Abstract
A major problem in cancer treatment is the development of resistance to chemotherapeutic agents, multidrug resistance (MDR), associated with increased activity of transmembrane drug transporter proteins which impair cytotoxic treatment by rapidly removing the drugs from the targeted cells. Previously, it has been shown that heparin treatment of cancer patients undergoing chemotherapy increases survival. In order to determine whether heparin is capable reducing MDR and increasing the potency of chemotherapeutic drugs, the cytoxicity of a number of agents toward four cancer cell lines (a human enriched breast cancer stem cell line, two human breast cancer cell lines, MCF-7 and MDA-MB-231, and a human lung cancer cell line A549) was tested in the presence or absence of heparin. Results demonstrated that heparin increased the cytotoxicity of a range of chemotherapeutic agents. This effect was associated with the ability of heparin to bind to several of the drug transport proteins of the ABC and non ABC transporter systems. Among the ABC system, heparin treatment caused significant inhibition of the ATPase activity of ABCG2 and ABCC1, and of the efflux function observed as enhanced intracellular accumulation of specific substrates. Doxorubicin cytoxicity, which was enhanced by heparin treatment of MCF-7 cells, was found to be under the control of one of the major non-ABC transporter proteins, lung resistance protein (LRP). LRP was also shown to be a heparin-binding protein. These findings indicate that heparin has a potential role in the clinic as a drug transporter modulator to reduce multidrug resistance in cancer patients.
Introduction
A major problem associated with breast cancer chemotherapy is the subsequent development of resistance to chemotherapeutic agents, known as multidrug resistance (MDR), which often leads to patient relapse. There are several ways by which tumor cells can become resistant to anti-cancer agents including alteration in drug metabolism (uptake, efflux, and detoxification), modification of drug targets, enhanced DNA repair, and dysregulation of apoptosis.Citation1,Citation2 Of these, the commonest and most widely studied form of resistance is alteration of drug uptake and efflux by the tumor due to variations in the cellular transport of drugs. The importance of transport of the drug arises because most chemotherapeutic agents exert their effects after cellular entry, a function which is controlled by the transporter proteins within the cell membrane. The activity of the cell transporter system has often been shown to be enhanced in cancer cells, which leads to the relatively rapid removal of the drug from the cell, thereby hindering the accumulation of the drug at an intracellular level which is cytotoxic.Citation3,Citation4 Since it was recognized that the drug transporter system plays an important role in modulating drug potency, one strategy that has been proposed to overcome the MDR associated with high levels of transporter activity is to co-administer drugs that can act as MDR modulators or MDR chemosensitizers.Citation4 In principle, such transporter modulators could be used in combination with chemotherapeutics to increase the effective intracellular concentration of anticancer drugs. However, of the compounds which have been investigated as MDR modulators, none has, as yet, been recommended for clinical use in cancer patients because of severe side effects.Citation3,Citation5
Venous thromboembolism (VTE) in cancer patients can occur as the first indication of malignant disease or arise during chemo- or radiotherapy.Citation6,Citation7 The efficacy of heparin in treating VTE in cancer patients has been investigated in numerous clinical trials from which it has also been concluded that heparin treatment that extends for over a month or more, prolongs the survival of cancer patients, even in the absence of overt thrombosis.Citation8-Citation11 Heparin is a negatively charged polysaccharide, a highly sulphated form of heparan sulfate (HS), which is able to bind, by virtue of its charge, to a large number of extracellular proteins including growth factors and extracellular matrix components and thereby modulate their activity. In investigating a possible mechanism for the apparent anti-tumorigenic action of heparin, we have recently shown that heparin treatment reduced the pro-tumorigenic properties of breast cancer cells, genotypically and phenotypically.Citation12 With these observations in mind, we hypothesized that heparin treatment may also reduce the inherent level of drug resistance of the cells and enhance the efficacy of chemotherapy. According to this hypothesis, the degree of MDR in cancer patients would be consequently reduced when chemotherapy occurred in combination with heparin treatment.
There are two principal families of transporter proteins—the ATP-binding cassette transporters (ABC-transporter proteins) and the non-ABC-transporter proteins.Citation5,Citation13 In normal physiology, ABC transporter proteins, which require energy provided by ATP hydrolysis to carry out drug transport, play an important protective function preventing the accumulation of toxic compounds in a variety of cells and tissues. Their expression is often observed to be enhanced in malignant cells.Citation4 Three of the ABC transporter group of proteins account for the majority of the cases of MDR in cancer patients, namely P-glycoprotein (ABCB1/P-gp/MDR1), multidrug resistance associated protein 1 (ABCC1/MRP1), and breast cancer resistance protein (ABCG2/BRCP).Citation4,Citation5 Relatively high levels of expression of ABCG2 have been demonstrated in many types of cancer in samples taken from patients, and have been shown to correlate with decrease in the response to a variety of chemotherapeutic drugs as well as a reduction in survival rate.Citation1,Citation3 It has been proposed that the level of ABCG2 expression may be used as a reliable indicator of prognosis in cancer patients.Citation14,Citation15
Apart from ABC-transporter superfamily there are also a small number of non-ABC-transporter proteins which has been shown to be implicated in MDR, namely: lung resistance related protein (LRP—also called major vault protein, MVP), Topoisomerase IIα (TopoIIα), Annexin A1, Glutathione S-transferase (GST-π), and Ral binding protein (RalBP-1or RLIP76).Citation16-Citation18 The mechanism of action and specificity as well as and the mechanisms involved in the regulation/inhibition of the non-ABC-transporter proteins are still largely unknown. The expression of LRP has been found to correlate with the degree of malignancy in certain cancer types, suggesting a direct involvement in tumor development and/or progression. LRP is also found to be overexpressed in some of chemotherapy resistant cell lines, including ovarian, lung, breast cancer cell carcinoma, and acute myeloid leukemia, most of them are ABC transporter (ABCG1/Pgp)-negative MDR cancer cell.Citation19-Citation21
In the first part of the study we established that the addition of heparin enhanced the toxicity of a number of chemotherapeutic drugs toward breast cancer cells and that this was due, at least in part, to the ability of heparin to block the efflux of the drug from the cell. In the second part of the study we examined the mechanism by which heparin was able to achieve this by investigating possible interactions with the drug transporter system looking separately at the ABC and non-ABC-transport systems. The underlying mechanism was then investigated by determining which proteins of the drug transporter system were able to interact with heparin. These results indicate heparin is able to bind and inhibit several of drug transport proteins that influence the distribution and toxicity of chemotherapeutic agents and thereby possesses the potential to reduce the effect of MDR in breast cancer cells.
