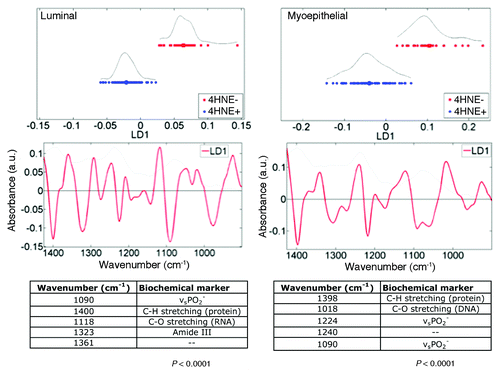
Figure 6. Principal component analysis-linear discriminant analysis (PCA-LDA) of luminal and basal, myoepithelial mammary epithelial cells in 4-hydroxy-2-nonenal (4HNE) immunopositive (4HNE+) and 4HNE immunonegative (4HNE−) breast terminal ductal lobular units (TDLUs). Tissue sections were categorized as 4HNE+ and 4HNE− as described in Methods and in legends to and . 4HNE+ and 4HNE− TDLUs (n = 3) were interrogated by synchrotron radiation-based Fourier-transform IR (SR‑FTIR) microspectroscopy via a 10 μm × 10 μm beam aperture in a 10 μm step-wise fashion to derive image maps; extracted infrared (IR) spectra of either luminal (n = 195) or basal epithelial cells (n = 90) of 4HNE+ and 4HNE− TDLUs were then obtained. Wavenumbers in the DNA/RNA region (1450 cm−1–900 cm−1) of the IR spectrum were incorporated into PCA-LDA. The PCA-LDA scores plots show good segregation between IR spectra derived from both luminal and basal epithelial cells of 4HNE+ vs. 4HNE− TDLUs. From the consequent cluster vectors plots, the five wavenumbers contributing most to segregation were prioritized. Significance of category segregation was determined using an unpaired t test.
Discussion
In this study, SR-FTIR microspectroscopy enabled comparing the spectral signatures derived from 4HNE+ and 4HNE− cells most directly relevant to breast carcinogenesis; namely, the luminal and basal ME cells lining ductules within TDLUs and the spectral signatures of intralobular and interlobular stroma. In this, as in our previous study in which we compared the relative levels of 84 OxS-relevant transcripts in 4HNE+ and 4HNE− breast parenchyma, the breast tissues interrogated were obtained from healthy young women chosen from either end of the spectrum of immunostaining of cells for 4HNE adducts.Citation43 The SR-FTIR spectra were obtained from the mid-IR frequency that is known to encompass IR wavelengths with frequencies absorbed by molecules in each of four major categories of biomolecules: proteins, lipids, carbohydrates and nucleic acids. Computational analyses of the data identified highly significant differences between the spectral signatures of 4HNE+ vs. 4HNE− ME cells in TDLUs and stromal cells and stroma within and surrounding the TDLUs. The findings supports the hypothesis that 4HNE immunostaining can identify loci in histologically normal breast parenchyma where cells in the latent promotional stage of carcinogenesis might be residing. The findings also add to the growing body of evidence attesting to IR spectroscopy’s ability, and of the related technique of Raman spectroscopy, to identify an altered biomolecular composition of tissues associated with different physiological and pathophysiological states, including neoplastic and pre-neoplastic states.Citation62,Citation63,Citation65-Citation73 By identifying disease-specific spectral signatures, these studies are laying the foundation for molecular diagnosis of diverse diseases based on vibrational spectroscopy.
In the studies cited above, the tissues analyzed were categorized based on histological or histopathological criteria. To the best of our knowledge, this is the first study in which categorization was based on the presence or absence of a biomarker and in which the technology was used to test the validity and utility of a candidate biomarker. The findings supports the hypothesis that 4HNE immunostaining can serve as a fiducial marker for identifying in breast tissues of healthy young women where cells that have entered latent stages of carcinogenesis might be localized. The findings suggest also that SR-FTIR microspectroscopy can provide a new approach to test the value of a biomarker as a guide to identify loci where pathophysiological processes are ongoing before they reveal themselves by histological changes.
Understanding the central role and alterations therein of the tissue microenvironment in the patho-physiology of cancer development is critical.Citation47-Citation53 Previous biospectroscopy studies of relatively normal prostate tissue from UK (high-risk) vs. India (low-risk) demonstrated that stromal-derived spectra from cellular as well as from collagenous matrix exhibit alterations in addition to those derived from epithelial cells.Citation74 This maps well with pioneering work by Malins and coworkers who demonstrated that oxidative DNA base lesions in breast occurred in cells in both the epithelial and stromal compartments.Citation34,Citation35 In addition, their environmental exposure studies clearly demonstrated the role of exogenous contaminants in inducing such DNA lesion.Citation37‑Citation42 The identification in the present study by high-resolution spectral imaging of SR-FTIR microspectroscopy adds to the growing recognition of the important role of the microenvironment in carcinogenesis.
