Abstract
Aberrant overexpression of the enhancer of zeste homolog 2 (EZH2), a histone methyltransferase inhibiting targets expression via epigenetic mechanisms, is associated with an invasive phenotype and drug resistance in ovarian cancer. Breast cancer 1 (BRCA1) gene is a well-recognized tumor suppressor, whose downregulation plays a key role in the development of ovarian cancer. In the present study, we found depletion of EZH2 increased BRCA1 protein expression and promoted its nuclear translocation, but decreased BRCA1 mRNA expression. Treatment with the Akt-1 activator insulin-like growth factor-1 (IGF-1) prevented EZH2-induced BRCA1 nuclear/cytoplasmic shuttling. Loss of BRCA1 partially rescued the effects of EZH2 downregulation on proliferation, G2/M transition, and migration in ovarian cancer cells. However, in a cisplatin-resistant sub-line of A2780 (A2780/DDP), both EZH2 and BRCA1 were overexpressed compared with parental A2780 cells and depletion of EZH2 reduced BRCA1 expression at both mRNA and protein levels. Downregulation of EZH2 or BRCA1 sensitized A2780/DDP cells to cisplatin, whereas simultaneous inhibition of them only resulted in modest resensitization instead of showing any synergistic effect because EZH2 expression was reactivated when BRCA1 expression was very low. Accordingly, our results suggest the expression of BRCA1 is modulated by EZH2 in epithelial ovarian cancer and BRCA1 is required for the effects of EZH2 downregulation on biological behaviors of tumor cells.
Introduction
Ovarian cancer is one of the most lethal gynecologic cancers. Despite advances in therapeutic strategies, the 5-year survival rate remains lower than 50%,Citation1 due to the absence of effective screening techniques and the development of chemotherapy resistance.Citation2,Citation3 However, the precise molecular mechanisms leading to ovarian cancer are still not clear. The first breast and ovarian cancer susceptibility gene BRCA1 is a recognized key tumor suppressor in ovarian cancer, which regulates the activation of cell cycle checkpoints, DNA repair, and maintenance of chromosome stability. Women carrying a heterozygous mutation in the BRCA1 gene have approximately 40% risk of developing ovarian cancer in life time.Citation4 Although such mutation is not common in sporadic tumors, loss of BRCA1 occurs in approximately 90% of both hereditary and sporadic ovarian cancers,Citation5,Citation6 indicating there are other mechanisms underlying BRCA1 downregulation.
The enhancer of zeste homolog 2 (EZH2) protein is an active component of the polycomb repressive complex 2/3 (PRC2/3)Citation7 that exhibits an intrinsic histone lysine methyltransferase activity on histone H3 Lys-9 and -27 or histone H1 Lys-26.Citation8,Citation9 Additionally, EZH2 can serve as a recruitment platform for DNA methyltransferase and is essential for DNA methylation of EZH2-target promoters.Citation10 Furthermore, it can repress gene expression by binding to the promoter of the target genes directly, such as P57, RUNX3, and KLF2.Citation11-Citation13
Overexpression of EZH2 has been observed in breast, prostate, and bladder cancer, and is associated with a poor prognosis.Citation14-Citation16 In two previously published works, we have shown that EZH2 expression was upregulated in ovarian cancer and associated with advanced FIGO (International Federation of Gynecology and Obstetrics) stage and poorer cell differentiation, and downregulation of EZH2 inhibited cell proliferation and migration of ovarian cancer and sensitized tumor cells to cisplatin.Citation17,Citation18
Previous studies revealed that epigenetic mechanisms, especially promoter hypermethylation should be in charge of the inactivation of BRCA1 in sporadic ovarian tumor.Citation19,Citation20 The cytoplasmic retention of BRCA1 protein was induced by Akt-1 activation in breast cancer cells.Citation21 Furthermore, Gonzalez et al. reported that the effect of EZH2 on ER-negative breast cancer cell proliferation and G2/M transition required BRCA1, and EZH2 induced an Akt-dependent BRCA1 inhibition.Citation22,Citation23 Thus, we were interested in whether BRCA1 is regulated by EZH2 and plays a role in the biological function of EZH2 in epithelial ovarian cancer.
