Abstract
Present work mainly evaluated the inhibitory effects of lidamycin (LDM), an enediyne antibiotic, on angiogenesis or glioma-induced angiogenesis in vitro and in vivo, especially its synergistic anti-angiogenesis with temozolomide (TMZ). LDM alone efficiently inhibited proliferations and induced apoptosis of rat brain microvessel endothelial cells (rBMEC). LDM also interrupted the tube formation of rat brain microvessel endothelial cells (rBMEC) and rat aortic ring spreading. The blockade of rBMEC invasion and C6 cell-induced rBMEC migration by LDM was associated with decrease of VEGF secretion in a co-culture system. TMZ dramatically potentiated the effects of LDM on anti-proliferation, apoptosis induction, and synergistically inhibited angiogenesis events. As determined by western blot and ELISA, the interaction of tumor cells and the rBMEC was markedly interrupted by LDM plus TMZ with synergistic regulations of VEGF induced angiogenesis signal pathway, tumor cell invasion/migration, and apoptosis signal pathway. Immunofluorohistochemistry of CD31 and VEGF showed that LDM plus TMZ resulted in synergistic decrease of microvessel density (MVD) and VEGF expression in human glioma U87 cell subcutaneous xenograft. This study indicates that the high efficacy of LDM and the synergistic effects of LDM plus TMZ against glioma are mediated, at least in part, by the potentiated anti-angiogenesis.
Introduction
The pro-angiogenesis factors of tumor microenvironment play critical role in tumor growth and metastasis. Angiogenesis, controlled by the balance of positive and negative regulatory molecules, is one of the important events in tumor microenvironment for tumor growth and metastasis, and has been recognized as an applicable target for cancer chemoprevention and therapy.Citation1-Citation6 Glioma is the most common primary brain cancer. Although many aggressive therapies were applied, patients with nervous system tumors still had poor and dismal prognosis with high mortality. The median survivals of patients with malignant glioma are usually less than one year; meanwhile, the common therapy schedules are palliative with dismal results.Citation7-Citation9 Gliomas are highly vascularized and angiogenesis-dependent human cancers,Citation10-Citation13 and current reports showed that the vascular status in microenvironment of glioma plays a central role in glioma progression, invasion, diagnosis and effectiveness of clinic treatment.Citation14-Citation17 Many preclinical and clinical assessments of various strategies were performed to evaluate the anti-angiogenic effects on glioma suppression; therefore, targeting angiogenesis factors in tumor microenvironment is promising and clinically feasible strategies for glioma therapy.Citation13,Citation17-Citation21
Lidamycin (LDM, also named C-1027) is a member of the enediyne antitumor antibiotics family produced by a streptomyces strain isolated in China.Citation22 LDM showed extremely potent cytotoxicity, anti-angiogenic activity, and tumor growth inhibition in mice.Citation23-Citation25 The results in pharmacodynamics, pharmacokinetics, and toxicology have promoted LDM into clinical trial in China.Citation26 Our previous work has reported the meaningful effects of lidamycin on glioma inhibition, in which the glioma U87 cell in culture and the xenograft were observed.Citation27 The present study is set to investigate whether anti-angiogenesis is involved into the anti-glioma effect of LDM.
Temozolomide (TMZ) is a highly efficient drug for malignant gliomas therapy with significant survival increase. Despite of the encouraging results, the therapeutic effects of TMZ are far less ideal.Citation8,Citation28 Combination therapies of TMZ with other agents have been introduced to achieve higher efficacy on glioma. Combinations of TMZ plus direct and indirect angiogenesis inhibitors have been demonstrated to be effective against glioma growth.Citation29-Citation31
The benefits of combination therapy of TMZ with some other agents to glioma patients are well accepted. In our present work, we postulate that anti-angiogenesis might involve in the anti-glioma effect of LDM, which may be potentiated by TMZ co-administration. MTT assay and Annexin V-FITC/PI were performed to investigate the effects of LDM plus TMZ on rBMEC cell proliferation and apoptosis. Meanwhile, a co-culture system of rat brain microvessel endothelial cells with rat glioma C6 cells was applied to examine endothelial migration, invasion, and VEGF secretion. Angiogenesis associated endothelial events were also investigated with tube formation assay and rat aortic ring assay. Furthermore, immunohistochemical examination of CD31 and VEGF were performed to evaluate the synergistic anti-angiogenesis effects of LDM plus TMZ. The in vivo antitumor efficacy of LDM plus TMZ was determined with human glioma U87 xenograft model. Our work demonstrated that the synergy in anti-angiogenesis by LDM plus TMZ may partially contribute to glioma growth delay, which would shed light on how to improve the efficacy of glioma therapy.
