Abstract
Prognostic factor analysis has been conducted to determine whether the parameters of clinical data and biomarkers would predict differential progression-free survival (PFS) or overall survival (OS) from lapatinib-based therapy in patients with primary or acquired resistance to trastuzumab. Treatment with lapatinib plus capecitabine for HER2-positive metastatic breast cancer (MBC) with primary or acquired resistance to trastuzumab was analyzed retrospectively. Tumor biomarkers, which came from the biopsies before the starting of lapatinib therapy, were evaluated by immunohistochemistry (IHC). Prognostic factors related to PFS or OS of the lapatinib therapy were assessed by univariate and multivariate analysis. Ki-67 index and liver metastases were the significant prognostic factors for predicting PFS of subsequent lapatinib therapy in the univariate analysis and the multivariate analysis. The risk for disease progression in patients who had a Ki-67 index < 40% was 59% less than that in patients had Ki-67 ≥ 40 (HR = 0.41, 95% CI, 0.23–0.74, P = 0.003). TTP of prior trastuzumab therapy, liver metastases, and the number of metastatic sites were three independent prognostic factors of subsequent lapatinib therapy. Ki-67 index was the significant prognostic factors for predicting PFS of the subsequent second line targeted therapy in patients with trastuzumab resistance.
Introduction
Approximately 25–30% of invasive breast cancers overexpress the human epidermal growth factor receptor-2 (HER2), which is associated with increased risk of recurrence, and poor prognosis.Citation1 Trastuzumab (Herceptin), a monoclonal antibody against HER2, has been found significantly to improve PFS and OS of patients with HER2-positive advanced breast cancer in clinical practice,Citation2-Citation4 so a trastuzumab-based regimen has become a standard therapy for patients with HER2-positive breast cancer. However, almost all of these patients have diseases that develop resistance to trastuzumab alone or trastuzumab in combination with chemotherapy. As shown in two early studies of taxane plus trastuzumab therapy (H0648 g and M77001),Citation2,Citation3 the overall response rate (ORR) of MBC to trastuzumab in combination with taxanes as the first line therapy was only 50–61%. In addition, with prolonged therapy, the disease progressed in patients initially responsive to trastuzumab. It has been proven that patients with resistance to trastuzumab-based therapy still require anti-HER2 therapy for effective control of the disease. Lapatinib, a small-molecule tyrosine kinase inhibitor (TKI) targeting HER1 (EGFR, epidermal growth factor receptor) and HER2, has a different mechanism of action from trastuzumab. In the present study, we analyzed prognostic factors in patients receiving lapatinib-based regimen to treat HER2-positive MBC with resistance to trastuzumab.
Results
The characteristics of the patients
From July 2008 to July 2012, 56 patients who were treated with lapatinib plus capecitabine therapy at our institution were enrolled. The characteristics of the 56 patients with resistance to trastuzumab were shown in . The median age of all patients included in the study was 49 y. Twenty-five patients (45%) had hormone receptor positive disease. Forty-nine patients (87%) had visceral metastases. Twenty-six patients (47%) had ≥3 metastatic sites. All patients had been treated with anthracyclines and taxanes, and all patients had received trastuzumab therapy for metastatic disease until disease progression. Thirty-seven patients (66%) had been exposed to prior capecitabine therapy. Twenty-seven patients (48%) had ≥3 lines of chemotherapy for metastatic disease. The median TTP of trastuzumab therapy was 5.3 mo. Twenty patients (36%) had TTP of trastuzumab therapy ≤3 mo (4 cycles), 9 patients (16%) had TTP of 3–6 mo (4–8 cycles) and 27 patients (48%) had TTP ≥6 mo (8 cycles). The histological specimen of metastatic sites from 56 patients with trastuzumab resistance were obtained and analyzed about the biomarkers before starting the treatment of lapatinib: PTEN loss in 95% patients, EGFR-positive in 29% patients, and p53-positive in 45% patients. The median of Ki-67 index is 35%. The low-intermediate of Ki-67 index (<40%) in 57% patients and high (≥40%) in 43% patients. The median duration of lapatinib plus capecitabine treatment is 4.7 mo (95% CI 4.2, 5.3).