Results
Loss of EZH2 increases BRCA1 protein level and induces its nuclear translocation but decreases BRCA1 mRNA level
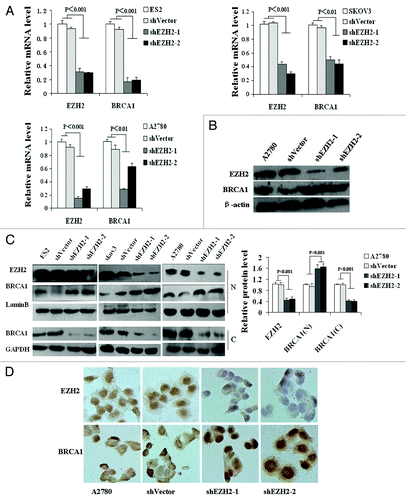
To test whether EZH2 modulates BRCA1 gene expression in epithelial ovarian cancer, EZH2 expression was downregulated in ovarian cancer cell lines using either stable (in A2780) or transient (in ES2 and SKOV3) transfection of shEZH2 and the responses of BRCA1 mRNA and protein levels to loss of EZH2 were assessed. Unexpectedly, the cells transfected with the shEZH2 showed significant decrease in BRCA1 mRNA level as revealed by qRT-PCR (, P < 0.01) but apparent increase in protein level as revealed by western blotting (). Of note, the expression of BRCA1 protein in nucleus was elevated, whereas cytoplasmic protein was decreased (, P < 0.001), suggesting the depletion of EZH2 resulted in nuclear translocation of BRCA1 protein. This result was further supported by immunocytochemistry analyses ().
Figure 1. Inhibition of EZH2 decreases BRCA1 mRNA level but increases BRCA1 protein level. (A) The mRNA level of BRCA1 was decreased in shEZH2 transfected cells compared with untransfected ES2, SKOV3, and A2780 cells. (B) Immunoblot assays revealed that total protein expression of BRCA1 was increased in stable transfected shEZH2-A2780 cells. (C) Left, loss of EZH2 increased BRCA1 nuclear protein level, but decreased BRCA1 cytoplasmic protein level. N and C represent nuclear and cytoplasmic protein respectively. Right, quantization of western blot. (D) Immunocytochemistry analysis shown that stable inhibition of EZH2 in A2780 cells increased BRCA1 protein expression and induced BRCA1 nuclear localization (×400).
Loss of BRCA1 partially rescues the biological effects of EZH2 downregulation in epithelial ovarian cancer in vitro
Three siRNAs (siBRCA1-1, siBRCA1-2, and siBRCA1-3) were transfected in A2780 cells to knock down BRCA1 expression. QRT-PCR and western blotting revealed dramatic decreases (approximately 80%) in BRCA1 expression in cells transfected with siBRCA1-2 and -3 (, P < 0.001).
Figure 2. Depletion of BRCA1 partially rescues the biological effects of EZH2 inhibition in ovarian cancer. (A and B) The efficiencies of siBRCA1s silencing in A2780 cells were detected at mRNA (A) and protein (B) levels by qRT-PCR and western blotting respectively. (C) The shEZH2-mediated BRCA1 overexpression was abolished by cotransfection of siBRCA1-2 or -3. (D) Quantization of western blot. (E and F) Cell proliferation and cell cycle analyses were detected by MTT assay and flow cytometry respectively; showing that cotransfection of siBRCA1 partly rescued the reduction in cell proliferation and depressed the elevated percentage of G2/M phase caused by shEZH2 transfection in A2780 and ES2 cells. (G) Transwell migration assays revealed that inhibition of BRCA1 inversed the suppression in cell migration in A2780 and ES2 cells with shEZH2 transfection (×200).
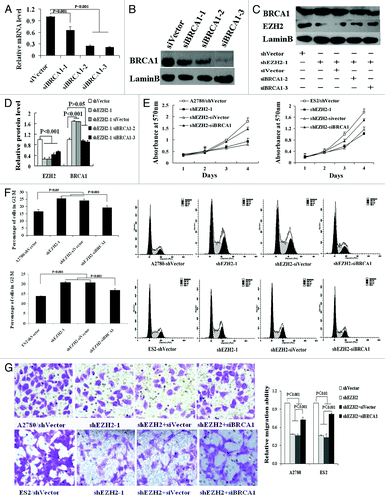
To ascertain whether the effects caused by EZH2 in ovarian cancer cells require BRCA1 protein, we treated A2780 and ES2 cells with shEZH2 alone or in combination with siBRCA1. As shown in , shEZH2 depleted EZH2 and increased nuclear BRCA1 protein level and the shEZH2-mediated BRCA1 overexpression could be abolished by cotransfection of siBRCA1. Then siBRCA1-3 transfection was performed in the following studies. Functionally, siBRCA1 partially rescued the inhibition of cell proliferation and migration induced by depletion of EZH2. Survival curves based on the results of MTT assays showed the survival rates of shEZH2-A2780 and shEZH2-siBRCA1-A2780 cells were reduced by 57.8% and 19.6% 96 h after transfection, respectively. Similar results were observed in transfected ES2 cells (, P < 0.01). Moreover, the G2/M cell-cycle arrests in shEZH2-A2780 (25.56%) and shEZH2-ES2 (20.73%) cells were remitted in those cotransfected with siBRCA1 (19.28% and 16.88% respectively, , P < 0.01). Additionally, in vitro migration assays revealed that the inhibition of cell migration by EZH2 depletion could be partially relieved by BRCA1 downregulation (, P < 0.01). These results suggest that the effects of EZH2 on ovarian cancer cell proliferation and migration require BRCA1.