Results
Synergistic cytotoxicity and potentiated induction of apoptosis in rBMEC cells by combination of LDM and TMZ
MTT assay showed that the IC50 value of LDM to rBMEC was 1.06 × 10−11 M (), which presented the highly potent anti-proliferation effect of LDM on endothelial cells. After 72 h exposure to LDM (10−10 M, 10−11 M, 10−12 M, 10−13 M, and 10−14 M), TMZ (60 μM, 90 μM, 120 μM, and 150 μM) or different combinations, the inhibitory effects in MTT assay were evaluated with isobolographic analysis (), which suggested that LDM plus TMZ has synergistic effects on inhibiting rBMEC cell proliferation. By Annexin V-FITC/PI double-staining, the results of apoptosis induction in rBMEC cells treated with TMZ, LDM, and combination indicated the potent proapoptotic effect of LDM on rBMECs, and the enhanced apoptosis by the combination was obtained (). The combinations resulted in a higher percentage of apoptotic cells with significant differences from respective single treatments (), and the CI value was 0.83 ().
Figure 1. The cytotoxicity and cell apoptosis of rBMECs treated with various dosing regimens were determined. (A) MTT assay showed the potently inhibitory effects of LDM on rBMECs proliferation. The dash line showed the IC50 value. (B) The normalized isobologram analysis showed synergistic interactions of LDM (10−10 to 10−14 M) with TMZ (60 μM, 90 μM, 120 μM, and 150 μM) on rBMECs cell proliferation. A large number of combinations below the additivity line indicated the synergism of LDM and TMZ. (C) The apoptosis assay was performed with Annexin V-FITC/PI double staining, the representative images of the rBMECs apoptosis treated with indicated dosing regimens were shown. The apoptosis ratio was calculated and plotted. Combinations of the two drugs exerted enhanced apoptosis ratios to rBMEC cells (D). **P < 0.01, and ***P < 0.001 between the indicated groups.
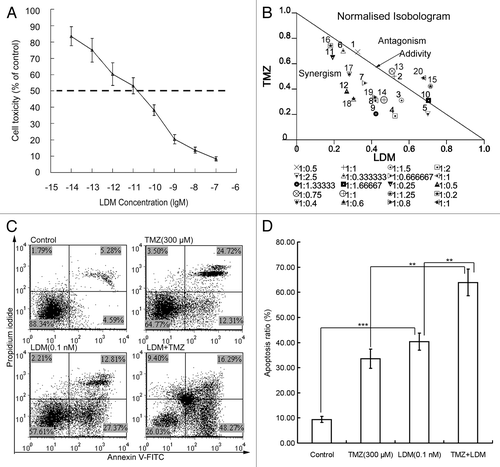
Table 1. Evaluation of synergistic effects on events associated with angiogenesis in vitro and in vivo.
Inhibition of rBMEC invasion by the combination of LDM and TMZ
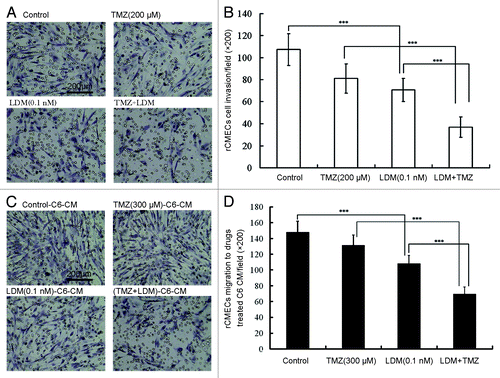
With the Boyden chamber assay, LDM and TMZ were administrated to the rBMECs cells as the indicated protocol. The results showed that LDM significantly inhibited rBMECs invasion (, P < 0.001, vs control). In comparison with single drug, drug combination significantly decreased the invasion ratio of rBMECs (, P < 0.001, vs LDM, P < 0.001, vs TMZ). Significant synergy was presented with CI value of 0.68 ().
Figure 2. Lidamycin acted in synergism with TMZ on rBMECs invasion and rBMECs migration induced by drugs-treated C6 cell condition medium. The invasive rBMECs were photographed (A) and enumerated (B), ***P < 0.001 between the indicated groups; the migrated rBMECs induced by drug-treated C6 cell condition medium were photographed (C) and enumerated (D), ***P < 0.001 between the indicated groups. In all the photos, the scale bar = 200 μm.