The spectral signatures of the 4HNE+ and 4HNE− cell populations obtained by SR-FTIR microspectroscopy represent a superposition of the unique spectral fingerprint of the many thousands of molecular constituents of the cells interrogated. Additional studies are needed to identify the contribution made by individual biomolecules to the differences identified between spectral signatures of 4HNE+ and 4HNE− cells. Many of the known effects of OxS are likely to be reflected in the spectral signature of 4HNE+ cells: these include conformational changes caused by the interaction of biomolecules with reactive oxidizing species, post-translational modifications of proteins, quantitative changes in levels of biomolecules resulting from altered gene expression and products of oxidative damage to the different classes of biomolecules, including genomic and mitochondrial DNA.Citation75 The challenge now is how to link the differences identified by SR-FTIR spectroscopy to the plethora of molecular mechanisms associated with carcinogenesis identified in laboratory studies during the first reductionist phase of the genomic era. This will require being able to correlate the IR spectroscopic findings with the molecular phenotype of different cell populations within TDLUs and associated stroma. Microscopic technologies with multiplexing, “omics” capabilities that make this possible are becoming available.Citation76 One promising relevant imaging technology is based on mass spectrometry (IMS).Citation77-Citation80 However, its current lateral resolution would have to be increased to at least 10 μm for it to provide a molecular profile of individual cells in tissue section. The Toponome Imaging System (TISTM) is currently the only mature technology with both the requisite multiplexing capability and resolution needed to accomplish this: it has the demonstrated ability to provide a map up to 100 proteins at ~40 nm lateral resolution, the “toponome”, of cells in a single tissue section.Citation81
In summary, the “black box” state of the decades-long latent stages of breast carcinogenesis prior to histological changes is attributable to two factors; first, the lack of fiducial biomarker(s) needed to identify cell populations that are undergoing molecular changes relevant to carcinogenesis in histologically normal tissues, and second, the lack of technologies needed to obtain molecular profiles of cell populations in situ in tissue sections in their native microenvironment. The present study provides evidence that the first obstacle might be overcome by using a cytochemical marker of chronic OxS, such as 4HNE, while the second obstacle can be overcome using new and emerging biomolecular microscopic techniques, such as used in this study and referred to above. Together, they make it possible to open up for study latent stages of breast carcinogenesis in histologically normal cell populations in situ in their native microenvironment. Knowledge gained from these studies is needed to develop effective, stage specific and mechanism based strategies for preventing breast and other prevalent cancers.
Materials and Methods
Tissue source
Breast tissues were obtained from young women undergoing reduction mammoplasty for simple macromastia. None of them were known to have any specific risk factors for BC over and above that of the population residing in central Pennsylvania, a population with a high breast cancer incidence typical for the US. The tissues were processed under uniform conditions; they were freed from excess fat by blunt dissection and trimming with surgical scissors, fixed in freshly prepared 10% neutral buffered formalin for 12–24 h and embedded in paraffin. Tissues used were selected from subjects between ages 20–30 y at either end of the spectrum of 4HNE immunostaining of ME cells in breast tissue sections using criteria described previously.Citation43 Those in the 4HNE+ category were from subjects whose tissue sections showed consistently a preponderance of TDLUs occupied by many 4HNE+ ME cells, while those in the 4HNE− category were from subjects whose tissue sections showed consistently at most a few TDLUs occupied by a small number of 4HNE+ ME cells. Use of tissues for research was authorized by the Institutional Review Board of The Pennsylvania University College of Medicine.
Immunocytochemistry
FFPE tissues were sectioned at 4 μm, transferred onto Fisher Superfrost Plus slides, deparaffinized, rehydrated, and then subjected to antigen retrieval using Vector Antigen Unmasking Solution, pH 6.0 (Vector Laboratories) for 1 h at 80 °C. The sections were incubated in a humidity chamber overnight at 4 °C with a mouse monoclonal antibody developed against 4HNE-modified low-density lipoprotein (clone NA59) that binds lysine adducts of 4HNE.Citation82 Immunocytochemistry was completed using Vector Alkaline Phosphatase Universal Kit with Vector Alkaline Phosphatase Substrate Kit IV chromogen. Negative controls included adjacent sections from each sample, which were incubated with medium without the primary antibody. Sections of aortas with atherosclerotic plaques served as positive controls.
Preparations of sections for SR-FTIR micro-spectroscopy
Ten-micrometer-thick tissue sections were floated onto BaF2 slides (Photox Optical Systems), dewaxed by immersion in three sequential washes of fresh xylene (5 min) and then washed and cleared in acetone (5 min). Parallel 4 μm tissue sections were also taken and stained with hematoxylin and eosin (H&E) to confirm tissue architecture in unstained sections subjected to analysis by SR-FTIR microspectroscopy. Additional parallel 4 μm sections were immunostained for 4HNE adducts to confirm correct categorization of tissues as 4HNE+ and 4HNE−.