EZH2-induced BRCA1 nuclear/cytoplasmic shuttling necessitates the activation of Akt-1
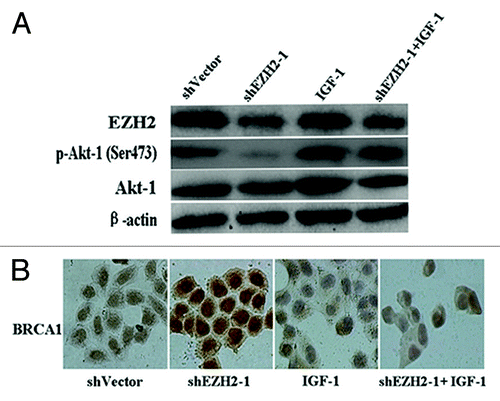
Western blot analyses shown that inhibition of EZH2 in A2780 cells decreased the level of Akt-1 phosphorylated at Ser473 (p-Akt-1[Ser473]), which was required to promote its maximal activation. While insulin-like growth factor-1 (IGF-1) treatment of A2780 cells increased p-Akt-1(Ser473) level, and IGF-1 treatment of shEZH2-A2780 abolished p-Akt-1(Ser473) decrease mediated by depletion of EZH2 (, P < 0.01). Expectedly, as shown by immunocytochemistry analysis, activation of Akt-1 inverted EZH2-mediated BRCA1 nuclear/cytoplasmic shuttling ().
Figure 3. EZH2-induced BRCA1 nuclear/cytoplasmic shuttling requires the activation of Akt-1. (A) Western blot analyses showed that the protein level of p-Akt-1(Ser473) was decreased by downregulation of EZH2 and inceased by IGF-1 treatment. (B) Immunocytochemistry analyses showed that activation of Akt-1 inversed EZH2-mediated BRCA1 nuclear/cytoplasmic shuttling (×400).
Interaction of EZH2 and BRCA1 in ovarian cancer cells with acquired cisplatin resistance
Although BRCA1 is downregulated in most ovarian cancers, its upregulation is associated with repair-mediated resistance to cisplatin.Citation24 Therefore, we were interested in whether the regulation of BRCA1 expression by EZH2 in cisplatin-resistant tumor cells is different from that in sensitive cells. Compared with parental A2780 cells, the A2780 cells with acquired cisplatin resistance (A2780/DDP) expressed significantly more EZH2 and BRCA1. As shown in , EZH2 and BRCA1 mRNA expression were increased 3.2- and 4.8-fold, respectively, and protein expression were increased 2.1- and 2.5-fold, respectively (P < 0.001).
Figure 4. Interaction of EZH2 and BRCA1 in acquired cisplatin-resistant ovarian cancer cells. (A and B) The elevated mRNA (A) and protein (B) expression of EZH2 and BRCA1 were detected by qRT-PCR and western blotting respectively. (C) QRT-PCR shown the mRNA levels of EZH2 and BRCA1 in A2780/DDP cells with shEZH2 and siBRCA1 transfection alone or simultaneously. (D) Left, western blotting shown the protein expression of EZH2 and BRCA1 in cells with shEZH2 and siBRCA1 transfection independently or simultaneously. Right, quantization of western blot. (E) Following cisplatin treatment, MTT assays assessed the survival rates of cells with different expression of EZH2 and BRCA1.