Synergistic inhibition of glioma cell-induced rBMEC migration by LDM plus TMZ
With a Boyden chamber, co-culture of C6 cells and rBMEC was performed to detect glioma cell-induced rBMECs migration. Treated with the indicated dosing regimens for 72 h, the conditioned medium-induced rBMEC migration was detected. The representative images were shown and the data were plotted (). Migration ratio of rBMECs co-cultured with C6 cells was decreased significantly by LDM alone (P < 0.001, vs control). Compared with single drug-treated condition medium, the migration ratio of cells in combination-treated conditioned medium was significantly decreased (P < 0.001, vs LDM, P < 0.001, vs TMZ). Significant synergy was presented with CI value of 0.72 ().
LDM and TMZ synergistically inhibit VEGF secretion in glioma C6 cells
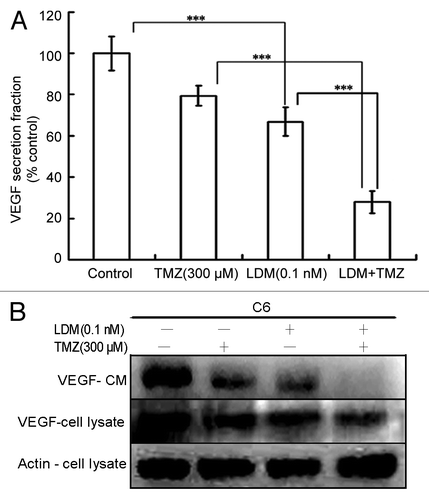
As shown (), the VEGF level was significantly reduced by LDM (P < 0.001, vs control). Compared with the two respective single drug treatments, the combination treatment potentiated the inhibitory effect (P < 0.001 vs LDM, and P < 0.001 vs TMZ). Western blot of VEGF in cells treated with C6 conditioned medium and C6 cell lysate showed similar and coincident results () with ELISA assay. Significant synergy was observed with CI value of 0.53 ().
Figure 3. Combination of Lidamycin with TMZ synergistically inhibit VEGF secretion of C6 cells. The VEGF concentrations were measured with ELISA assay, and the data were plotted (A). Meanwhile, the VEGF expressions of C6 glioma cells and the conditioned medium were detected by western blot (B). ***P < 0.001 between the indicated groups.
LDM potentiated the anti-angiogenesis effects of TMZ in vitro
In tube formation assay, single administration of LDM disrupted tube formation efficiently (P < 0.001 vs control). Tube formation ratio in cells by combination treatment was significantly decreased as compared with respective single drug treatment (, P < 0.01 vs LDM; P < 0.001 vs TMZ), and significant synergism was obtained with CI value of 0.86 ().
Figure 4. Lidamycin plus TMZ exhibited synergistic anti-angiogenesis effects in vitro. (A) The tube formation inhibitions of different treatments were photographed (200×), then the intact tubes were enumerated and plotted, and the scale bar = 200 μm (B). The aortic rings were treated and photographed [(C) 40×], then the endothelial cell spreading were analyzed and plotted, and the scale bar = 40 μm (D). **P < 0.01, and ***P < 0.001, between the indicated groups.
![Figure 4. Lidamycin plus TMZ exhibited synergistic anti-angiogenesis effects in vitro. (A) The tube formation inhibitions of different treatments were photographed (200×), then the intact tubes were enumerated and plotted, and the scale bar = 200 μm (B). The aortic rings were treated and photographed [(C) 40×], then the endothelial cell spreading were analyzed and plotted, and the scale bar = 40 μm (D). **P < 0.01, and ***P < 0.001, between the indicated groups.](/cms/asset/99b44bc8-a66d-422b-9b82-d507ea98afb5/kcbt_a_10927626_f0004.gif)
To further confirm the anti-angiogenesis effects of the two drugs, rat aortic ring sprouting assay was also performed. Single administration of LDM inhibited the endothelial cell spreading from the aortic rings (P < 0.001 vs control), and the sprouting in combination treatment was significantly decreased, compared with respective single drug treatment (, P < 0.001 vs LDM; P < 0.001 vs TMZ). Significant synergism was observed with CI value of 0.81 ().