SR-FTIR microspectroscopy
SR-FTIR data were obtained on the IR beamline at the Swiss Light Source, Paul Scherrer Institut, Switzerland (http://www.psi.ch/sls/). Spectra were acquired using a Bruker Vertex 70 spectrometer coupled to a Bruker Hyperion 3000 microscope (Bruker Optics) containing a liquid nitrogen cooled mercury cadmium telluride detector. A 36× objective was used for spectral collection in transmission mode with a 10 μm × 10 μm beam aperture for point and image map acquisition. Spectra were collected at 4 cm−1 spectral resolution, 256 scan co-additions, measured over the region 4000–400 cm−1 and converted to absorbance using OPUS 6.5 (Bruker Optics). Image maps (from which IR spectra were extracted) were acquired using a 10 μm × 10 μm beam aperture in a 10 μm step-wise fashion. Background spectra were taken every 10 spectra to compensate for atmospheric and beam current changes. Raw spectra were processed using 13-point smoothing, cut (1800 cm−1–900 cm−1), baseline corrected and normalized to amide I (1650 cm−1) using Bruker OPUS software (Bruker Optics).
Spectra were obtained from tissue sections from three subjects with at most few minimally 4HNE+ ME cells in TDLUs (ages 20, 21, and 24 y) and from two subjects with many strongly 4HNE+ ME cells in TDLUs (ages 23 and 25 y). From each of nine TDLUs in sections from each subject, 60 IR spectra were acquired per luminal and basal epithelial cells (n = 540 total), 10 spectra per intra-lobular stroma (n = 90 spectra total) and 20 spectra per inter-lobular stroma (n = 180 total). Point map spectra of epithelia were acquired in a staggered fashion across the epithelium to account for both luminal epithelial and basal myoepithelial cells in a location-by-location basis; these data were used for –. In order to segregate between different cell types within TDLUs, three additional image maps were acquired from HNE+ (n = 2) and HNE− (n = 1) tissue sections; this allowed us to extract additional spectra representing luminal (n = 195) and basal (n = 90) epithelial cells, which were used for and .
Computational analysis
Exploratory PCA was first performed as an unsupervised technique and used to generate the scores and loadings plots from derived principal components (PCs) of the mean-centered, pre-processed spectra (Matlab, The MathWorks Inc.). Each PC was examined individually to determine which represented the best segregation of classes for 4HNE adduct status and/or cell type. Derived PCA loadings plots determined the wavenumbers (vibrational modes) responsible for segregation of classes. Further application of the supervised technique of LDA to the output process of PCA up to the first 10 PCs (~99% variance) was applied (i.e., PCA-LDA); LDA maximizes the inter-category variance in relation to the intra-category variance based on pre-assigned class labels, giving optimal class segregation. A scores plot was produced to visualize segregation of the classes, while derived cluster vectors plots were used to determine the wavenumbers responsible for segregation. Feature selection allowed visualizing single wavenumbers extracted as significant by PCA-LDA cluster vectors plots as scores plots. Statistical significance of each PC and LDA analysis contributing to inter-category segregation were determined by the unpaired t test (Graphpad Prism 5). Any residual paraffin peaks were excluded from computational analyses (1425–1485 cm−1). IRootLab, a free and open-source MATLAB toolbox for vibrational biospectroscopy data analysis has recently become available.Citation83
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Acknowledgments
Funding for these studies in Prof FL Martin’s laboratory was provided by Rosemere Cancer Foundation (II Patel) and BBSRC (SW Fogarty) and in part under a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds (J Weisz). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. We thank Dr Linda Curtiss, Scripps Research Institute, La Jolla, CA, for the antibody against 4-hydroxy-2-nonenal lysine adducts used in this study. We wish to acknowledge the support of the Department of Obstetrics and Gynecology of the Penn State University College of Medicine that was instrumental in enabling the completion of this study.
Note Added in Proof
This year the Shimadzu Corporation of Japan introduced the first matrix-assisted laser desorption/ionization imaging system with a high enough spatial resolution (5 μm) to enable identifying biomolecules in situ in individual cells or tissue section (http://www.shimadzu.com/about/pressrelease/5iqj1d000001u3r0.html). The system has been tested in the course of its development by researchers at Hamamatsu University School of Medicine, Japan. See references Citation79 and Citation84.
References
- Muir CS. Epidemiology of cancer in ethnic groups. Br J Cancer Suppl 1996; 29:S12 - 6; PMID: 8782793
- Park SK, Kim Y, Kang D, Jung EJ, Yoo KY. Risk factors and control strategies for the rapidly rising rate of breast cancer in Korea. J Breast Cancer 2011; 14:79 - 87; http://dx.doi.org/10.4048/jbc.2011.14.2.79; PMID: 21847401
- Pott P. Chirurgical observations. London: L Hawes, W Clark, and R Collins., 1775.
- Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980; 46:2736 - 40; http://dx.doi.org/10.1002/1097-0142(19801215)46:12<2736::AID-CNCR2820461233>3.0.CO;2-L; PMID: 7448712
- Selikoff IJ. Lessons for living in a chemical world. Bull Environ Contam Toxicol 1984; 33:682 - 95; http://dx.doi.org/10.1007/BF01625600; PMID: 6394072
- Weiss W. Cigarette smoking and lung cancer trends. A light at the end of the tunnel?. Chest 1997; 111:1414 - 6; http://dx.doi.org/10.1378/chest.111.5.1414; PMID: 9149602
- Miyakawa M, Tachibana M, Miyakawa A, Yoshida K, Shimada N, Murai M, Kondo T. Re-evaluation of the latent period of bladder cancer in dyestuff-plant workers in Japan. Int J Urol 2001; 8:423 - 30; http://dx.doi.org/10.1046/j.1442-2042.2001.00342.x; PMID: 11555006
- Archer VE, Coons T, Saccomanno G, Hong DY. Latency and the lung cancer epidemic among United States uranium miners. Health Phys 2004; 87:480 - 9; http://dx.doi.org/10.1097/01.HP.0000133216.72557.ab; PMID: 15551786
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res 2007; 168:1 - 64; http://dx.doi.org/10.1667/RR0763.1; PMID: 17722996
- Finkelstein MM. Absence of radiographic asbestosis and the risk of lung cancer among asbestos-cement workers: Extended follow-up of a cohort. Am J Ind Med 2010; 53:1065 - 9; http://dx.doi.org/10.1002/ajim.20881; PMID: 20672325
- Karabela-Bouropoulou V, Liapi-Avgeri G, Iliopoulou E, Agnantis NJ. Histological findings in breast tissue specimens from reduction mammoplasties. Pathol Res Pract 1994; 190:792 - 8; http://dx.doi.org/10.1016/S0344-0338(11)80427-9; PMID: 7831156
- Ishag MT, Bashinsky DY, Beliaeva IV, Niemann TH, Marsh WL Jr.. Pathologic findings in reduction mammaplasty specimens. Am J Clin Pathol 2003; 120:377 - 80; http://dx.doi.org/10.1309/4KD652HN739XTLM3; PMID: 14502800
- Colwell AS, Kukreja J, Breuing KH, Lester S, Orgill DP. Occult breast carcinoma in reduction mammaplasty specimens: 14-year experience. Plast Reconstr Surg 2004; 113:1984 - 8; http://dx.doi.org/10.1097/01.PRS.0000122212.37703.6E; PMID: 15253187
- Dotto J, Kluk M, Geramizadeh B, Tavassoli FA. Frequency of clinically occult intraepithelial and invasive neoplasia in reduction mammoplasty specimens: a study of 516 cases. Int J Surg Pathol 2008; 16:25 - 30; http://dx.doi.org/10.1177/1066896907307176; PMID: 18203780
- Hassan FE, Pacifico MD. Should we be analysing breast reduction specimens? A systematic analysis of over 1,000 consecutive cases. Aesthetic Plast Surg 2012; 36:1105 - 13; http://dx.doi.org/10.1007/s00266-012-9919-9; PMID: 22678135
- Brown MH, Weinberg M, Chong N, Levine R, Holowaty E. A cohort study of breast cancer risk in breast reduction patients. Plast Reconstr Surg 1999; 103:1674 - 81; PMID: 10323701
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 2003; 42:318 - 43; http://dx.doi.org/10.1016/S0163-7827(03)00014-6; PMID: 12689622
- Jürgens G, Chen Q, Esterbauer H, Mair S, Ledinski G, Dinges HP. Immunostaining of human autopsy aortas with antibodies to modified apolipoprotein B and apoprotein(a). Arterioscler Thromb 1993; 13:1689 - 99; http://dx.doi.org/10.1161/01.ATV.13.11.1689; PMID: 7692957
- Waeg G, Dimsity G, Esterbauer H. Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic Res 1996; 25:149 - 59; http://dx.doi.org/10.3109/10715769609149920; PMID: 8885333
- Rosenfeld ME, Palinski W, Ylä-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis 1990; 10:336 - 49; http://dx.doi.org/10.1161/01.ATV.10.3.336; PMID: 1693069
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A 1996; 93:2696 - 701; http://dx.doi.org/10.1073/pnas.93.7.2696; PMID: 8610103
- Clawson GA, Benedict CM, Kelley MR, Weisz J. Focal nuclear hepatocyte response to oxidative damage following low dose thioacetamide intoxication. Carcinogenesis 1997; 18:1663 - 8; http://dx.doi.org/10.1093/carcin/18.8.1663; PMID: 9276646