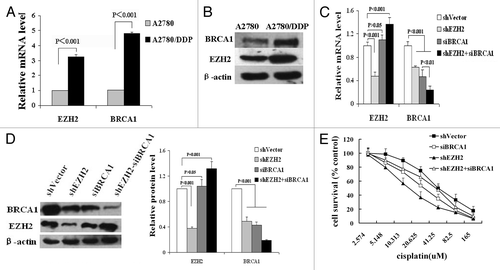
Transfection of A2780/DDP cells with shEZH2 resulted in a 37.3% decrease in BRCA1 mRNA expression. Nevertheless, different from that in parental A2780, the protein level of BRCA1 was also reduced (51%, P < 0.001). Transfection with siBRCA1-3 decreased mRNA (approximately 54%) and protein (about 57%) expression of BRCA1 and lead to a slightly rise in EZH2 expression which did not meet statistical significance (P = 0.544). Interestingly, the transcription of EZH2 was reactivated (about 1.32-fold increased compared with shVector transfected A2780/DDP, P = 0.001) in A2780/DDP cells as BRCA1 protein expression was further suppressed (approximately 81.3%) through cotransfection with shEZH2 and siBRCA1 ().
The effect of manipulating expression of EZH2 and BRCA1 on cell response to cisplatin was assessed by MTT assays. As shown in , A2780/DDP cells transfected with shEZH2 exhibited the highest sensitivity to cisplatin treatment with a 60% decrease in the 50% inhibitory concentration (IC50) of cisplatin when compared with control. Transfection with siBRCA1 could also increase the cytotoxicity of cisplatin in A2780/DDP significantly (IC50 decreased 31%, P < 0.01). However, shEZH2 and siBRCA1 cotransfection resensitized A2780/DDP slightly instead of showing any synergy. The IC50 of cisplatin for cells in this group was only decreased 14% compared with control, which might be due to the elevated EZH2 level.
Discussion
In this study, we found that inhibition of EZH2 in ovarian cancer cells increased BRCA1 protein expression with an adverse decrease in BRCA1 mRNA expression. It is not surprising given previous studies showing that the expression of mRNA and protein was discordant. And one key reason for this may be the processing of the protein or posttranslational mechanism.Citation25,Citation26 Additionally, our data shown that EZH2 depletion could induce BRCA1 to localize into nucleus, the place where BRCA1 protein located predominantly and could be activated,Citation19 suggesting that depletion of EZH2 can reactivate the function of BRCA1 in epithelial ovarian cancer. Given that EZH2 regulates gene expression negatively through epigenetic mechanism, the decrease in BRCA1 mRNA reduced by EZH2 depletion indicates BRCA1 is likely regulated by EZH2 via an indirect pathway. Researchers have pointed out that Akt activation could induce the cytoplasmic retention of tumor suppressor proteins including FOXO3a, p21 Cip1/WAF1, and BRCA1.Citation21,Citation27,Citation28 Additionally, EZH2 could regulate Akt-1-dependent BRCA1 expression in breast cancer cells.Citation23 By using IGF-1 treatment, we revealed that the effect of EZH2 on BRCA1 intracellular localization also required the activation of Akt-1 in epithelial ovarian cancer cells.
Previously published works revealed that downregulation of EZH2 inhibited cell proliferation, migration, and induced G2/M cell cycle arrest in ovarian cancer.Citation17,Citation18 Inversely, loss of BRCA1 resulted in an increase in cell proliferation and a transition of cell cycle,Citation29,Citation30 and contributed to breast cancer cell migration by affecting its main regulators, such as caveolin-1.Citation31,Citation32 Our data in the present study revealed that the above-mentioned effects of EZH2 downregulation require the expression of BRCA1, as inhibition of BRCA1 partly rescued the decrease in cell proliferation, migration, and the delay in G2/M transition caused by EZH2 depletion. Consistently, preceding studies reported that EZH2 could modulate BRCA1-mediated cell proliferation in breast cancer.Citation14,Citation22,Citation33 Meanwhile, we found the effects of EZH2 downregulation were not completely blocked by BRCA1 inhibition, which may be due to the incomplete knockdown of BRCA1 expression and/or the existence of other mechanisms underlying. As reported previously, a set of genes associated with cell proliferation (such as RUNX3 and CDKN1C)Citation11,Citation12 and cell migration (such as FOXC1 and CDH1)Citation34,Citation35 were regulated by EZH2 through a direct or indirect pathway.
In a previous study, we found that the overexpression of EZH2 was associated with resistance to cisplatin in ovarian cancer cells via promoting H3K27 tri-methylation, which can affect expression of drug resistance-related genes.Citation18 Although BRCA1 is downregulated in most cancers, its upregulation is associated with repair-mediated resistance to cisplatin. The epithelial ovarian cancer cell line SKOV3 undergoing cisplatin-induced DNA damage expressed more BRCA1 to enhance DNA repair and suppression of BRCA1 in SKOV3/DDP cells increased the cytotoxicity of cisplatin through reducing the proficiency of DNA damage repair and promoting cisplatin-induced apoptosis.Citation24 In the present study, in line with previous reports, we found an increased expression of EZH2 and BRCA1 in acquired cisplatin-resistant ovarian cancer cell line A2780/DDP and separate inhibition of them resensitized A2780/DDP cells to cisplatin to varying degree. The reactivation of EZH2 expression was observed in cells cotransfected with shEZH2 and siBRCA1 where BRCA1 expression level was extremely low. Puppe et al. reported that BRCA1-deficiency could elevate the expression of EZH2 through upstream regulators in breast tumor cells.Citation33 EZH2 overexpression can impair chromosome stability, e.g., through decreasing expression of five RAD51 paralogous proteins involved in homologous recombination repair of DNA double-strand breaks,Citation36,Citation37 attenuating the effect of BRCA1 knockdown on cell sensibility to cisplatin.