Results
The cytotoxicity of chemotherapeutic agents toward breast cancer stem cells is enhanced when used in combination with heparin
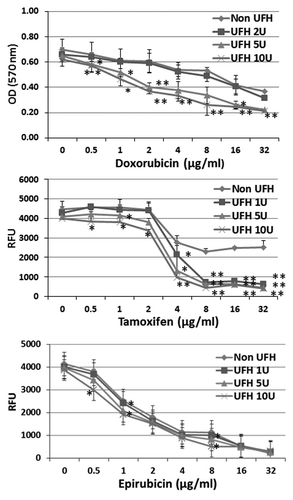
Overall, in this study, we have used two human breast cancer cell lines (MCF-7 and MDA-MB-231), and a human lung cancer cell line (A549) as well as a human breast adenocarcinoma enriched cancer stem cell line (BioMedicure). Cancer stem cells (CSCs) are of particular interest since they tend to be relatively resistant to drug treatment and the ABCG2+ subset of tumor cells is often enriched in cells with a cancer stem cell-like phenotype. For the first part of the study, therefore, in which we investigated whether the cytoxicity of a range of chemotherapeutic agents was enhanced by the addition of heparin, we used the stem cell line. CSCs were treated with each of three chemotherapeutic agents that are known substrates of the ABC transporter system, namely, epirubicin, tamoxifen, and doxorubicin, respectively. The drugs were added over a range of concentrations (as indicated) in the presence or absence of heparin at 1, 5, or 10 IU/ml. After incubation for 24 h, the degree of cytotoxicity was determined using the CyQUANT NF cell proliferation assay kit (Invitrogen), which is based upon measurement of cellular DNA content via fluorescent dye binding by viable cells. In the case of doxorubicin, the MTT assay was used, as the fluorescent dye of the proliferation assay kit and doxorubicin share the same excitation and emission wavelengths. The results demonstrated that the percentage of viable cells was slightly reduced upon heparin treatment alone, but the cytotoxicity of tamoxifen and doxorubicin were each enhanced significantly when used in combination with heparin (). Taking into account the effect of treatment with heparin alone, the potency of doxorubicin was increased around 4-fold at 10 IU heparin/ml. In the case of tamoxifen, the percentage of viable cells was reduced to a minimum of around 50% at concentrations above 4 μg/ml of the drug in the absence of heparin but around 90% in the presence of heparin at 1, 5, and 10 IU/ml. The cytotoxicity of epirubicin was also observed to be slightly increased when used in combination with heparin (). The results indicate that the acute toxicity of three of the chemotherapeutic agents tested can be enhanced when used in combination with heparin in a manner which is possibly synergistic in nature in the case of CSCs.
Figure 1. The influence of heparin upon the cytotoxicity of chemotherapeutic drugs tested on breast cancer CSCs. CSCs were treated with doxorubicin or tamoxifen or epirubicin respectively at a series of concentrations (as indicated) in the presence or absence of heparin at 1, 5, or 10 U/ml and incubated for 24 h. The cytoxicity was determined using the CyQUANT NF cell proliferation assay kit, based upon the identification of viable cells using a fluorescent dye. In the case of doxorubicin, the MTT assay was used. Asterisks indicate the degree of statistical significance (*P < 0.05; **P < 0.01) as determined in comparing the cytotoxic index of cultures treated with drug alone and to drug in combination with heparin.
Heparin treatment decreases the rate of efflux of substrates of the drug transporter system in cancer cells
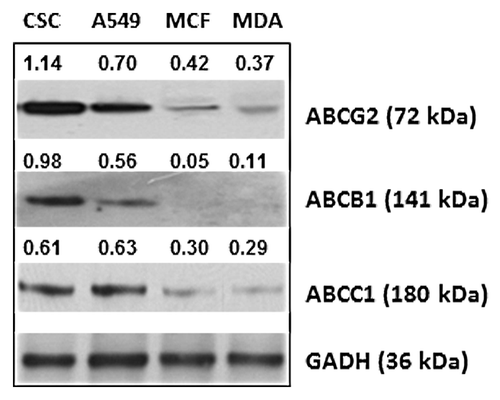
The effect of heparin on the function of drug transporter proteins was investigated by measuring the efflux of each of a number of reporter substrates in the presence or absence of heparin using flow cytometry. Several substrates of drug transporter system were used, including two dyes, rhodamine 123 and Ph-A, and four chemotherapeutic drugs, namely, epirubicin, mitoxantrone, tamoxifen, and doxorubicin. Cells were pre-incubated with each of the substrates individually for 30 min at 37 °C to permit uptake by the cell. The medium containing substrate was removed by centrifugation, and the cells washed and then resuspended in fresh medium containing heparin (UFH or LMWH at 1 or 10 IU/ml). The efflux of substrate was then allowed to occur for 30 min at 37 °C before cells were subjected to cytometric analysis and the residual levels in the heparin treated and untreated cells determined from the emission intensity setting that of the control sample at an arbitrary value of 1. The results showed that both UFH and LMWH were able to act as inhibitors of the drug transporter system since the residual intracellular level of each of the substrates was significantly higher in the heparin treated cells in the majority of the experiments (75% of the individual experiments reported in ). It was also noted that the level of residual substrate observed in the presence of heparin differed between each of the four tested cancer cell lines. These differences probably arise, at least in part, from variation in the level of expression of the ABC drug transporter proteins between each of the four cell lines. The relative level of expression of ABCG2, ABCB1, and ABCC1 as determined from western blot analysis of whole cell lysate of each of the cell lines was observed to be CSCs >> A549 > MCF-7 > MDA-MB-231 (). These levels would be expected to coincide with the relative rate of efflux of each of the drugs tested over a 30 min incubation period. In point of fact, the overall difference in residual levels of drug was observed to correspond to the level of expression of ABCG2 protein in each of the four cancer cell lines tested, but not with that of the ABCB1 and ABCC1 proteins. Thus the residual level was highest in CSCs in comparison to that of A549, MCF-7, and MDA-MB-231 cell lines, respectively and MDA-MB-231, the cell line with the lowest level of expression exhibited the lowest level of residual substrate. The one exception was the residual level of doxorubicin in MCF-7 cell which was close to that of the CSCs—further work (described below) demonstrated that a non-ABC transporter protein was primarily responsible for doxorubicin transport. It was also observed that the level of residual substrate was directly dependent upon the concentration of heparin—an effect that was particularly marked in the case of CSCs. Heparin had therefore been demonstrated to be capable of inhibiting the function of the ABC transporter system by reducing the rate of efflux of substrate from cancer cells.