Table 1. The characteristics of the patients with resistance to trastuzumab (N = 56)
Univariate analysis for the prognostic factors of lapatinib therapy
The PFSs in all 56 patients were available, and the OSs in 40 patients were available until the date of analysis. The present study included 14 influence factors of lapatinib plus capecitabine therapy. Univariate analysis showed that Ki-67 index <40% and without liver metastases were significantly associated with longer median PFS (P = 0.008, P = 0.01 respectively); TTP of trastuzumab therapy >3 mo (4 cycles), without liver metastases and number of metastatic sites <3 were significantly associated with longer median OS (P = 0.005, P = 0.006, P = 0.0006, respectively) ().
Table 2. Univariate analysis for the prognostic factors of lapatinib therapy in the patients with resistance to trastuzumab (N = 56)
Multivariate analysis for the prognostic factors of lapatinib therapy
All of the factors listed in were fed into a Cox regression model for multivariate analysis. The analysis revealed that Ki-67 index and liver metastases were the significant prognostic factors for predicting PFS of lapatinib therapy (). This observation suggests that the risk for disease progression of lapatinib therapy in patients who had Ki-67 index <40% was 59% less than the risk in patients who had Ki-67 ≥40 (HR = 0.41, 95% CI, 0.23–0.74, P = 0.003) ();the risk for disease progression of lapatinib therapy in patients without liver metastases was 43% less than the risk in patients with liver metastases (HR = 0.57, 95% CI, 0.33–0.98, P = 0.04). Multivariate analysis for OS revealed that TTP of trastuzumab therapy, liver metastases, and the number of metastatic sites were three independent prognostic factors of lapatinib therapy in patients with trastuzumab resistance (). This observation suggests that the risk of death for patients who had TTP of trastuzumab therapy >3 mo (4 cycles) was 54% less than the risk for patients who had TTP of trastuzumab therapy ≤3 mo (4 cycles) (HR = 0.46, 95% CI, 0.28–0.76, P = 0.002); the risk of death for patients without liver metastases was 50% less than the risk for patients with liver metastases(HR = 0.5, 95% CI, 0.3–0.81, P = 0.006); the risk of death for patients had the number of metastatic sites <3 was 45% less than the risk for patients had the number of metastatic sites ≥3 (HR = 0.55, 95% CI, 0.34–0.88, P = 0.014).
Table 3. Multivariate analysis for the prognostic factors of PFS of lapatinib therapy in the patients with resistance to trastuzumab (N = 56)
Figure 1. Kaplan–Meier estimates for PFS of lapatinib plus capecitabine therapy in patients with trastuzumab resistance. PFS was significantly longer in patients who had a Ki-67 index <40% than in patients who had Ki-67 ≥40% (HR = 0.41, 95% CI, 0.23–0.74, P = 0.003 by Cox regression model).
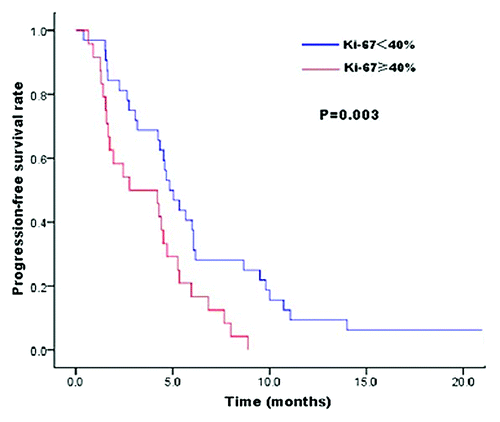
Table 4. Multivariate analysis for the prognostic factors of OS of lapatinib therapy in the patients with resistance to trastuzumab (N = 56)
Discussion
Lapatinib, a small-molecule dual tyrosine kinase inhibitor (TKI) of both HER2 and EGFR (HER1), can bind to the intercellular ATP-binding domain of both receptors and inhibit receptor auto-phosphorylation. Therefore, lapatinib prevents receptor activation and leads to the blocking of downstream signal transduction, such as MAPK or PI3K/Akt pathway, which is responsible for modulation of tumor cell proliferation and apoptosis.Citation5,Citation6 Lapatinib is active against trastuzumab-resistant cell lines in preclinical study;Citation7 furthermore, several studies demonstrated that treatment with lapatinib is effective in some patients with HER2-positive MBC resistant to trastuzumab-based therapy,Citation8-Citation11 which may be attributed to differences between the mechanism of trastuzumab and lapatinib in the modulation of cell signaling.Citation12 These data suggest that the clinical use of lapatinib is necessary for continued tumor suppression in HER2-positive MBC with trastuzumab resistance. Cameron reported that lapatinib may be more effective in patients who had received only one prior trastuzumab-containing regimen.Citation13 In spite of this, to date the analyses about the prognostic factors in patients receiving the second line targeted therapy (lapatinib) to treat HER2-positive MBC after resistance to the first line targeted therapy (trastuzumab) are insufficient. In the present study, prognostic factor analysis has been conducted to determine whether parameters of prior therapy or related biomarkers would predict differential PFS or OS from subsequent lapatinib in patients with primary or acquired resistance to trastuzumab.