In conclusion, our results suggest the expression of BRCA1 is modulated by EZH2 in epithelial ovarian cancer and is required for the effects of EZH2 on proliferation, migration, and response to cisplatin of tumor cells.
Materials and Methods
Cell culture
Three human epithelial ovarian cancer cell lines, A2780, SKOV3, and ES2 were purchased from China Center for Type Culture Collection (CCTCC). SKOV3 and A2780 are ovarian adenocarcinoma cell lines, obtained from malignant ascites and tumor tissue of untreated patients with ovarian cancer respectively. ES2 cell line was established from a surgical tumor specimen of a patient with poorly differentiated ovarian clear cell carcinoma. Cisplatin-resistant A2780 (A2780/DDP) was established by our laboratory as previously described.Citation18 All cell lines were maintained at 37 °C in RPMI-1640 medium (Gibco) with 10% fetal calf serum (Gibco) under 5% CO2 atmosphere. To active Akt, IGF-1(100 ng/ml, Peprotech) was given to incubate A2780 cells for one hour following a serum starvation overnight.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cultured cells by Trizol reagent (Invitrogen) according to the manufacturer’s protocol. Before cDNA was synthesized using a reverse transcription kit (Toyobo), quality and quantity of total RNA were evaluated by NanoDrop 2000/2000C (Thermo Scientific). The sequences of primers were as follows: BRCA1 upstream 5′-GAATAGGCTG AGGAGGAAGT-3′ and downstream 5′-GGAAAGTATC GCTGTCATGT C-3′, EZH2 upstream 5′-TTGTTGGCGG AAGCGTGTAA AATC-3′ and downstream 5′-TCCCTAGTCC CGCGCAATGA GC-3′. Expression of both GAPDH and β-actin were used for normalization. PCR reactions were performed on an Applied Biosystems StepOnePlus Real-time PCR system (Applied Biosystems), using SYBR Green Real-time PCR Master Mix (Toyobo). Before the PCR experiments, standard curves were made to make sure that the amplification efficiencies of the four primers were approximately 100%. The reactions for BRCA1, β-actin, and GAPDH were performed by two-step thermal cycling method: 95 °C for 1 min, 40 PCR cycles at 95 °C for 15 s, 60 °C for 1 min, followed by a melting curve. The reaction condition for EZH2 was similar but the annealing temperature was 57 °C. The results were calculated using the Ct (2−ΔΔCt) method.Citation38 Each reaction was performed in triplicate.
Western blotting
Cells were collected in cold radioimmune precipitation assay (RIPA) buffer including 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitory cocktail for 30 min, centrifuged at 13 000 g for 10 min, and the supernatants were collected. Nuclear and cytoplasmic proteins were respectively isolated with a nuclear and cytoplasmic protein exacted kit (KeyGEN Biotech) according to the instructions. Protein concentration was measured by bicinchoninic acid (BCA) assay. Samples were separated by 8% sodium dodecyl sulfate-PAGE (SDS-PAGE) and transferred onto a PVDF membrane. The membranes were blocked with 5% nonfat milk in Tris-buffered saline with Tween-20 (TBST) for 1 h at room temperature, incubated with rabbit anti-EZH2 (1:500; Invitrogen), rabbit anti-BRCA1 (1:2000; Millipore), rabbit anti-Akt1 (1:1000 dilution, Cell Signaling Technology Inc.), or anti-P-Akt1 S473 (1:1000 dilution, Cell Signaling Technology Inc.) at 4 °C overnight. Rabbit anti-β-actin (1:1000; Epitomics, Inc.), rabbit anti-GAPDH (1:1000; Epitomics, Inc.), and goat anti-LaminB (1:200; Santa Cruz Biotechnology) were used to normalize total, cytoplasmic, and nuclear protein respectively. Horseradish peroxidase-conjugated anti-rabbit or anti-goat secondary antibodies (1:5000; Santa Cruz Biotechnology) were used for detection as appropriate. Samples were visualized by an enhanced chemiluminescence kit (Pierce) and gray values of bands were quantified by Quantity One Software (Bio-Rad) or Molecular Imager® ChemiDocTM XRS+ with Image LabTM Software (Bio-Rad).