- Ando Y, Brännström T, Uchida K, Nyhlin N, Näsman B, Suhr O, Yamashita T, Olsson T, El Salhy M, Uchino M, et al. Histochemical detection of 4-hydroxynonenal protein in Alzheimer amyloid. J Neurol Sci 1998; 156:172 - 6; http://dx.doi.org/10.1016/S0022-510X(98)00042-2; PMID: 9588853
- Meng J, Sakata N, Takebayashi S, Asano T, Futata T, Nagai R, Ikeda K, Horiuchi S, Myint T, Taniguchi N. Glycoxidation in aortic collagen from STZ-induced diabetic rats and its relevance to vascular damage. Atherosclerosis 1998; 136:355 - 65; http://dx.doi.org/10.1016/S0021-9150(97)00238-4; PMID: 9543107
- Khan MF, Wu X, Tipnis UR, Ansari GA, Boor PJ. Protein adducts of malondialdehyde and 4-hydroxynonenal in livers of iron loaded rats: quantitation and localization. Toxicology 2002; 173:193 - 201; PMID: 11960672
- Temma K, Shimoya K, Zhang Q, Kimura T, Wasada K, Kanzaki T, Azuma C, Koyama M, Murata Y. Effects of 4-hydroxy-2-nonenal, a marker of oxidative stress, on the cyclooxygenase-2 of human placenta in chorioamnionitis. Mol Hum Reprod 2004; 10:167 - 71; http://dx.doi.org/10.1093/molehr/gah030; PMID: 14981143
- Dalfó E, Portero-Otín M, Ayala V, Martínez A, Pamplona R, Ferrer I. Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J Neuropathol Exp Neurol 2005; 64:816 - 30; http://dx.doi.org/10.1097/01.jnen.0000179050.54522.5a; PMID: 16141792
- Rytilä P, Rehn T, Ilumets H, Rouhos A, Sovijärvi A, Myllärniemi M, Kinnula VL. Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPD. Respir Res 2006; 7:69; http://dx.doi.org/10.1186/1465-9921-7-69; PMID: 16646959
- Chu J, Tong M, de la Monte SM. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol 2007; 113:659 - 73; http://dx.doi.org/10.1007/s00401-007-0199-4; PMID: 17431646
- Hattoria N, Wanga M, Taka H, Fujimura T, Yoritaka A, Kubo S, Mochizuki H. Toxic effects of dopamine metabolism in Parkinson’s disease. Parkinsonism Relat Disord 2009; 15:Suppl 1 S35 - 8; http://dx.doi.org/10.1016/S1353-8020(09)70010-0; PMID: 19131041
- Malins DC, Holmes EH, Polissar NL, Gunselman SJ. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer 1993; 71:3036 - 43; http://dx.doi.org/10.1002/1097-0142(19930515)71:10<3036::AID-CNCR2820711025>3.0.CO;2-P; PMID: 8387875
- Malins DC, Polissar NL, Nishikida K, Holmes EH, Gardner HS, Gunselman SJ. The etiology and prediction of breast cancer. Fourier transform-infrared spectroscopy reveals progressive alterations in breast DNA leading to a cancer-like phenotype in a high proportion of normal women. Cancer 1995; 75:503 - 17; http://dx.doi.org/10.1002/1097-0142(19950115)75:2<503::AID-CNCR2820750213>3.0.CO;2-0; PMID: 7812921
- Malins DC, Polissar NL, Schaefer S, Su Y, Vinson M. A unified theory of carcinogenesis based on order-disorder transitions in DNA structure as studied in the human ovary and breast. Proc Natl Acad Sci U S A 1998; 95:7637 - 42; http://dx.doi.org/10.1073/pnas.95.13.7637; PMID: 9636202
- Malins DC, Anderson KM, Jaruga P, Ramsey CR, Gilman NK, Green VM, Rostad SW, Emerman JT, Dizdaroglu M. Oxidative changes in the DNA of stroma and epithelium from the female breast: potential implications for breast cancer. Cell Cycle 2006; 5:1629 - 32; http://dx.doi.org/10.4161/cc.5.15.3098; PMID: 16880742
- Anderson KM, Jaruga P, Ramsey CR, Gilman NK, Green VM, Rostad SW, Emerman JT, Dizdaroglu M, Malins DC. Structural alterations in breast stromal and epithelial DNA: the influence of 8,5′-cyclo-2′-deoxyadenosine. Cell Cycle 2006; 5:1240 - 4; http://dx.doi.org/10.4161/cc.5.11.2816; PMID: 16760644
- Malins DC, Anderson KM, Gilman NK, Green VM, Barker EA, Hellström KE. Development of a cancer DNA phenotype prior to tumor formation. Proc Natl Acad Sci U S A 2004; 101:10721 - 5; http://dx.doi.org/10.1073/pnas.0403888101; PMID: 15249662
- Malins DC, Haimanot R. 4,6-Diamino-5-formamidopyrimidine, 8-hydroxyguanine and 8-hydroxyadenine in DNA from neoplastic liver of English sole exposed to carcinogens. Biochem Biophys Res Commun 1990; 173:614 - 9; http://dx.doi.org/10.1016/S0006-291X(05)80079-8; PMID: 2175601