Synergistic effect of LDM and TMZ in regulations of angiogenic pathway
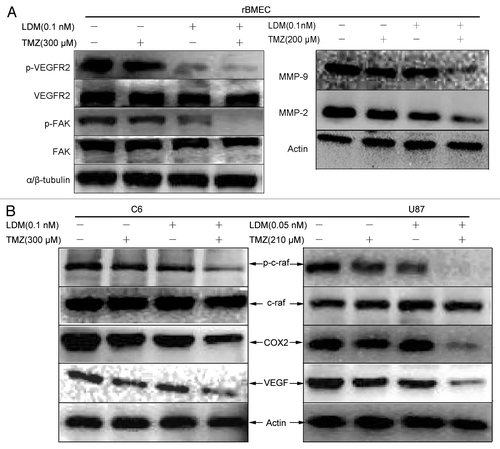
To confirm the anti-angiogenesis potentials and gain further insight into the effects of LDM and TMZ combination, VEGF signal pathway was analyzed by western blot. The combination of LDM and TMZ inhibited VEGFR2, FAK, MMP-2, and MMP-9 activity in rBMEC () more efficiently than respective single drugs, which was coincident with rBMEC invasion/migration assay (). As shown, VEGF expressions, c-raf activities and COX-2 expression in C6 cells and U87 cells () were also significantly and synergistically inhibited by the combination.
Figure 5. The synergistic regulations of angiogenesis pathway by combination of LDM with TMZ. The enhanced anti-angiogenic effect of LDM combined with TMZ is associated with VEGF signal cascades. (A):For rBMEC treated with indicated regimens, the regulations of p-VEGFR2, VEGFR2, p-FAK, FAK, and MMPs were determined by western blot with corresponding primary antibodies; the results indicated that combination more efficiently inhibit angiogenic and invasive/migratory factors expression than single treatments. (B) The inhibitory effects of LDM and its synergism with TMZ on angiogenic factors secreted by C6 cells and U87 cells were determined, in which the c-raf, COX2, and VEGF were immunoblotted. Combination treatment inhibited the expressions of VEGF and COX2 more significant than single drugs; meanwhile, the activity of c-raf was downregulated sharply.
Synergy of LDM and TMZ on angiogenesis inhibition in glioma U87 xenograft
LDM, TMZ, or combination of the two drugs decreased the tumor microvessel density (MVD) compared with control. In particular, the combination of LDM and TMZ significantly and synergistically decreased the MVD of the xenograft tumors compared with single drug treated groups (). The CI values were 0.53 for combination of LDM 25 μg/kg with TMZ (), indicating the synergistically inhibitory effects on tumor MVD in vivo. Meanwhile, the immunohistochemistry staining showed that the combination of LDM and TMZ also synergistically inhibited VEGF expression in the tumor () with the CI = 0.92 for LDM 25 μg/kg plus TMZ (), which confirmed the synergistic anti-angiogenesis effects of LDM plus TMZ.
Figure 6. Synergistic enhancement of anti-angiogenesis effect of lidamycin with TMZ in subcutaneous U87 tumor sections. The microvessle density (MVD) was determined by CD31 immunofluorostain assay, and the images were photographed [(A), 200×] and enumerated (B). Red, CD31-positive blood vessels stained with Cy3-labeled secondary antibody; blue, the cell nuclei counter-stained with 4,6-diamidino-2-phenylindole (DAPI). The VEGF secretion related to angiogenesis were detected by VEGF immunofluorostain in glioma section [(C), 200×). Red, VEGF-positive areas stained with Cy3-labled secondary antibody; blue, the cell nuclei counter-stained with 4,6-diamidino-2-phenylindole (DAPI). The photos were enumerated and plotted (D). Each column represented the mean ± s.d. ***P < 0.001 between indicated groups. In all the photos, the scale bar = 200 μm.