Table 1. Heparin (UFH or LMWH) is able to inhibit the ABC drug transporter system and increase the intracellular accumulation of ABC transporter substrates in cancer cells
Figure 2. The expression of ABC transporter proteins by cancer cell lines. Equivalent amounts of protein in lysates prepared from breast CSCs, lung cancer cell line A549, and breast cancer cell lines MCF-7 and MDA-MB-231, respectively, were subjected to SDS-PAGE followed by western blot analysis with anti-ABCG2 or anti-ABCB1 or anti-ABCC1, respectively. The number shown alongside each sample is the level of expression values, calculated from densitometric analysis in respect of the level of GAPDH in controls.
ABCG2 and ABCB1 are heparin-binding proteins
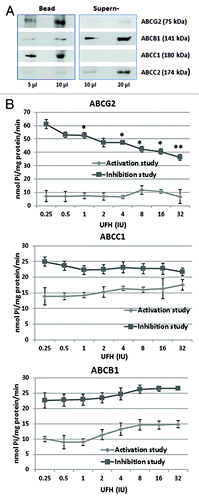
The ABC drug transporter proteins are glycoproteins principally located in the plasma membrane. To test whether heparin was able to bind directly to certain of the ABC transporter proteins and thereby influence their function, CSCs lysates were incubated with heparin–agarose beads. After separation by centrifugation, the fractions containing proteins which bound to the washed beads were analyzed and compared with those remaining in the supernatant, by SDS-PAGE and western blot analysis employing antibodies against each of the four principal ABC transport proteins, namely: ABCC1, ABCC2, ABCB1, and ABCG2. The results showed that a major fraction of the ABCG2 and the ABCC1 content bound to the beads. In contrast the greater part of the ABCB1 content of the cell remained in the supernatant as well as almost all of the ABCC2 (). When lysates were extracted with agarose beads type I alone as controls, no significant binding was observed for any of the four ABC proteins. These results demonstrate that only ABCG2 and ABCC1 are heparin binding proteins.
Figure 3. Heparin interaction with ABC transport proteins. (A) The binding of ABC transporter proteins by heparin–agarose. Breast CSCs lysates were incubated with heparin–agarose beads for 2 h, then the beads and supernatant were separated by centrifugation and subjected to western blot analysis using anti-ABCG2, anti-ABCB1, anti-ABCC1, or anti-ABCC2 primary antibody. The results show that heparin has an affinity for ABCG2 (BRCP) and ABCC1 (MRP1), but little or no affinity for ABCB1 (P-gp) and ABCC2 (MPR2). (B) The influence of heparin upon the vanadate sensitive ATPase activity of drug transporter proteins. The ATPase activity of ABCG2, ABCC1, and ABCB1 protein was respectively measured using the specific PREDEASY TM ATPase kit (Solvo Biotechnology, Budapest, Hungary) according to the manufacturer's instructions. ATPase activity was determined in the presence of 1.2 mM sodium orthovanadate either in the absence (termed the activation study) or the presence of substrate (termed the inhibition study) at increasing concentrations of UFH. Significance values (*P < 0.05; **P < 0.01) are shown at a particular heparin concentration where the activity observed in the activation study differed from the activity in the inhibition study (activation/inhibition study are defined by the kit manufacturer). The results show that heparin causes significant inhibition of the ATPase activities of ABCG2, and to a lesser extent that of ABCC1, but has no effect on the activity of ABCB1.
Heparin inhibits the ATPase activity of ABCG2 and ABCC1
ABC transporter proteins are active transporters, pumping their substrates against a concentration gradient using the energy provided by ATP hydrolysis.Citation5 With most of the ABC transporter proteins, the ATPase activity is stimulated by substrate recognition.Citation13 Since heparin was able to bind to the ABCG2 and ABCC1 proteins, the possibility existed that this would influence their ATPase activity and cause the inhibition of the efflux function, we chose to investigate the effect of heparin upon the ATPase activity of ABCG2, ABCC1, and ABCB1, respectively. The assays were performed in the presence of a range of heparin concentrations (0.25–32 IU/ml) using ATPase assay kits which have been developed specifically for these three major members of the drug transporter system. Each assay is comprised of two different tests which are performed on the same plate. In what is termed by the manufacturers as the activation study, the baseline, vanadate sensitive ATPase activity of separate membrane preparations of each of the ABC proteins (ABCG2, ABCC1, or ABCB1) was measured in the presence of increasing concentrations of heparin. In what is termed the inhibition study, the maximum vanadate sensitive ATPase activity of activated membrane ABC protein was determined (ABCG2, ABCC1, or ABCB1 respectively—each performed with the respective activator) in the presence of increasing concentrations of heparin. The occurrence of inhibition due to heparin was then determined by comparing the graphically represented data in both the inhibition and activation tests. The results showed that in the activation study, the ATPase activity of ABCG2 was not significantly affected by heparin, but in the inhibition assay the level of ATPase activity of ABCG2 was reduced in a concentration-dependent manner by as much as 2-fold at 32 IU/ml (). In the activation study of ABCC1, ATPase activity was slightly enhanced with an increasing concentration of heparin and in the inhibition study, the reduction of ATPase activity of ABCC1 was slight, only reaching significance at 32 IU/ml of heparin (). In the case of ABCB1, ATPase activity in both the activation and inhibition study sides of the experiments progressively increased at heparin levels above 2 IU/ml. Since a similar pattern of change was observed in both the activation and inhibition study, it can be concluded that there was no inhibition of activity by heparin (). The results therefore demonstrate that heparin is able to inhibit the ATPase activities of ABCG2 significantly and, to a lesser extent, that of ABCC1, but not that of ABCB1. Considering our evidence that ABCG2 and ABCC1 are heparin binding proteins, we propose that the influence of heparin upon the functionality of the ABC transporter system is mainly due to an inhibitory effect on the ABCG2 activity with a lesser influence on that of ABCC1.