The univariate analysis and the multivariate analysis relating to OS revealed that TTP of prior trastuzumab therapy, liver metastases and the number of metastatic sites were three independent prognostic factors of lapatinib therapy in patients with trastuzumab resistance.The risk of death for patients who had TTP of trastuzumab therapy >3 mo (4 cycles) was 54% less than the risk for patients who had TTP of trastuzumab therapy ≤3 mo (4 cycles) (HR = 0.46, 95% CI, 0.28–0.76, P = 0.002). The current data confirmed that a shorter TTP of prior trastuzumab therapy was associated with a shorter overall survival of subsequent lapatinib treatment, that is to say, the patients who had short TTP of prior trastuzumab therapy would have short overall survival from the total anti-HER2 therapy and poorer prognosis.
The proliferation biomarker Ki-67 is suggested to be a prognostic factor for breast cancer in a lot of studies.Citation14 The cut-off of Ki-67 in several survival analysis of different cancers was 40%. In anorectal malignant melanoma, with a cut-off point of 40%, patients with lower Ki-67 scores showed survival advantage over those with higher Ki-67 scores by multivariate analysis.Citation15 In rectal/recto sigmoid cancer, Ki-67 was divided into high (>40%) and low (≤40%) expression and high expression of Ki-67 was associated with better survival.Citation16 In breast cancer, the median Ki-67 index of HER2-positive tumors was 40%, and patients with a high Ki-67 index had significantly poor disease-free survival (DFS) and overall survival.Citation17 Thus, in our study, according to median Ki-67 value, we used 40% as the cut point of Ki-67.
The results of our study suggested that Ki-67 index and liver metastases were the significant prognostic factors for predicting PFS of lapatinib therapy in the univariate analysis and the multivariate analysis. The risk for disease progression of lapatinib therapy in patients who had a Ki-67 index <40% was 59% less than the risk in patients had Ki-67 ≥40 (HR = 0.41, 95% CI, 0.23–0.74, P = 0.003); thus, high Ki-67 index was correlated with poor prognosis in patients in lapatinib treatment. On the other hand, there were no significant correlations between patient survival and other biomarkers such as PTEN, EGFR, and p53 status in lapatinib therapy. The proliferative gene Ki-67, as a prognostic and predictive marker, was understood well recently in breast cancer. In endocrine therapy, changes in proliferation biomarker Ki-67 have been proposed as a surrogate marker of clinical activity.Citation18 Dowsett et al. draw the conclusion that Ki-67 as a marker of treatment benefit and predictor of long-term outcome and Ki-67 as a marker of acquired resistance of endocrine therapy.Citation19 Our study supported that the Ki-67 index was the significant prognostic factors for predicting PFS of lapatinib therapy and high Ki-67 index was correlated with poor prognosis.
In conclusion, in patients with trastuzumab resistant tumors, PFS was longer in patients with a low vs. high Ki-67 with lapatinib plus capecitabine treatment, which is a potential biomarker to indicating the benefit from changing to lapatinib therapy for HER2-positive MBC patients after occurring the resistance to trastuzumab therapy.
Patients and Methods
Study population
The major eligibility criteria included the following: female, aged 18–75 y; histologically confirmed metastatic and invasive breast cancer; presence of a HER2-positive tumor, defined as immunohistochemistry (IHC) test +++ or fluorescence in situ hybridization (FISH) test-positive (HER2/CEN-17 > 2.2) (Abbott Laboratories); has progressed after treatment with regimens that included an anthracycline, a taxane, and developed resistance to trastuzumab-based regimen, which was defined as “tumor progression of metastatic breast cancer occurred during trastuzumab treatment”; according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, has at least one measurable lesion; Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; the left ventricular ejection fraction assessed using echocardiography is equal to or larger than the lower limit of normal value; the key indicators for blood test, liver, and kidney function are in the normal range. The institutional review board of Affiliated Hospital of Academy of Military Medical Sciences approved the study protocol. All patients gave written informed consent.