- Malins DC, Gunselman SJ. Fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry reveal a remarkable degree of structural damage in the DNA of wild fish exposed to toxic chemicals. Proc Natl Acad Sci U S A 1994; 91:13038 - 41; http://dx.doi.org/10.1073/pnas.91.26.13038; PMID: 7809168
- Malins DC, Polissar NL, Garner MM, Gunselman SJ. Mutagenic DNA base modifications are correlated with lesions in nonneoplastic hepatic tissue of the English sole carcinogenesis model. Cancer Res 1996; 56:5563 - 5; PMID: 8971153
- Malins DC, Polissar NL, Gunselman SJ. Infrared spectral models demonstrate that exposure to environmental chemicals leads to new forms of DNA. Proc Natl Acad Sci U S A 1997; 94:3611 - 5; http://dx.doi.org/10.1073/pnas.94.8.3611; PMID: 9108025
- Malins DC, Stegeman JJ, Anderson JW, Johnson PM, Gold J, Anderson KM. Structural changes in gill DNA reveal the effects of contaminants on Puget Sound fish. Environ Health Perspect 2004; 112:511 - 5; http://dx.doi.org/10.1289/ehp.6719; PMID: 15064153
- Malins DC, Anderson KM, Stegeman JJ, Jaruga P, Green VM, Gilman NK, Dizdaroglu M. Biomarkers signal contaminant effects on the organs of English sole (Parophrys vetulus) from Puget Sound. Environ Health Perspect 2006; 114:823 - 9; http://dx.doi.org/10.1289/ehp.8544; PMID: 16759979
- Weisz J, Shearer DA, Murata E, Patrick SD, Han B, Berg A, Clawson GA. Identification of mammary epithelial cells subject to chronic oxidative stress in mammary epithelium of young women and teenagers living in USA: implication for breast carcinogenesis. Cancer Biol Ther 2012; 13:101 - 13; http://dx.doi.org/10.4161/cbt.13.2.18873; PMID: 22231390
- Martyniuk CJ, Feswick A, Spade DJ, Kroll KJ, Barber DS, Denslow ND. Effects of acute dieldrin exposure on neurotransmitters and global gene transcription in largemouth bass (Micropterus salmoides) hypothalamus. Neurotoxicology 2010; 31:356 - 66; http://dx.doi.org/10.1016/j.neuro.2010.04.008; PMID: 20438755
- Martyniuk CJ, Kroll KJ, Doperalski NJ, Barber DS, Denslow ND. Genomic and proteomic responses to environmentally relevant exposures to dieldrin: indicators of neurodegeneration?. Toxicol Sci 2010; 117:190 - 9; http://dx.doi.org/10.1093/toxsci/kfq192; PMID: 20584760
- Freeman TA, Parvizi J, Della Valle CJ, Steinbeck MJ. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis Tissue Repair 2009; 2:5; http://dx.doi.org/10.1186/1755-1536-2-5; PMID: 19912645
- Cunha GR, Ricke WA. A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differentiation 2011; 82:168 - 72; http://dx.doi.org/10.1016/j.diff.2011.04.002; PMID: 21723032
- Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol 1993; 2:79 - 89; PMID: 8353596
- Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 1996; 76:69 - 125; PMID: 8592733
- Rønnov-Jessen L, Bissell MJ. Breast cancer by proxy: can the microenvironment be both the cause and consequence?. Trends Mol Med 2009; 15:5 - 13; http://dx.doi.org/10.1016/j.molmed.2008.11.001; PMID: 19091631
- Haviv I, Polyak K, Qiu W, Hu M, Campbell I. Origin of carcinoma associated fibroblasts. Cell Cycle 2009; 8:589 - 95; http://dx.doi.org/10.4161/cc.8.4.7669; PMID: 19182519
- Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev 2008; 18:27 - 34; http://dx.doi.org/10.1016/j.gde.2007.12.006; PMID: 18282701
- Ricke EA, Williams K, Lee YF, Couto S, Wang Y, Hayward SW, Cunha GR, Ricke WA. Androgen hormone action in prostatic carcinogenesis: stromal androgen receptors mediate prostate cancer progression, malignant transformation and metastasis. Carcinogenesis 2012; 33:1391 - 8; http://dx.doi.org/10.1093/carcin/bgs153; PMID: 22535887
- Kelly JG, Trevisan J, Scott AD, Carmichael PL, Pollock HM, Martin-Hirsch PL, Martin FL. Biospectroscopy to metabolically profile biomolecular structure: a multistage approach linking computational analysis with biomarkers. J Proteome Res 2011; 10:1437 - 48; http://dx.doi.org/10.1021/pr101067u; PMID: 21210632
- Patel II, Martin FL. Discrimination of zone-specific spectral signatures in normal human prostate using Raman spectroscopy. Analyst 2010; 135:3060 - 9; http://dx.doi.org/10.1039/c0an00518e; PMID: 20949203