The upregulation of LRP by treatment of MCF-7 cells with 17-β-estradiol shows that LRP was responsible for the inhibition of the efflux of doxorubicin and impacted by heparin
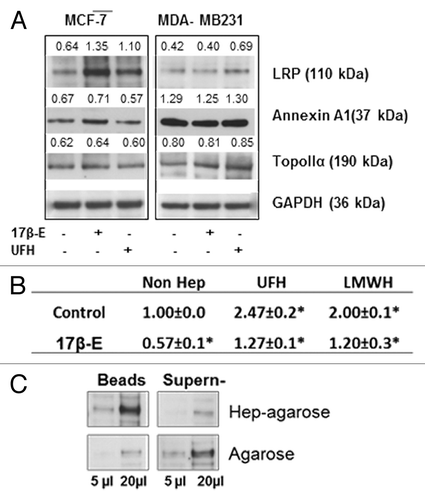
Since heparin inhibition of the rate of efflux of doxorubicin by MCF-7 cells did not correlate with the level of expression of ABC transporter proteins unlike what was observed with the other cell lines tested, such as MDA-MB-231 (), it was considered that other non- ABC -transport proteins may predominate in the intracellular movement of doxorubicin by MCF-7 cells. One of major difference in genotype/phenotype between MCF-7 and MDA-MB-231 cell lines is that MCF-7 cells express the estradiol receptor (ER) and MDA-MB-231 cells do not express ER. The expression of certain of the non-ABC drug transporter proteins such as LRP and annexin A1 has been found to be increased after 17-β-estradiol treatment, as a consequence of the activation of the ER receptor.Citation22,Citation23 To clarify whether the high efflux of doxorubicin in MCF-7 cells was due to non-ABC drug transport proteins, MCF-7 and MDA-MB-231 cells were treated with 17-β-estradiol overnight in culture medium containing steroid stripped serum (Sigma). The treated cells were analyzed for the expression of non-ABC drug transporter proteins by western blot, as well as functionally by measuring the efflux rate of doxorubicin. As expected, the expression of both LRP and annexin A1 were increased after 17-β-estradiol treatment, which was correlated with a reduced accumulation of doxorubicin in the treated MCF-7 cells (). The 17-β-estradiol treatment also reduced the heparin induced accumulation of doxorubicin in MCF-7 cells (). No significant change in the levels of these proteins or doxorubicin accumulation was observed in 17-β-estradiol treated MDA-MB-231 cells. These observations suggested that the non ABC transporter proteins may also play a principal role in doxorubicin efflux from MCF-7 cells prior to 17-β-estradiol treatment. In fact, western blot analysis showed that the expression level of LRP in MCF-7 cell is higher than that in MDA-MB-231 cells (). It was also noted that the level of expression level of annexin A1 by MDA-MB-231 cell is much higher than that of MCF-7 cells (), and does not correlate with the relatively lower rate of efflux activity of doxorubicin by MDA-MB-231 cells. No change was observed in the level of expression of topoisomerase II, another non-ABC transporter protein, after estradiol treatment. Taking these points into consideration, it was therefore considered that LRP was probably principally responsible for the efflux of doxorubicin from MCF-7 cells. Since both UFH and LMWH inhibited the efflux of doxorubicin in 17-β-estradiol treated MCF-7 cells, it was decided to determine if LRP is a heparin binding protein. The results confirmed this showing that the major part of LRP in cell lysates bound to heparin–agarose beads, while in control experiments that used agarose beads most of the LRP content of the cell remained in the supernatant ()
Figure 4. The upregulation of LRP by 17-β-estradiol shows that interaction with LRP was responsible for the heparin induced inhibition of the efflux of doxorubicin from MCF-7 cells. (A) Western blot of non-ABC proteins expression regulated by 17-β-estradiol. MCF-7 cells were treated with 17-β-estradiol with or without heparin for 12 h, lysates were prepared and western blot analysis performed with a range of non ABC protein antibodies, as indicated. The number above each band is the level of expression calculated in respect of the level of GAPDH; (B) Efflux assay demonstrates that the intracellular accumulation of doxorubicin in MCF-7 cell was reduced by 17-β-estradiol treatment. Data shown are the -mean ± SD of 3 separate experiments as calculated relative to the control. *P = 0.01 compared with control cultures. (C). Extraction of cells lysates with heparin–agarose shows that LRP is a heparin binding protein, The experiment was performed as described in . Binding by agarose beads type II are shown as a control.
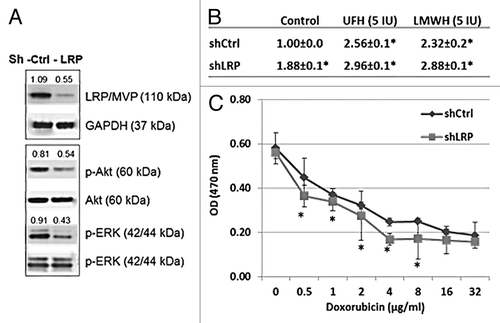
LRP gene knockdown by shRNA demonstrates that LRP is affected by heparin and responsible for controlling doxorubicin transport and cytotoxicity in MCF-7 cells
To further confirm that LRP is responsible for doxorubicin transport in MCF-7 cells, cells were prepared in which the LRP gene was knocked down by shRNA transfection. Cells treated with an ineffective scrambled shRNA cassette contained in the same vector were used as controls. The level of expression of LRP protein by each of the transfected cell lines was determined by western blot (). Since ERK and Akt activations may depend on LRP.Citation24-Citation26 an observed reduction in the level of p-ERK and p-Akt further confirmed the functional effect of the knockdown of LRP in shLRP-MCF-7 cells (). When the rate of accumulation of doxorubicin by the LRP gene knockdown cells was determined by efflux assay, it was observed that the residual level of intracellular doxorubicin was significantly increased in the shLRP MCF-7 cell, consistent with the lower expression level of LRP protein in these cell lines (). Upon treatment with heparin, the rate of accumulation of doxorubicin by the shLRP cell line was shown to be further increased (). This enhanced accumulation coincided with an increase in the cytotoxic effect of doxorubicin on shLRP MCF-7 cells when compared with effect on control cells transfected with the scrambled shRNA. The 50% median effective dose (ED50) of doxorubicin in sh-Control and sh-LRP cell lines were significantly different, at 1.02 g/ml and 0.75 g/ml, respectively (). These findings are consistent with the conclusion that LRP is primarily responsible for the efflux of doxorubicin from MFC-7 cells and therefore highly contributory to the observed fall in efflux in the presence of heparin presumably due to heparin binding by the protein.
Figure 5. LRP gene knockdown by shRNA demonstrates that LRP is responsible for the transport and cytotoxicity effect of doxorubicin in MCF-7 cells and the doxorubicin transport is inhibited by heparin treatment. (A) Western blot shows the expression of LRP was reduced in the LRP gene knockdown cells following treatment with shLRP. The levels of p-Akt and p-ERK are also reduced in the LRP gene knockdown cells, further evidence of the overall biological-effect of LRP gene knockdown in MCF-7 cells. The number shown are the levels calculated with respect to the level of levels of GAPDH or Akt or ERK in control cells. (B) The intracellular accumulation of doxorubicin was increased in LRP gene knockdown cells. Data represent the fluorescence intensity mean (G mean) ± SD of 3 separate experiments calculated relative to the control. *P = 0.01 compared with control. (C) The toxicity of doxorubicin was reduced in the LRP gene knockdown MCF-7 cells. ED 50 of doxorubicin is 0.75 µg/ml in shControl cells and 1.02 µg/m in shLRP cells.