Major ineligibility criteria included the following: presence of central nervous system metastases with symptoms (the patients whose central nervous system metastasis was stabilized after treatment were included); presence of gastrointestinal functional damage or disease that can significantly affect the absorption of orally administered lapatinib or capecitabine; has active heart disease or cardiac dysfunction; or has other types of malignant tumors.
Treatment and assessment
All patients received trastuzumab 8 mg/kg IV with first dose followed by trastuzumab 6 mg/kg IV every 21 d. All patients with resistance to trastuzumab received therapy consisting of lapatinib (1250 mg/d, oral) plus capecitabine (2000 mg/m2/d; oral; on days 1–14) in a 21-d cycle.
Before entering into the study,all patients received a baseline assessment, including a complete medical history and physical examination, chest CT, abdominal CT or MRI scan, bone scan, and other appropriate procedures were also performed as needed. Therapeutic efficacy was evaluated once every 6 wk (2 cycles of treatment) during the treatment according to the RECIST 1.1 criteria. Of the lesions observed prior to treatment, the short axis of two measurable lesions from each metastasized organ up to a total of five lesions was selected as target lesions. In cases of partial or complete response, a confirmative CT scan was performed 4 wk later, and this was followed by a CT scan after every two treatment cycles. Tumor-related symptoms were assessed at baseline and before each cycle. The efficacy criteria included complete remission (CR), partial remission (PR), stable disease (SD), and progression disease (PD). The study index included the PFS, defined as the time from initiation of study medication until the earliest date of disease progression or death from any cause; the OS, defined as the time from initiation of study medication until death due to any cause.
Biomarker analysis
Tumor samples from the patients’ metastatic cancer mass after progression of the trastuzumab therapy but before starting the lapatinib treatment were evaluated by the pathology department of our institution for expression of EGFR, PTEN, P53, and Ki-67 by IHC. The biopsy sites included liver (18, 32%), lung (3, 5%), lymph node (22, 40%), breast (7, 12.5%), soft tissues (4, 7%; chest wall, 3; cervical part, 1), brain (2, 3.5%).
The operation of IHC was in accordance with the standard procedures. Paraffin-embedded 4 um thick tissue sections were dewaxed and rehydrated. Slides were incubated with the primary antibodies for 30 min at room temperature. The four antigens were detected with the following primary antibodies: EGFR, PTEN, P53, and Ki-67 (Beijing Zhongshanjinqiao Biotech. Co. Ltd).Then the slides were incubated for 5 min in 3% H2O2 and were washed in TBS and incubated with the primary antibody diluted in TBS with 0.5% casein and 5% normal goat serum, for 60 min at room temperature. In the negative controls, an irrelevant primary antibody was used.
PTEN was scored semiquantitatively using the immunoreactive score (IRS), which was calculated by multiplying the percentage of PTEN-positive cells (scored 0–4) with the PTEN staining intensity (0–3). PTEN loss was defined as an IRS < 9.Citation20 Tumors were considered EGFR positive if the percentage of positive tumor cells was ≥1%. Only clear staining of the tumor cell membranes was scored positive; diffuse cytoplasmatic or granular staining was diagnosed as negative. p53-positive was indicated by the homogeneous and diffuse staining, or focal staining in >5% of tumor cells. Ki-67 index was determined to be low-intermediate if the proportion of cells was <40% and high if the proportion was ≥40% by counting at least 500 tumor cells.Citation21
Statistical analysis
Prognostic factors related to PFS or OS of the lapatinib plus capecitabine regimen were assessed in patients with resistance to trastuzumab. The log-rank test was used for univariate analysis, and a Cox regression model was employed for multivariate analysis. The Kaplan–Meier method was applied for delineation of the survival curve. A value of P < 0.05 was considered statistically significant. Statistical analyses were calculated with SPSS 16.0 statistical analysis software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235:177 - 82; http://dx.doi.org/10.1126/science.3798106; PMID: 3798106