- Pang W, Li J, Ahmadzai AA, Heppenstall LD, Llabjani V, Trevisan J, Qiu X, Martin FL. Identification of benzo[a]pyrene-induced cell cycle-associated alterations in MCF-7 cells using infrared spectroscopy with computational analysis. Toxicology 2012; 298:24 - 9; http://dx.doi.org/10.1016/j.tox.2012.04.009; PMID: 22561278
- Miller LM, Dumas P. From structure to cellular mechanism with infrared microspectroscopy. Curr Opin Struct Biol 2010; 20:649 - 56; http://dx.doi.org/10.1016/j.sbi.2010.07.007; PMID: 20739176
- Miller LM, Dumas P. Chemical imaging of biological tissue with synchrotron infrared light. Biochim Biophys Acta 2006; 1758:846 - 57
- German MJ, Pollock HM, Zhao B, Tobin MJ, Hammiche A, Bentley A, Cooper LJ, Martin FL, Fullwood NJ. Characterization of putative stem cell populations in the cornea using synchrotron infrared microspectroscopy. Invest Ophthalmol Vis Sci 2006; 47:2417 - 21; http://dx.doi.org/10.1167/iovs.05-1254; PMID: 16723451
- Patel II, Harrison WJ, Kerns JG, Filik J, Wehbe K, Carmichael PL, Scott AD, Philpott MP, Frogley MD, Cinque G, et al. Isolating stem cells in the inter-follicular epidermis employing synchrotron radiation-based Fourier-transform infrared microspectroscopy and focal plane array imaging. Anal Bioanal Chem 2012; 404:1745 - 58; http://dx.doi.org/10.1007/s00216-012-6314-y; PMID: 22945554
- Walsh MJ, Hammiche A, Fellous TG, Nicholson JM, Cotte M, Susini J, Fullwood NJ, Martin-Hirsch PL, Alison MR, Martin FL. Tracking the cell hierarchy in the human intestine using biochemical signatures derived by mid-infrared microspectroscopy. Stem Cell Res 2009; 3:15 - 27; http://dx.doi.org/10.1016/j.scr.2009.02.003; PMID: 19393589
- Walsh MJ, Singh MN, Pollock HM, Cooper LJ, German MJ, Stringfellow HF, Fullwood NJ, Paraskevaidis E, Martin-Hirsch PL, Martin FL. ATR microspectroscopy with multivariate analysis segregates grades of exfoliative cervical cytology. Biochem Biophys Res Commun 2007; 352:213 - 9; http://dx.doi.org/10.1016/j.bbrc.2006.11.005; PMID: 17141660
- Fogarty SW, Patel II, Trevisan J, Nakamura T, Hirschmugl CJ, Fullwood NJ, Martin FL. Sub-cellular spectrochemical imaging of isolated human corneal cells employing synchrotron radiation-based Fourier-transform infrared microspectroscopy. Analyst 2013; 138:240 - 8; http://dx.doi.org/10.1039/c2an36197c; PMID: 23152953
- Walsh MJ, Fellous TG, Hammiche A, Lin WR, Fullwood NJ, Grude O, Bahrami F, Nicholson JM, Cotte M, Susini J, et al. Fourier transform infrared microspectroscopy identifies symmetric PO(2)(-) modifications as a marker of the putative stem cell region of human intestinal crypts. Stem Cells 2008; 26:108 - 18; http://dx.doi.org/10.1634/stemcells.2007-0196; PMID: 17901405
- Lee LS, Lin SY, Chi CW, Liu HC, Cheng CL. Non-destructive analysis of the protein conformational structure of human pituitary adenomas using reflectance FT-IR microspectroscopy. Cancer Lett 1995; 94:65 - 9; http://dx.doi.org/10.1016/0304-3835(95)03825-H; PMID: 7621446
- Dukor RK, Liebman MN, Johnson BL. A new, non-destructive method for analysis of clinical samples with FT-IR microspectroscopy. Breast cancer tissue as an example. Cell Mol Biol (Noisy-le-grand) 1998; 44:211 - 7; PMID: 9551652
- Lasch P, Haensch W, Naumann D, Diem M. Imaging of colorectal adenocarcinoma using FT-IR microspectroscopy and cluster analysis. Biochim Biophys Acta 2004; 1688:176 - 86; http://dx.doi.org/10.1016/j.bbadis.2003.12.006; PMID: 14990348
- Krafft C, Steiner G, Beleites C, Salzer R. Disease recognition by infrared and Raman spectroscopy. J Biophotonics 2009; 2:13 - 28; http://dx.doi.org/10.1002/jbio.200810024; PMID: 19343682
- Diem M, Papamarkakis K, Schubert J, Bird B, Romeo MJ, Miljković M. The infrared spectral signatures of disease: extracting the distinguishing spectral features between normal and diseased states. Appl Spectrosc 2009; 63:307A - 18A; http://dx.doi.org/10.1366/000370209789806894; PMID: 19891826