![Figure 6. Synergistic enhancement of anti-angiogenesis effect of lidamycin with TMZ in subcutaneous U87 tumor sections. The microvessle density (MVD) was determined by CD31 immunofluorostain assay, and the images were photographed [(A), 200×] and enumerated (B). Red, CD31-positive blood vessels stained with Cy3-labeled secondary antibody; blue, the cell nuclei counter-stained with 4,6-diamidino-2-phenylindole (DAPI). The VEGF secretion related to angiogenesis were detected by VEGF immunofluorostain in glioma section [(C), 200×). Red, VEGF-positive areas stained with Cy3-labled secondary antibody; blue, the cell nuclei counter-stained with 4,6-diamidino-2-phenylindole (DAPI). The photos were enumerated and plotted (D). Each column represented the mean ± s.d. ***P < 0.001 between indicated groups. In all the photos, the scale bar = 200 μm.](/cms/asset/696877da-a195-4ec8-8c92-68dd8221fc90/kcbt_a_10927626_f0006.gif)
Discussion
Glioma growth and metastasis are highly angiogenesis-dependent. The vascular status of glioma microenvironment plays a critical role in tumor progression, and so, it is important to understand the changes of tumor microenvironment for improvement of tumor therapeutic efficacy and exploration of new therapy strategies.Citation14,Citation32 Lidamycin (C-1027), an enediyne agent with mechanisms of dsDNA cleavage, proapoptosis and anti-angiogenesis, and so on,Citation24,Citation33-Citation35 has ever been tested for glioma growth inhibition in our previous report.Citation27 As to TMZ, its applications in clinic and preclinical have obtained encouraging results with anti-angiogenic and anti-tumor effects in glioma therapy,Citation36,Citation37 and the reported experimental and clinic practices has confirmed the potential role of TMZ combined with other agents in glioma growth delay.Citation29,Citation38,Citation39
The anti-glioma effects of LDM as single agent on U87 cells in culture and subcutaneous xenograft have been depicted in our previously work,Citation27 in which the involvement of angiogenesis was not at all concerned. In the present study, a profound investigation of LDM, and especially the combination of TMZ and LDM on angiogenesis and glioma-cell-induced angiogenesis in vitro and in vivo was performed. The widely reports on the prominent results of TMZ combination therapies motivate us to validate the postulation that synergistic inhibitory effects on glioma growth by the combination of LDM with TMZ may relate to the involvement of potentiated anti-angiogenesis. A previous report showed that anti-angiogenic effect of a VEGFR inhibitor can be potentiated by the combination of TMZ, which implied the importance of anti-angiogenesis in the potentiated anti-glioma efficacy of TMZ-based combination therapy.Citation40
As reported, glioma cells secreted generous pro-angiogenesis factors into the tumor microenvironment of glioma, which induced endothelial cell proliferation, migration and tube formation.Citation32,Citation41 The interaction of tumor cells and endothelial cells is important for angiogenesis of malignant glioma, in which VEGF, bFGF, and other cytokines play pivotal roles in promoting angiogenesis.Citation42,Citation43 Likewise, to truly simulate the interaction of the cells in glioma microenvironments, a co-culture system of rBMEC and C6 cells was established in the present study for migration or invasion assay. When C6 cells were treated with LDM and TMZ, the VEGF secretion was inhibited synergistically (), which in turn induced a decrease of rBMEC migration (). Similarly, the combination of LDM and TMZ dramatically inhibited rBMEC invasion (). As reported, overexpression of VEGF was significantly correlated with upregulation of MMP-2 and MMP-9 in human gliomas,Citation44 which facilitates glioma cells invasion. In our study, a correlation of VEGF and MMPs was also confirmed with decreased expressions of MMP-2, MMP-9, VEGF, and VEGFR2 by western blot of drug-treated rBMEC cells (). To further investigate the anti-angiogenesis of the combination regimen, tube formation assay and rat aortic ring spreading were performed. rBMECs and rat aortic rings were treated with LDM, TMZ, and the combination. As expected, the combination regimen inhibited the angiogenesis events more significantly than the respective single drug did, of which synergistic effects were displayed ().
It has been addressed that the antitumor activity of TMZ may,Citation45 at least in part, be due to its anti-angiogenic properties. Some in vivo animal experiments also indicated that the anti-glioma effects of TMZ alone or in combination therapies are associated with anti-angiogenesis.Citation40,Citation46 The involvement of anti-angiogenesis in the synergistic anti-glioma effects is still worth to being explored. In our study, by determination of the microvessel density (MVD) and VEGF expression with immunohistochemical staining in drugs-treated tumor sections, the data revealed the synergistic anti-angiogenesis effects of LDM plus TMZ (). The results of the in vivo anti-angiogenesis assay further confirmed and highlighted the potentiated involvement of anti-angiogenesis in the synergistic anti-glioma efficacy of LDM plus TMZ.
The major finding of present study is that the combination of LDM with TMZ results in synergistic modulation of glioma angiogenesis associated events in vitro and in vivo, which may partially contributed to the significant potentiation of glioma growth delay. To our knowledge, synergy of enediyne agents combined with TMZ has not been reported previously. Hence, our data demonstrate that the LDM itself displays strong anti-glioma effects, and the higher efficacy against glioma of LDM plus TMZ could be related to, at least in part, the enhancement of anti-angiogenesis, which strongly suggest that LDM plus TMZ may be of values for improvement of the efficacy of glioma therapy. More efforts should be made to this potential strategy, and further investigation is warranted.