Discussion
In this study, we have observed that the cytotoxic effect of each of three chemotherapeutic drugs on breast cancer stem cells was enhanced, but to different degrees when used in combination with heparin. A previous report investigated the ability of heparin to modulate the cellular accumulation of doxorubicin in ABCC1 (MRP1) overexpressing HL60/doxo cells.Citation27 The same laboratory also reported that a high concentration of UFH (20 IU/ml) was able to increase the cellular accumulation and cytotoxicity of doxorubicin in MDA-MB-231 cells and concluded that the increased accumulation and cytotoxicity of doxorubicin in the presence of heparin occurred via the inhibition by heparin of ABCB1 mediated efflux.Citation28 However in our hands and also according to others, MB-MB-231 cells exhibit a very low level expression of ABCB1Citation29 and also there is no evidence to show how heparin might be having such an effect.Citation28 Our results, however, have demonstrated that heparin has little or no interaction with ABCB1 neither binding to the protein nor inhibiting the ATPase activity. In contrast to a lack of interaction with ABCB1, we have shown that treatment with heparin at relatively low concentrations inhibits the ATPase activity of ABCG2 and at higher concentrations inhibits the ATPase activity of ABCC1 in accordance with the observed affinity of each of these proteins for heparin. Since the ATPase activity of ABC transporters is crucial to their transport activity, and the ability of heparin to enhance the cytotoxicity of the ABC transporter substrates coincides with the level of expression of ABCG2 protein in each of the four cancer cell lines, we conclude that out of the major ABC transporter proteins, the interaction with ABCG2 is primarily responsible for the observed effect of heparin on drug transport and efficacy in breast cancer cells.
The actual mechanism of the ABC transporter system is still largely unknown particularly as to how it recognizes and transports a large number of structurally and chemically unrelated drugs and xenobiotics. In mammals, the functionally active ABC proteins comprise a minimum of four core domains, including two transmembrane domains (TMDs) that form the permeation pathway for the transport of substrates and two intracellular nucleotide binding domains (NBDs) in which ATP binding and hydrolysis occurs.Citation5 Currently it is considered that the specific binding of the substrate enhances the ATPase activity of the transporter probably by communication between the TMD and NBD through conformational changes.Citation5,Citation14,Citation15 In regard to substrate binding, it has been reported that there are multiple drug binding sites in ABC proteins. For example, doxorubicin binding by ABCG2 occurs in one area of the protein and rhodamine 123 binding in another area while the binding site for mitoxantrone may partially overlap with both of these binding sites.Citation15 Our results found that the transport of several substrates and the ATPase activities of ABCG2 and ABCC1 were inhibited by heparin. ABC transport activities require the energy of ATP hydrolysis which is controlled by drug interaction and coupled directly to the actual substrate translocation. With these features of the mechanism of action in mind, we conclude that heparin by binding to ABCG2 and ABCC1 is able to block the binding sites of each the substrates which may well occur over a wide area of the molecule and thereby also inhibit the ATPase activity of the transporters.
In contrast to the ubiquity as well as the rather broad selectivity of the ABC transporter system toward substrates, non-ABC transporter proteins, have often been shown to be responsible for transport of an individual drug in a particular cell line, for example, the efflux of doxorubicin in MCF-7 was shown to be significantly higher than in MDA-MB-231 cells, a property that was associated with differences in the expression level of LRP. A number of the non-ABC drug transport proteins have been shown to interact with heparin e.g., Annexin A1;Citation30 Other examples which have been shown to be inhibited by heparin are topoisomerase II (topo II)Citation31 and the Glutathione S-transferases (GSTs—ubiquitous multifunctional enzymes, which play a key role in cellular detoxification).Citation32 It has been reported that the level of LRP in tumors may be highly predictive of a poor response to chemotherapy as well as adverse clinical outcome in several different types of cancer.Citation21,Citation33 Our work has demonstrated in MCF-7 cells in which LRP expression has been upregulated by 17-β-estradiol treatment or downregulated by shLRP transfection, that the LRP levels corresponded with the rate of intracellular accumulation and cytotoxicity of doxorubicin and that a high concentration of heparin (>5 IU/ml) is capable of exerting an inhibitory effect on the efflux of doxorubicin from shLRP MCF-7 cells. The significance of LRP in controlling drug sensitivity in cancer is still unresolved. A number of previous investigations have shown that the level of LRP was closely linked to MDR in various cancers;Citation21,Citation26,Citation33 however, it has also been reported that LRP neither affects drug sensitivity nor intracellular sequestration, and that LRP knockout mouse do not show enhanced hypersensitivity to cytostatics.Citation34,Citation35 More recent reports observed that LRP knockdown in human bladder cancer cell lineCitation36 and human breast cancer MCF-7 cell lineCitation37 allowed doxorubicin access to the nucleus, and resulted in an enhanced cytotoxicity. Based on our present work we propose that LRP makes a partial contribution to doxorubicin transport, and that other transporter proteins, which are also affected by heparin treatment, may also be involved in the doxorubicin efflux from MCF-7 cells. This catalog of contradictory observations as regards the functional role of LRP may be because the studies have been made with a variety of cancer cell lines in which variation of LRP protein level, together with variation in non-LRP transporter proteins, may compromise a proper assessment of the overall role of LRP to influence drug efflux.
The three major proteins of the ABC drug transporter system: ABCB1, ABCC1, and ABCG2, form a powerful drug efflux pump, which plays a central role in protecting the organism from the toxicity of a variety of endogenous and exogenous molecules. All three of the ABC proteins demonstrate overlapping drug substrate specificity and share the ability to transport a wide range of substrates out of cell, but each transporter protein also handles unique substrates. The substrates used in this study include two dyes and four drugs, of which rhodamine 123, mitoxantrone, epirubicin, and doxorubicin are overlapping substrates of ABCG2, ABCB1, and ABCC1. Tamoxifen, an anti-estrogen anticancer drug, is an inhibitory substrate for ABCB1, ABCC1, and ABCG2.Citation3,Citation5,Citation13,Citation38 Only pheophorbide-A is a specific substrate for ABCG2 alone.Citation39 Many of the currently used chemotherapeutic drugs could be overlapping substrates among both the ABC and non-ABC transporter systems. An ideal modulator of MDR should be able to target the activity of a wide range of both the ABC and non ABC transporter proteins. We have now established that heparin is able to bind a wide range of the ABC and non-ABC transporters. Thus, based on our work, heparin can be added to the list of MDR modulators which are able to influence ABC, and non-ABC proteins with the added advantage that heparin, in particular LMWHs, has been used extensively during cancer treatment with no evidence of the severe side effects reported for other candidates investigated as MDR modulators. Our findings support the setting up of clinical studies to establish if chemotherapy in combination with heparin leads to a reduction in MDR and improvement in therapeutic response.