Immunocytochemistry
Cells were seeded on coverslips in triplicate, fixed with 4% paraformaldehyde for 30 min at room temperature. 0.1% Trixon-100 and 0.3% H2O2 were used to break cell membrane and inhibit endogenous peroxidase activity respectively. Then the coverslips were blocked with 5% BSA at room temperature for 30 min and incubated with rabbit anti-EZH2 (1:100) or rabbit anti-BRCA1 (1:300) at 4 °C overnight, followed by incubation with biotinylated goat anti-rabbit secondary antibody (1:100) for 1 h. Immunostaining was performed with diaminobenzidine (DAB), using the streptavidin–biotin complex/horseradish peroxidase (sABC-HRP) method. Positive expression was defined as brown-yellow granules distributed in the cytoplasm or nucleus, with stain intensity higher than the nonspecific background.
Stable transfection of EZH2 shRNA
Two short hairpin RNAs targeting EZH2 (shEZH2-1, 5′-GCAACACCCA ACACTTATAA G-3′; shEZH2-2, 5′-GGCTCCTCTA ACCATGTTTA C-3′) and control shRNA (5′-GTTCTCCGAA CGTGTCACGT-3′) were synthesized by GenePharma. Twenty-four hours before transfection, A2780 cells were seeded in a 24-well plate at a concentration of 1.5 × 105 cells per well. Transfection was performed using LipofectamineTM 2000 (Invitrogen) according to the instructions. Cells were passaged at a 1:10 dilution into fresh medium 24 h after transfection. Three hundred micrograms per milliliter G418 was added into the medium to kill untransfected cells in the following days for 3 wk. To verify the interferential efficiency, mRNA and protein of cells were extracted for qRT-PCR and western blot analysis as described above.
Transient transfection of EZH2 shRNA and BRCA1 siRNA
Three different 21-nucleotides duplex small interfere RNAs for BRCA1 (siBRCA1 ID 2167, 2806, and 3207) and a control siRNA were synthesized by GenePharma. The three siBRCA1s were abbreviated as siBRCA1-1, siBRCA1-2, and siBRCA1-3 respectively. Cells were seeded in 6-well plates at a concentration of 5 × 105 cells per well. Transient transfections of shEZH2, shVector, siBRCA1, and siVector were performed using Lipofectamine 2000 according to the introduction. At suitable time points after transfection, cells were harvested and subjected to following analysis.
Cell proliferation assay
Cells were seeded in five copies at a density of 2000 cells per well in 96-well plates. At indicated times, 20 µl of 5 mg/ml 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to incubate cells for 4 h. After this period, cells were dissolved in 150 µl dimethyl sulfoxide (DMSO) for 10 min. The absorbance at 570 nm was measured by a microplate reader (Bio-Rad). Wells without cells were used as blank.
Cell cycle analysis
Cell cycle was assessed by using a LSR II flow cytometer (Becton Dickinson) following propidium iodide (PI, Antgene) staining according to the manufacturer’s instructions. The result was analyzed by ModFit LT software (Verity Software House).
Cell migration assay
Cell migration ability was evaluated by transwell assay. Cells were suspended in serum free medium and adjusted to 1 × 106/ml. The transwell inserts (8 µm pore size, 6.5 mm diameter with polycarbonate membrane; Corning Costar) were added with 100 µL cell suspensions, and the lower chambers were added with 600 µL RPMI 1640 medium containing 10% FBS. Transwell assays were terminated when about 10 cells were observed in lower chambers. Migrated cells on the lower surface of the membrane were fixed with methanol followed by staining with 0.1% Crystal Violet and subsequently observed under microscopy. Cells in three random fields per well were counted (×200).
Drug cytotoxicity assay
MTT assays were performed to evaluate cell response to cisplatin in ovarian cancer cells.Citation39 Cells were treated with cisplatin at concentrations ranging from 2.5 to 165 µM. The assays were performed following procedure described in a previous study.Citation18
Statistical analysis
All statistical analysis was performed using SPSS 13.0 statistic software (SPSS). Numerical datas were expressed as mean ± standard deviation. Differences between two and multiple groups were analyzed by independent samples t test and one-way analysis of variance (ANOVA) respectively. Difference with P < 0.05 was considered statistically significant.
| Abbreviations: | ||
| EZH2 | = | enhancer of zeste homolog 2 |
| BRCA1 | = | breast cancer 1 |
| PRC2/3 | = | polycomb repressive complex 2/3 |
| IGF-1 | = | insulin-like growth factor-1 |
| p-Akt-1(Ser473) | = | Akt-1 phosphorylated at Ser473 |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by JX5A07 from the key Project of Health Department of Hubei Province.