Materials and Methods
Chemicals and animals
Lidamycin (LDM, c-1027) was kindly provided by Prof Jin Lianfang of the Chinese Academy of Medical Science, China. Temozolomide (TMZ), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and type II collagenase were purchased from Sigma-Aldrich. Annexin V-FITC/PI was purchased from BaoSai Biotechnology Co. Matrigel and 8 μm pore size cell culture insert were purchased from Becton Dickinson Co. EGF, ECGF, bFGF, and protease inhibitors cocktail were purchased from Roche Diagnostics. VEGF ELISA kit was purchased from Boster. The primary antibodies and second antibodies were purchased from Becton Dickinson, Cell Signaling Technology, Inc., and Santa Cruz as needed. Polyvinylidene difluoride (PVDF) membrane and enhanced chemiluminescence detection system were purchased from Millipore. The specific-pathogen-free (SPF) male athymic nude mice (18–22 g), purchased from Vitalriver, were housed under pathogen-free conditions. All animal studies were performed according to the protocol approved by the Animal Care and Use Committee of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College.
Cell lines, rBMEC isolation, and cultivation
Rat C6 cells and human glioma U87 cells were purchased from Cell Center, Peking Union Medical College, China. Rat brain microvessel endothelial cells (rBMECs) were primarily isolated from the cerebral gray matter of rat brains as described previously,Citation47,Citation48 with minor modifications. In brief, the isolated rat cerebral gray matter was homogenized and filtrated with 145 μm and 70 μm nylon meshes, the matter on the 70 μm nylon mesh was collected and digested with type II collagenase (0.1%) at 37 °C for 30 min, and then centrifuged at room temperature for 10 min (200 × g). The pellet was re-suspended and maintained in DMEM/F12 containing 20% defined fetal bovine serum, with EGF, ECGF, and bFGF supplemented. On day 5, the rBMEC was subcultured at 37 °C. All experiments using rBMEC were performed between passage 3 and passage 6.
Cell proliferation assay
Rat brain microvessels endothelial cells (2 × 103 cells/well) were plated in 96 well plates. After incubation for 24 h, the cells were treated with appropriate final concentrations of LDM (10−10 to 10−14 M) and TMZ (60 μM, 90 μM, 120 μM, and 150 μM) or mixture of LDM and TMZ for further 72 h incubation. Then the culture medium was removed and 20 μl of 5 mg/ml MTT were added. With incubation for 4 h at 37 °C, the supernatant was discarded and 150 μl of DMSO were added. The mixture was measured at 570 nm using Multiskan MK3 microplate reader (Thermo Labsystem). The above experiments were triple performed.
Cell apoptosis detection with Annexin V-FITC/PI double staining assay
As per the manufacturer’s instruction, cell apoptosis was detected with commercial Annexin V-FITC/PI kit. Briefly, the cells were seeded into 6-well plates, allowed to attach for 24 h, then the rat brain microvessel endothelial cells (rBMEC) were treated with LDM 0.1 nM and TMZ 300 μM or combination. After 72 h incubation, all the cells were harvested. After double staining with fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide (PI), the cells were analyzed by flow cytometry. The above experiments were performed in triplicate.
rBMEC invasion assay
To assay the effects of LDM combined with TMZ on rBMEC cell invasion, 8-μm-pore size polyethylene tetraphthalate (PET) membrane Millicell inserts for 24-well plates were coated with Matrigel and located to the wells.Citation49 The rBMECs (4 × 104 cells/100 μl) were seeded to the upper chambers and treated with LDM (0.1 nM), TMZ (200 μM), or combination of the two drugs. The system was incubated for 24 h to allow the cells to migrate through the membrane. After incubation, the cells were fixed, stained, and quantified with Leica Qwin Pro 3.0. The above experiments were repeated three times.
rBMEC chemotactic migration induced by rat C6 glioma cell in co-culture system
To assay C6 cell induced rBMEC cell chemotaxis, a co-culture system assay was performed in Boyden chambers as previous description.Citation50 Briefly, C6 cells (2 × 105 cells/500 μl) cells were seeded to 24-well plate, 24 h later, the culture medium was replaced with fetal bovine serum (FBS)-free medium in the presence of LDM (0.1 nM) and TMZ (300 μM) alone or combination treatment. After 48 h incubation, 8-μm-pore size polyethylene tetraphthalate (PET) membrane inserts for 24-well plates (no matrigel coated) were located to the wells. The rBMECs (2 × 104 cells/100 μl) were seeded to the upper chambers. After 24 h incubation, the migrated cells were analyzed as the above description, and the drug-conditioned mediums of C6 cells were centrifuged and collected for ELISA assay and western blot assay of VEGF. The above experiments were repeated three times.