Apart from the direct interaction with transporter that probably affects substrate recognition sites, MDR reversal by MDR modulators may also result from indirect effects, such as changes in gene expressionCitation40 and cell signaling pathways.Citation41,Citation42 The function of ABC transport proteins has been found to be affected by the activity of cell signaling pathways, for example the inhibition of the elevated Akt activity of tumor cells has been shown to downregulate ABCG2 expression and efflux activity.Citation41,Citation42 Interestingly we have also established that heparin treatment of MCF-7, MDA-MB-231, and CSCs cell lines for 30 min reduces the level of intracellular phosphorylation including the level of p-AktCitation12 opening up the possibility that heparin treatment may also regulate ABC transporter system through inhibition of Akt activity. There are also reports of LRP binding several phosphatases and kinases including PTEN, SHP-2, as well as ERK, and evidence suggesting that LRP might be involved in the regulation of cell signaling pathways including the PI3K/Akt and the MAPK pathways.Citation24-Citation26 In this investigation, reduced levels of p-ERK and p-Akt were found in the LRP knockdown MCF-7 cells (). Therefore apart from the ability to affect drug transporter activity by direct binding to individual transporter proteins, heparin may also influence drug efflux by regulating crucial signaling pathways.
In summary, heparin acts as an inhibitor of a range of ABC and non-ABC transporter proteins, by blocking transporter protein activity and modulating the efflux and toxicity of their drug substrates, which has been confirmed in this study for the ABCG2, ABCC1, and LRP transport proteins. Our findings suggest that the clinical response to chemotherapeutic drugs may be enhanced in patients who are also receiving heparin as adjuvant clinical treatment, which may be responsible, at least in part, for the increased survival of heparin treated cancer patients.
Materials and Methods
Chemical reagents
Rhodamine 123, pheophorbide a (Ph-A), mitoxantrone, tamoxifen, and doxorubicin were obtained from Sigma and epirubicin from Hospira. Unfractionated heparin (UFH) of porcine origin was obtained from Sigma and low molecular heparin (LMWH, Fragmin) from Pfizer.
Cell lines
A human enriched breast cancer stem cell (CSC) line was purchased from BioMedicure. Human breast cancer cell lines, MCF-7 and MDA-MB-231 and human lung cancer line A549 were obtained from the Health Protection Agency culture collection (UK). CSCs, MCF-7, and A549 cells were maintained in DMEM medium supplemented with 15% fetal bovine serum (PAA), 100 U/ml penicillin, and 100 mg/ml streptomycin, and cultured in a humidified atmosphere of 5% CO2 in air. MDA-MB-231 cells were cultured in Leibovitz L-15 medium with the above same supplements and conditions but in the absence of CO2.
Cytotoxicity assay
The CyQUANT® NF cell proliferation assay kit (Invitrogen) was used to determine the effect of heparin upon the cytotoxicity of chemotherapeutic agents according to the manufacturers’ instruction. Breast cancer cells were plated onto 96 well plates at a density of 5000 cells/per well and allowed to attach overnight. Chemotherapeutic agents: mitoxantrone, epirubicin, tamoxifen, and doxorubicin were added at varying concentrations in the presence or absence of varying concentrations of heparin (1, 5, or 10 IU /ml) and the plates were incubated at 37 °C for 24 h. After removing the medium, the cells were further incubated with the CyQUANT dye binding solution (50 µl/well) for 30 min before the fluorescence intensity of each sample was read using a SpectraMax M2 microplate reader (Molecular Devices) with excitation set at 485nm and emission detection set at 530 nm. The cytotoxicity of doxorubicin was also assessed using a cell viability assay based upon the direct measurement of the cleavage of a tetrazolium salt (MTT), added to the culture medium (Cell Proliferation Kit I Roche) according to the manufacturer instructions.
Assay of substrate efflux by the drug transporter system
Cells at 90% confluence were harvested by trypsinization, washed with serum free medium containing 2% Lactalbumin Hydrolysate (Sigma) and resuspended in the same medium at a cell density of 106 cells/ml. The uptake of substrates of the drug transporter system by the cell was investigated by incubating 2 ml of cell suspension with either rhodamine 123 (2 µM), or Ph-A (10 µM), or mitoxantrone (10 µM/L), or epirubicin (5 µM), or tamoxifen (5 µM), or doxorubicin (4 µM), respectively, at 37 °C for 30 min. Cells were then separated, washed, and resuspended in fresh medium containing UFH or LMWH (1–10 IU/ml) and incubated at 37 °C to allow efflux to occur using cells suspended in medium alone as a control. After 30 min, the intracellular concentration of each substrates was determined using a Cytomics FC 500 (Beckman Coulter), with excitation wavelength set at 488 nm and emission wavelength set at 525 nm for rhodamine 123 and Ph-A, at 560 nm for epirubicin, tamoxifen, and doxorubicin and at 670 nm for mitoxantrone.
Heparin binding and western blot analysis
Cell suspensions (2 × 106 cells) were washed twice with ice-cold PBS before resuspension in 200 µl of CelLytic lysis buffer (Sigma). After incubation at 4 °C for 10 min, cell debris was removed by centrifugation. The supernatant (lysate) was incubated with 100 µl of heparin–agarose type I beads (Sigma) suspended in PBS for 2 h at room temperature. Agarose type I beads (Sigma) were used to extract the lysates in control analyses. The beads and supernatant were separated by centrifugation and the beads washed with PBS. Beads and supernatant were respectively mixed with 200 µl of 2× SDS PAGE electrophoresis sample buffer and then run on SDS-PAGE gels prior to western blot analysis respectively using anti-ABCG2, anti-ABCB1, anti-ABCC1, anti-ABCC2, anti-LRP, anti-Annexin A1, and anti-TopoIIα primary antibody (Abcam), together with the appropriate HRP-conjugated secondary antibody (Cell Signaling). The expressions of ABCG2, ABCB1, ABCC1, LRP, Annexin A1, and TopoIIα in different cell lines were also investigated by western blot analysis of unfractionated cell lysates obtained from each of the cell lines.Citation43 Densitometry was performed using Quantity One software (Bio-Rad) as previously described.Citation43
Assay of drug transporter ATPase activity
Measurement of the effect of heparin on the ATPase activity of each of the ABC transporter proteins (ABCG2, ABCB1, and ABCC1) was performed using individual kits, namely SB-BCRP(ABCG2)-, SB-P-gp(ABCB1)-, or SB-MRP1(ABCC1)- PREDEASY TM ATPase kits (Solvo Biotechnology) according to the manufacturer’s instructions. The assays were performed using purified membrane vesicles from insect cells or mammalian cells, which express high levels of the selected human ABC transporter protein. The membrane vesicles were incubated with various concentrations of UFH as indicated in the text. ATPase activity was determined as the difference between the amount of inorganic phosphate liberated when measured in the presence and absence of 1.2 mM sodium orthovanadate, an inhibitor of ABC efflux pumps, respectively. Results are presented as vanadate-sensitive ATPase activities.