References
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61:212 - 36; http://dx.doi.org/10.3322/caac.20121; PMID: 21685461
- Moss C, Kaye SB. Ovarian cancer: progress and continuing controversies in management. Eur J Cancer 2002; 38:1701 - 7; http://dx.doi.org/10.1016/S0959-8049(02)00161-2; PMID: 12175685
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011; 61:183 - 203; http://dx.doi.org/10.3322/caac.20113; PMID: 21521830
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007; 25:1329 - 33; http://dx.doi.org/10.1200/JCO.2006.09.1066; PMID: 17416853
- Khoo US, Chan KY, Cheung AN, Xue WC, Shen DH, Fung KY, Ngan HY, Choy KW, Pang CP, Poon CS, et al. Recurrent BRCA1 and BRCA2 germline mutations in ovarian cancer: a founder mutation of BRCA1 identified in the Chinese population. Hum Mutat 2002; 19:307 - 8; http://dx.doi.org/10.1002/humu.9015; PMID: 11857749
- Russell PA, Pharoah PD, De Foy K, Ramus SJ, Symmonds I, Wilson A, Scott I, Ponder BA, Gayther SA. Frequent loss of BRCA1 mRNA and protein expression in sporadic ovarian cancers. Int J Cancer 2000; 87:317 - 21; http://dx.doi.org/10.1002/1097-0215(20000801)87:3<317::AID-IJC2>3.0.CO;2-B; PMID: 10897034
- Kuzmichev A, Jenuwein T, Tempst P, Reinberg D. Different EZH2-containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol Cell 2004; 14:183 - 93; http://dx.doi.org/10.1016/S1097-2765(04)00185-6; PMID: 15099518
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 2002; 111:185 - 96; http://dx.doi.org/10.1016/S0092-8674(02)00975-3; PMID: 12408863
- Müller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O’Connor MB, Kingston RE, Simon JA. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 2002; 111:197 - 208; http://dx.doi.org/10.1016/S0092-8674(02)00976-5; PMID: 12408864
- Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006; 439:871 - 4; http://dx.doi.org/10.1038/nature04431; PMID: 16357870
- Yang X, Karuturi RK, Sun F, Aau M, Yu K, Shao R, Miller LD, Tan PB, Yu Q. CDKN1C (p57) is a direct target of EZH2 and suppressed by multiple epigenetic mechanisms in breast cancer cells. PLoS One 2009; 4:e5011; http://dx.doi.org/10.1371/journal.pone.0005011; PMID: 19340297
- Fujii S, Ito K, Ito Y, Ochiai A. Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation. J Biol Chem 2008; 283:17324 - 32; http://dx.doi.org/10.1074/jbc.M800224200; PMID: 18430739
- Taniguchi H, Jacinto FV, Villanueva A, Fernandez AF, Yamamoto H, Carmona FJ, Puertas S, Marquez VE, Shinomura Y, Imai K, et al. Silencing of Kruppel-like factor 2 by the histone methyltransferase EZH2 in human cancer. Oncogene 2012; 31:1988 - 94; http://dx.doi.org/10.1038/onc.2011.387; PMID: 21892211
- Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 2003; 100:11606 - 11; http://dx.doi.org/10.1073/pnas.1933744100; PMID: 14500907
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419:624 - 9; http://dx.doi.org/10.1038/nature01075; PMID: 12374981
- Weikert S, Christoph F, Köllermann J, Müller M, Schrader M, Miller K, Krause H. Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med 2005; 16:349 - 53; PMID: 16012774
- Guo J, Cai J, Yu L, Tang H, Chen C, Wang Z. EZH2 regulates expression of p57 and contributes to progression of ovarian cancer in vitro and in vivo. Cancer Sci 2011; 102:530 - 9; http://dx.doi.org/10.1111/j.1349-7006.2010.01836.x; PMID: 21205084
- Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J, Xiao L, Wang Z. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol Ther 2010; 10:788 - 95; http://dx.doi.org/10.4161/cbt.10.8.12913; PMID: 20686362
- Scully R, Ganesan S, Brown M, De Caprio JA, Cannistra SA, Feunteun J, Schnitt S, Livingston DM. Location of BRCA1 in human breast and ovarian cancer cells. Science 1996; 272:123 - 6; http://dx.doi.org/10.1126/science.272.5258.123; PMID: 8600523
- Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, Karlan BY. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res 2000; 60:5329 - 33; PMID: 11034065
- Plo I, Laulier C, Gauthier L, Lebrun F, Calvo F, Lopez BS. AKT1 inhibits homologous recombination by inducing cytoplasmic retention of BRCA1 and RAD51. Cancer Res 2008; 68:9404 - 12; http://dx.doi.org/10.1158/0008-5472.CAN-08-0861; PMID: 19010915
- Gonzalez ME, Li X, Toy K, DuPrie M, Ventura AC, Banerjee M, Ljungman M, Merajver SD, Kleer CG. Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene 2009; 28:843 - 53; http://dx.doi.org/10.1038/onc.2008.433; PMID: 19079346
- Gonzalez ME, DuPrie ML, Krueger H, Merajver SD, Ventura AC, Toy KA, Kleer CG. Histone methyltransferase EZH2 induces Akt-dependent genomic instability and BRCA1 inhibition in breast cancer. Cancer Res 2011; 71:2360 - 70; http://dx.doi.org/10.1158/0008-5472.CAN-10-1933; PMID: 21406404
- Husain A, He G, Venkatraman ES, Spriggs DR. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II). Cancer Res 1998; 58:1120 - 3; PMID: 9515792
- Chen G, Gharib TG, Huang CC, Taylor JMG, Misek DE, Kardia SLR, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 2002; 1:304 - 13; http://dx.doi.org/10.1074/mcp.M200008-MCP200; PMID: 12096112
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 1999; 19:1720 - 30; PMID: 10022859
- Yang J-Y, Hung M-C. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res 2009; 15:752 - 7; http://dx.doi.org/10.1158/1078-0432.CCR-08-0124; PMID: 19188143
- Zhou BP, Liao Y, Xia W, Spohn B, Lee M-H, Hung M-C. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 2001; 3:245 - 52; http://dx.doi.org/10.1038/35060032; PMID: 11231573
- Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 1999; 286:1162 - 6; http://dx.doi.org/10.1126/science.286.5442.1162; PMID: 10550055
- Xu B, Kim St, Kastan MB. Involvement of Brca1 in S-phase and G(2)-phase checkpoints after ionizing irradiation. Mol Cell Biol 2001; 21:3445 - 50; http://dx.doi.org/10.1128/MCB.21.10.3445-3450.2001; PMID: 11313470
- Yasmeen A, Liu W, Dekhil H, Kassab A, Aloyz R, Foulkes WD, Al Moustafa AE. BRCA1 mutations contribute to cell motility and invasion by affecting its main regulators. Cell Cycle 2008; 7:3781 - 3; http://dx.doi.org/10.4161/cc.7.23.6993; PMID: 19098453
- Wang Y, Yu J, Zhan Q. BRCA1 regulates caveolin-1 expression and inhibits cell invasiveness. Biochem Biophys Res Commun 2008; 370:201 - 6; http://dx.doi.org/10.1016/j.bbrc.2008.03.031; PMID: 18343216
- Puppe J, Drost R, Liu X, Joosse SA, Evers B, Cornelissen-Steijger P, Nederlof P, Yu Q, Jonkers J, van Lohuizen M, et al. BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb Repressive Complex 2-inhibitor 3-deazaneplanocin A. Breast Cancer Res 2009; 11:R63; http://dx.doi.org/10.1186/bcr2354; PMID: 19709408
- Du J, Li L, Ou Z, Kong C, Zhang Y, Dong Z, Zhu S, Jiang H, Shao Z, Huang B, et al. FOXC1, a target of polycomb, inhibits metastasis of breast cancer cells. Breast Cancer Res Treat 2012; 131:65 - 73; http://dx.doi.org/10.1007/s10549-011-1396-3; PMID: 21465172
- Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani R-S, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008; 27:7274 - 84; http://dx.doi.org/10.1038/onc.2008.333; PMID: 18806826
- Zeidler M, Varambally S, Cao Q, Chinnaiyan AM, Ferguson DO, Merajver SD, Kleer CG. The Polycomb group protein EZH2 impairs DNA repair in breast epithelial cells. Neoplasia 2005; 7:1011 - 9; http://dx.doi.org/10.1593/neo.05472; PMID: 16331887
- Baumann P, Benson FE, West SC. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell 1996; 87:757 - 66; http://dx.doi.org/10.1016/S0092-8674(00)81394-X; PMID: 8929543
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 1987; 47:936 - 42; PMID: 3802100