VEGF quantification with ELISA assay for drugs-treated C6 conditioned medium
A commercially available rat VEGF enzyme-linked immunosorbent assay (ELISA) kit was used to measure VEGF concentrations of the drugs conditioned medium (CM) of C6 cells as the manufacturer recommended protocols and previous description.Citation51
Tube formation assay for rBMEC
The standard Matrigel assay to evaluate in vitro angiogenesis of rBMEC was performed as previously description with slight modification.Citation52 The rBMECs were seeded to 96-well plates (2 × 104 cells/well) previously coated with 100 μl Matrigel, then the cells were treated with LDM (0.1 nM), TMZ (200 μM), or a combination of both and incubated. At 16 h, the capillary-like structures formed by rBMECs were photographed with Nikon inverted microscope. The intact tubes were numerated to quantify the effects of drugs.
Rat aortic ring assay
This anti-angiogenesis assay was performed as described previously,Citation53 with some modifications. Briefly, a Sprague-Dawley rat (200~220 g) was sacrificed. Thoracic aorta was dissected in sterile hood. After removing the peri-aortic fibroadipose tissue under the stereomicroscope, the aorta was sectioned into 1 mm ring, the rings were located to Matrigel-coated wells of 24-well plates. Then the ring was covered with additional 50 μl Matrigel and cultured in 2 ml DMEM/F12 supplemented with 10% rat serum for 72 h. Then, the medium was removed and replaced with 2 ml serum-free DMEM/F12 medium. LDM (0.05 nM), TMZ (300 μM) or combination of two drugs were added to the medium. After drug treatment for 7 d, aortic rings were stained with MTT and photographed. The images were analyzed with Leica Qwin Pro 3.0 to determine the staining area as previous description. The experiments were repeated three times.
Western blot
The effects of LDM, TMZ alone, or combination on cell proliferation, apoptosis, and angiogenesis pathway of rBMEC, C6 cells and U87 cells were evaluated with western blot. After treated with different dosing regimens for 72 h, the cells were homogenized with RIPA lysis buffer (25 mM Tris [pH 7.8], 2 mM EDTA, 20% glycerol, 0.1% Nonidet P-40 [NP-40], 1 mM dithiothreitol) and protease inhibitors. The protein concentrations of cell supernatants were determined with the BCA protein assay kit from Hyclone-Pierce. Proteins (30 μg/lane) were equally loaded to and separated by SDS-PAGE gel. After electrophoresis, proteins were transferred to PVDF membrane. Then the membranes were probed with primary antibodies anti-VEGFR2, anti-p-VEGFR2, anti-MMP-2, anti-MMP-9, FAK, p-FAK, and anti-α/β-tubulin for rBMEC lysate, and anti-VEGF for drug-treated C6 condition medium and C6 cells lysate, c-raf, p-c-raf, COX2, VEGF, and actin for C6 and U87 cells lysate. All the primary antibodies were diluted at 1:1000. The membranes were incubated in horseradish peroxidase-labeled anti-mouse or anti-rabbit IgG secondary antibodies (1:5000), and then developed with enhanced chemiluminescence blot detection system.
Animal model and drugs administration
U87 glioma cells were inoculated subcutaneously (s.c.) into the flank of the nu/nu athymic nude mice (5 × 107 cells/200 μl PBS/mouse). After tumor established, the tumor pieces were implanted s.c. to the right flank of nu/nu athymic nude mice. When the tumor was reached 100~150 mm3, tumor-bearing animals were randomly divided into four groups (n = 6 per group)—control group, TMZ (0.5 mg/kg) group, LDM (25 μg/kg) group, and TMZ+LDM group. TMZ was administrated for two weeks (6 times/week) by intraperitoneal injection (i.p.). LDM was administrated for two weeks (once a week) via tail vein injection. At 30 d, the liquid nitrogen-fixed tumors were embedded in optimal cutting temperature medium and serially sectioned (6 μm) on a cryotome; the sections were stored at −80 °C until stained.