Knockdown MVP/LRP protein expression in MCF-7 cells
The retroviral silencing plasmid (pRS) from OriGene that contains four designed and sequence-verified shRNA vectors for the MVP/LRP gene was used to generate a stable MVP/LRP knockdown cell line according to manufacturer’s specifications. Briefly, MCF-7 cells were seeded onto collagen-coated 6-well plates. On the following day, the retroviral vector containing either four unique shRNA construct against MVP/LRP or a non-effective scrambled shRNA as control were transduced into MCF-7 cells respectively. When the cells were ready to be passaged, the transfected cells were replanted into a fresh plate with selection medium containing puromycin (1 ug/ml). Selected clonal populations of cells were grown and repassaged in the selection medium for 3–4 passages. The expression of MVP/LRP protein by the MVP/LRP knockdown MCF-7 cells was verified by western blotting with anti-MVP/LRP antibody (Abcam). The stably transduced cell line which exhibited low levels of expression of MVP/LRP was used for the investigation of the level of contribution of the LRP to the efflux of doxorubicin by MCF-7 cells as well as the associated cytotoxicity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Wind NS, Holen I. Multidrug resistance in breast cancer: from in vitro models to clinical studies. Int J Breast Cancer 2011; 2011:967419; http://dx.doi.org/10.4061/2011/967419; PMID: 22332018
- Petzinger E, Geyer J. Drug transporters in pharmacokinetics. Naunyn Schmiedebergs Arch Pharmacol 2006; 372:465 - 75; http://dx.doi.org/10.1007/s00210-006-0042-9; PMID: 16532306
- Jäeger W. Classical resistance mechanisms. Int J Clin Pharmacol Ther 2009; 47:46 - 8; http://dx.doi.org/10.5414/CPP47046; PMID: 19203536
- Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 2008; 9:105 - 27; http://dx.doi.org/10.2217/14622416.9.1.105; PMID: 18154452
- Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci 2001; 58:931 - 59; http://dx.doi.org/10.1007/PL00000912; PMID: 11497241
- Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, Quehenberger P, Zielinski C, Pabinger I. Prediction of venous thromboembolism in cancer patients. Blood 2010; 116:5377 - 82; http://dx.doi.org/10.1182/blood-2010-02-270116; PMID: 20829374
- Lee AY. Thrombosis in cancer: an update on prevention, treatment, and survival benefits of anticoagulants. Hematology Am Soc Hematol Educ Program 2010; 2010:144 - 9; http://dx.doi.org/10.1182/asheducation-2010.1.144; PMID: 21239784
- Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol 2004; 22:1944 - 8; http://dx.doi.org/10.1200/JCO.2004.10.002; PMID: 15143088
- Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol 2005; 23:2130 - 5; http://dx.doi.org/10.1200/JCO.2005.03.134; PMID: 15699479
- Cunningham MS, Preston RJ, O’Donnell JS. Does antithrombotic therapy improve survival in cancer patients?. Blood Rev 2009; 23:129 - 35; http://dx.doi.org/10.1016/j.blre.2008.10.002; PMID: 19046797
- von Tempelhoff GF, Harenberg J, Niemann F, Hommel G, Kirkpatrick CJ, Heilmann L. Effect of low molecular weight heparin (Certoparin) versus unfractionated heparin on cancer survival following breast and pelvic cancer surgery: A prospective randomized double-blind trial. Int J Oncol 2000; 16:815 - 24; PMID: 10717252
- Chen Y, Scully M, Dawson G, Goodwin C, Xia M, Lu X, Kakkar A. Perturbation of the heparin/heparin-sulfate interactome of human breast cancer cells modulates pro-tumourigenic effects associated with PI3K/Akt and MAPK/ERK signalling. Thromb Haemost 2013; 109:1148 - 57; http://dx.doi.org/10.1160/TH12-12-0935; PMID: 23571852
- Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv 2004; 1:27 - 42; http://dx.doi.org/10.2174/1567201043480036; PMID: 16305368
- Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003; 22:7340 - 58; http://dx.doi.org/10.1038/sj.onc.1206938; PMID: 14576842
- Ni Z, Bikadi Z, Rosenberg MF, Mao Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr Drug Metab 2010; 11:603 - 17; http://dx.doi.org/10.2174/138920010792927325; PMID: 20812902
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol 2005; 205:275 - 92; http://dx.doi.org/10.1002/path.1706; PMID: 15641020
- Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 2000; 92:1295 - 302; http://dx.doi.org/10.1093/jnci/92.16.1295; PMID: 10944550
- Wang J, Zhang J, Zhang L, Zhao L, Fan S, Yang Z, Gao F, Kong Y, Xiao GG, Wang Q. Expression of P-gp, MRP, LRP, GST-π and TopoIIα and intrinsic resistance in human lung cancer cell lines. Oncol Rep 2011; 26:1081 - 9; PMID: 21805041
- Ikeda K, Oka M, Yamada Y, Soda H, Fukuda M, Kinoshita A, Tsukamoto K, Noguchi Y, Isomoto H, Takeshima F, et al. Adult T-cell leukemia cells over-express the multidrug-resistance-protein (MRP) and lung-resistance-protein (LRP) genes. Int J Cancer 1999; 82:599 - 604; http://dx.doi.org/10.1002/(SICI)1097-0215(19990812)82:4<599::AID-IJC21>3.0.CO;2-R; PMID: 10404077
- Izquierdo MA, Scheffer GL, Flens MJ, Giaccone G, Broxterman HJ, Meijer CJ, van der Valk P, Scheper RJ. Broad distribution of the multidrug resistance-related vault lung resistance protein in normal human tissues and tumors. Am J Pathol 1996; 148:877 - 87; PMID: 8774142
- Scheffer GL, Wijngaard PL, Flens MJ, Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC, Scheper RJ. The drug resistance-related protein LRP is the human major vault protein. Nat Med 1995; 1:578 - 82; http://dx.doi.org/10.1038/nm0695-578; PMID: 7585126
- Bontenbal M, Sonneveld P, Foekens JA, Klijn JG. Oestradiol enhances doxorubicin uptake and cytotoxicity in human breast cancer cells (MCF-7). Eur J Cancer Clin Oncol 1988; 24:1409 - 14; http://dx.doi.org/10.1016/0277-5379(88)90329-X; PMID: 3181264