- Heraud P, Caine S, Campanale N, Karnezis T, McNaughton D, Wood BR, Tobin MJ, Bernard CC. Early detection of the chemical changes occurring during the induction and prevention of autoimmune-mediated demyelination detected by FT-IR imaging. Neuroimage 2010; 49:1180 - 9; http://dx.doi.org/10.1016/j.neuroimage.2009.09.053; PMID: 19796690
- Kelly JG, Nakamura T, Kinoshita S, Fullwood NJ, Martin FL. Evidence for a stem-cell lineage in corneal squamous cell carcinoma using synchrotron-based Fourier-transform infrared microspectroscopy and multivariate analysis. Analyst 2010; 135:3120 - 5; http://dx.doi.org/10.1039/c0an00507j; PMID: 20886154
- Caine S, Heraud P, Tobin MJ, McNaughton D, Bernard CC. The application of Fourier transform infrared microspectroscopy for the study of diseased central nervous system tissue. Neuroimage 2012; 59:3624 - 40; http://dx.doi.org/10.1016/j.neuroimage.2011.11.033; PMID: 22119649
- Pijanka J, Sockalingum GD, Kohler A, Yang Y, Draux F, Parkes G, Lam KP, Collins D, Dumas P, Sandt C, et al. Synchrotron-based FTIR spectra of stained single cells. Towards a clinical application in pathology. Lab Invest 2010; 90:797 - 807; http://dx.doi.org/10.1038/labinvest.2010.8; PMID: 20125083
- Patel II, Trevisan J, Singh PB, Nicholson CM, Krishnan RK, Matanhelia SS, Martin FL. Segregation of human prostate tissues classified high-risk (UK) versus low-risk (India) for adenocarcinoma using Fourier-transform infrared or Raman microspectroscopy coupled with discriminant analysis. Anal Bioanal Chem 2011; 401:969 - 82; http://dx.doi.org/10.1007/s00216-011-5123-z; PMID: 21643857
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 2007; 121:2381 - 6; http://dx.doi.org/10.1002/ijc.23192; PMID: 17893868
- Evans RG, Naidu B, Rajpoot NM, Epstein D, Khan M. Toponome imaging system: multiplex biomarkers in oncology. Trends Mol Med 2012; 18:723 - 31; http://dx.doi.org/10.1016/j.molmed.2012.10.003; PMID: 23122853
- Rohner TC, Staab D, Stoeckli M. MALDI mass spectrometric imaging of biological tissue sections. Mech Ageing Dev 2005; 126:177 - 85; http://dx.doi.org/10.1016/j.mad.2004.09.032; PMID: 15610777
- Balluff B, Schöne C, Höfler H, Walch A. MALDI imaging mass spectrometry for direct tissue analysis: technological advancements and recent applications. Histochem Cell Biol 2011; 136:227 - 44; http://dx.doi.org/10.1007/s00418-011-0843-x; PMID: 21805154
- Setou M, Kurabe N. Mass microscopy: high-resolution imaging mass spectrometry. J Electron Microsc (Tokyo) 2011; 60:47 - 56; http://dx.doi.org/10.1093/jmicro/dfq079; PMID: 21109523
- Lagarrigue M, Lavigne R, Guével B, Com E, Chaurand P, Pineau C. Matrix-assisted laser desorption/ionization imaging mass spectrometry: a promising technique for reproductive research. Biol Reprod 2012; 86:74; http://dx.doi.org/10.1095/biolreprod.111.094896; PMID: 22156474
- Schubert W, Gieseler A, Krusche A, Serocka P, Hillert R. Next-generation biomarkers based on 100-parameter functional super-resolution microscopy TIS. N Biotechnol 2012; 29:599 - 610; http://dx.doi.org/10.1016/j.nbt.2011.12.004; PMID: 22209707
- Palinski W, Koschinsky T, Butler SW, Miller E, Vlassara H, Cerami A, Witztum JL. Immunological evidence for the presence of advanced glycosylation end products in atherosclerotic lesions of euglycemic rabbits. Arterioscler Thromb Vasc Biol 1995; 15:571 - 82; http://dx.doi.org/10.1161/01.ATV.15.5.571; PMID: 7749871
- Trevisan J, Angelov PP, Scott AD, Carmichael PL, Martin FL. IRootLab: a free and open-source MATLAB toolbox for vibrational biospectroscopy data analysis. Bioinformatics 2013; 29:1095 - 7; http://dx.doi.org/10.1093/bioinformatics/btt084; PMID: 23422340
- Setou M, Shrivas K, Sroyraya M, Yang H, Sugiura Y, Moribe J, Kondo A, Tsutsumi K, Kimura Y, Kurabe N. Developments and applications of mass microscopy. Med Mol Morphol 2010; 43:1 - 5; http://dx.doi.org/10.10007/s00795-009-0489-0; PMID: 20339999