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783 - 92; http://dx.doi.org/10.1056/NEJM200103153441101; PMID: 11248153
- Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005; 23:4265 - 74; http://dx.doi.org/10.1200/JCO.2005.04.173; PMID: 15911866
- Schaller G, Fuchs I, Gonsch T, Weber J, Kleine-Tebbe A, Klare P, Hindenburg HJ, Lakner V, Hinke A, Bangemann N. Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol 2007; 25:3246 - 50; http://dx.doi.org/10.1200/JCO.2006.09.6826; PMID: 17577021
- Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther 2008; 30:1426 - 47; http://dx.doi.org/10.1016/j.clinthera.2008.08.008; PMID: 18803986
- Tevaarwerk AJ, Kolesar JM. Lapatinib: a small-molecule inhibitor of epidermal growth factor receptor and human epidermal growth factor receptor-2 tyrosine kinases used in the treatment of breast cancer. Clin Ther 2009; 31:2332 - 48; http://dx.doi.org/10.1016/j.clinthera.2009.11.029; PMID: 20110044
- Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther 2007; 6:667 - 74; http://dx.doi.org/10.1158/1535-7163.MCT-06-0423; PMID: 17308062
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355:2733 - 43; http://dx.doi.org/10.1056/NEJMoa064320; PMID: 17192538
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008; 112:533 - 43; http://dx.doi.org/10.1007/s10549-007-9885-0; PMID: 18188694
- Capri G, Chang J, Chen SC, Conte P, Cwiertka K, Jerusalem G, Jiang Z, Johnston S, Kaufman B, Link J, et al. An open-label expanded access study of lapatinib and capecitabine in patients with HER2-overexpressing locally advanced or metastatic breast cancer. Ann Oncol 2010; 21:474 - 80; http://dx.doi.org/10.1093/annonc/mdp373; PMID: 19815649
- Xu BH, Jiang ZF, Chua D, Shao ZM, Luo RC, Wang XJ, Liu DG, Yeo W, Yu SY, Newstat B, et al. Lapatinib plus capecitabine in treating HER2-positive advanced breast cancer: efficacy, safety, and biomarker results from Chinese patients. Chin J Cancer 2011; 30:327 - 35; http://dx.doi.org/10.5732/cjc.010.10507; PMID: 21527065
- Kostyal D, Welt RS, Danko J, Shay T, Lanning C, Horton K, Welt S. Trastuzumab and lapatinib modulation of HER2 tyrosine/threonine phosphorylation and cell signaling. Med Oncol 2012; 29:1486 - 94; http://dx.doi.org/10.1007/s12032-011-0025-7; PMID: 21769502
- Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist 2010; 15:924 - 34; http://dx.doi.org/10.1634/theoncologist.2009-0181; PMID: 20736298
- Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010; 11:174 - 83; http://dx.doi.org/10.1016/S1470-2045(09)70262-1; PMID: 20152769
- Ben-Izhak O, Bar-Chana M, Sussman L, Dobiner V, Sandbank J, Cagnano M, Cohen H, Sabo E. Ki67 antigen and PCNA proliferation markers predict survival in anorectal malignant melanoma. Histopathology 2002; 41:519 - 25; http://dx.doi.org/10.1046/j.1365-2559.2002.01444.x; PMID: 12460204
- Salminen E, Palmu S, Vahlberg T, Roberts PJ, Söderström KO. Increased proliferation activity measured by immunoreactive Ki67 is associated with survival improvement in rectal/recto sigmoid cancer. World J Gastroenterol 2005; 11:3245 - 9; PMID: 15929175
- Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med 2010; 1:747 - 54; http://dx.doi.org/10.3892/etm.2010.133; PMID: 22993598
- Bianchini G, Gianni L. Surrogate markers for targeted therapy-based treatment activity and efficacy. J Natl Cancer Inst Monogr 2011; 2011:91 - 4; http://dx.doi.org/10.1093/jncimonographs/lgr024; PMID: 22043050
- Dowsett M, Smith I, Robertson J, Robison L, Pinhel I, Johnson L, Salter J, Dunbier A, Anderson H, Ghazoui Z, et al. Endocrine therapy, new biologicals, and new study designs for presurgical studies in breast cancer. J Natl Cancer Inst Monogr 2011; 2011:120 - 3; http://dx.doi.org/10.1093/jncimonographs/lgr034; PMID: 22043057
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004; 6:117 - 27; http://dx.doi.org/10.1016/j.ccr.2004.06.022; PMID: 15324695
- Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med 2010; 1:747 - 54; http://dx.doi.org/10.3892/etm.2010.133; PMID: 22993598