- Ang EZ, Nguyen HT, Sim HL, Putti TC, Lim LH. Annexin-1 regulates growth arrest induced by high levels of estrogen in MCF-7 breast cancer cells. Mol Cancer Res 2009; 7:266 - 74; http://dx.doi.org/10.1158/1541-7786.MCR-08-0147; PMID: 19208747
- Langlois B, Perrot G, Schneider C, Henriet P, Emonard H, Martiny L, Dedieu S. LRP-1 promotes cancer cell invasion by supporting ERK and inhibiting JNK signaling pathways. PLoS One 2010; 5:e11584; http://dx.doi.org/10.1371/journal.pone.0011584; PMID: 20644732
- Geetha N, Mihaly J, Stockenhuber A, Blasi F, Uhrin P, Binder BR, Freissmuth M, Breuss JM. Signal integration and coincidence detection in the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) cascade: concomitant activation of receptor tyrosine kinases and of LRP-1 leads to sustained ERK phosphorylation via down-regulation of dual specificity phosphatases (DUSP1 and -6). J Biol Chem 2011; 286:25663 - 74; http://dx.doi.org/10.1074/jbc.M111.221903; PMID: 21610072
- Steiner E, Holzmann K, Elbling L, Micksche M, Berger W. Cellular functions of vaults and their involvement in multidrug resistance. Curr Drug Targets 2006; 7:923 - 34; http://dx.doi.org/10.2174/138945006778019345; PMID: 16918321
- Angelini A, Ciofani G, Baccante G, Di Febbo C, Carmine DI, Cuccurullo F, Porreca E. Modulatory effects of heparin on cellular accumulation and cytotoxicity of doxorubicin in MRP1-overexpressing HL60/doxo cells. Anticancer Res 2007; 27:1A 351 - 5; PMID: 17352253
- Angelini A, Di Febbo C, Ciofani G, Di Nisio M, Baccante G, Di Ilio C, Cuccurullo F, Porreca E. Inhibition of P-glycoprotein-mediated multidrug resistance by unfractionated heparin: a new potential chemosensitizer for cancer therapy. Cancer Biol Ther 2005; 4:313 - 7; http://dx.doi.org/10.4161/cbt.4.3.1503; PMID: 15876859
- Wang Y, Serfass L, Roy MO, Wong J, Bonneau AM, Georges E. Annexin-I expression modulates drug resistance in tumor cells. Biochem Biophys Res Commun 2004; 314:565 - 70; http://dx.doi.org/10.1016/j.bbrc.2003.12.117; PMID: 14733945
- Ori A, Wilkinson MC, Fernig DG. A systems biology approach for the investigation of the heparin/heparan sulfate interactome. J Biol Chem 2011; 286:19892 - 904; http://dx.doi.org/10.1074/jbc.M111.228114; PMID: 21454685
- Redwood C, Davies SL, Wells NJ, Fry AM, Hickson ID. Casein kinase II stabilizes the activity of human topoisomerase IIalpha in a phosphorylation-independent manner. J Biol Chem 1998; 273:3635 - 42; http://dx.doi.org/10.1074/jbc.273.6.3635; PMID: 9452492
- Rusnati M, Urbinati C, Caputo A, Possati L, Lortat-Jacob H, Giacca M, Ribatti D, Presta M. Pentosan polysulfate as an inhibitor of extracellular HIV-1 Tat. J Biol Chem 2001; 276:22420 - 5; http://dx.doi.org/10.1074/jbc.M010779200; PMID: 11304529
- Zurita AJ, Diestra JE, Condom E, García Del Muro X, Scheffer GL, Scheper RJ, Pérez J, Germà-Lluch JR, Izquierdo MA. Lung resistance-related protein as a predictor of clinical outcome in advanced testicular germ-cell tumours. Br J Cancer 2003; 88:879 - 86; http://dx.doi.org/10.1038/sj.bjc.6600803; PMID: 12644825
- Huffman KE, Corey DR. Major vault protein does not play a role in chemoresistance or drug localization in a non-small cell lung cancer cell line. Biochemistry 2005; 44:2253 - 61; http://dx.doi.org/10.1021/bi047948g; PMID: 15709737
- van Zon A, Mossink MH, Schoester M, Scheper RJ, Sonneveld P, Wiemer EA. Efflux kinetics and intracellular distribution of daunorubicin are not affected by major vault protein/lung resistance-related protein (vault) expression. Cancer Res 2004; 64:4887 - 92; http://dx.doi.org/10.1158/0008-5472.CAN-03-3891; PMID: 15256459
- Herlevsen M, Oxford G, Owens CR, Conaway M, Theodorescu D. Depletion of major vault protein increases doxorubicin sensitivity and nuclear accumulation and disrupts its sequestration in lysosomes. Mol Cancer Ther 2007; 6:1804 - 13; http://dx.doi.org/10.1158/1535-7163.MCT-06-0372; PMID: 17575109
- Han M, Lv Q, Tang XJ, Hu YL, Xu DH, Li FZ, Liang WQ, Gao JQ. Overcoming drug resistance of MCF-7/ADR cells by altering intracellular distribution of doxorubicin via MVP knockdown with a novel siRNA polyamidoamine-hyaluronic acid complex. J Control Release 2012; 163:136 - 44; http://dx.doi.org/10.1016/j.jconrel.2012.08.020; PMID: 22940126
- Daoud R, Kast C, Gros P, Georges E. Rhodamine 123 binds to multiple sites in the multidrug resistance protein (MRP1). Biochemistry 2000; 39:15344 - 52; http://dx.doi.org/10.1021/bi0020574; PMID: 11112520
- Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res 2004; 64:1242 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-03-3298; PMID: 14973080
- Wesołowska O. Interaction of phenothiazines, stilbenes and flavonoids with multidrug resistance-associated transporters, P-glycoprotein and MRP1. Acta Biochim Pol 2011; 58:433 - 48; PMID: 22187677
- Chu TS, Chen JS, Lopez JP, Pardo FS, Aguilera J, Ongkeko WM. Imatinib-mediated inactivation of Akt regulates ABCG2 function in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2008; 134:979 - 84; http://dx.doi.org/10.1001/archotol.134.9.979; PMID: 18794444
- An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells?. Expert Opin Drug Metab Toxicol 2009; 5:1529 - 42; http://dx.doi.org/10.1517/17425250903228834; PMID: 19708828
- Chen Y, Shi-Wen X, van Beek J, Kennedy L, McLeod M, Renzoni EA, Bou-Gharios G, Wilcox-Adelman S, Goetinck PF, Eastwood M, et al. Matrix contraction by dermal fibroblasts requires transforming growth factor-beta/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: insights into pathological scarring in chronic fibrotic disease. Am J Pathol 2005; 167:1699 - 711; http://dx.doi.org/10.1016/S0002-9440(10)61252-7; PMID: 16314481