Immunofluorohistochemistry for VEGF and CD31
Microvessel density (MVD) and VEGF secretion of tumor was quantified as described previously.Citation54 Briefly, frozen sections were fixed in ice-cold acetone and rinsed, then blocked with 5% goat serum for 30 min. The sections were incubated with anti-CD31 primary antibody (1:50) and anti-VEGF primary antibody. Following rinse and incubation with Cy3-labeled second antibody (1:100), the sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and mounted with PBS-glycerol. With Nikon inverted fluorescence microscope, the sections were photographed at 200× magnifications, in which the CD31-positive vessels and VEGF-positive area were numerated with Leica Qwin Pro 3.0 program.
Drugs interaction assay
The cytotoxicological interaction of MTT assay was evaluated with normalized isobologram analysis, for which the Calcusyn software (Biosoft) was used to.Citation55 For the drug interaction evaluation on other determination, combination index (CI) of LDM with TMZ was calculated as (observed fraction value of combination group) / (expected fraction value of combination group), in which the expected fraction value of combination group were estimated as (observed mean value of LDM) / (mean control value) × (observed mean value of TMZ) / (mean control value).Citation56,Citation57 The CI < 1 indicates a synergism, CI = 1 indicates an additive effect, and CI > 1indicates an antagonistic effect.
Statistical analysis
All quantitative data were presented as mean ± SD. The significance was tested by one-way ANOVA using the SPSS software for comparison of multiple groups. Significance was accepted at P < 0.05.
| Abbreviations: | ||
| LDM | = | lidamycin |
| TMZ | = | temozolomide |
| rBMEC | = | rat brain microvessel endothelial cell |
| CM | = | conditioned medium |
| CI | = | combination index |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The work was supported by “Significant new drug development” Science and Technology Major Projects of China (2012ZX09301002), National Natural Science Foundation of China (81202559) and Doctor Initial Foundation of Heilongjiang Bayi Agricultural University (B 2010-6).
References
- Norton L, Massagué J. Is cancer a disease of self-seeding?. Nat Med 2006; 12:875 - 8; http://dx.doi.org/10.1038/nm0806-875; PMID: 16892025
- Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 2007; 7:139 - 47; http://dx.doi.org/10.1038/nrc2067; PMID: 17218951
- Bridges EM, Harris AL. The angiogenic process as a therapeutic target in cancer. Biochem Pharmacol 2011; 81:1183 - 91; http://dx.doi.org/10.1016/j.bcp.2011.02.016; PMID: 21382350
- Said SS, Pickering JG, Mequanint K. Advances in growth factor delivery for therapeutic angiogenesis. J Vasc Res 2013; 50:35 - 51; http://dx.doi.org/10.1159/000345108; PMID: 23154615
- Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol 2012; 9:498 - 509; http://dx.doi.org/10.1038/nrclinonc.2012.120; PMID: 22850752
- Aggarwal C, Somaiah N, Simon G. Antiangiogenic agents in the management of non-small cell lung cancer: where do we stand now and where are we headed?. Cancer Biol Ther 2012; 13:247 - 63; http://dx.doi.org/10.4161/cbt.19594; PMID: 22481432
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008; 359:492 - 507; http://dx.doi.org/10.1056/NEJMra0708126; PMID: 18669428
- Arko L, Katsyv I, Park GE, Luan WP, Park JK. Experimental approaches for the treatment of malignant gliomas. Pharmacol Ther 2010; 128:1 - 36; http://dx.doi.org/10.1016/j.pharmthera.2010.04.015; PMID: 20546782
- Juratli TA, Schackert G, Krex D. Current status of local therapy in malignant gliomas--a clinical review of three selected approaches. Pharmacol Ther 2013; 139:341 - 58; http://dx.doi.org/10.1016/j.pharmthera.2013.05.003; PMID: 23694764
- Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 2005; 15:297 - 310; http://dx.doi.org/10.1111/j.1750-3639.2005.tb00115.x; PMID: 16389942
- Lakka SS, Rao JS. Antiangiogenic therapy in brain tumors. Expert Rev Neurother 2008; 8:1457 - 73; http://dx.doi.org/10.1586/14737175.8.10.1457; PMID: 18928341
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia 2012; 60:502 - 14; http://dx.doi.org/10.1002/glia.21264; PMID: 22379614
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci 2007; 8:610 - 22; http://dx.doi.org/10.1038/nrn2175; PMID: 17643088
- Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. Cancer Treat Res 2004; 117:249 - 62; http://dx.doi.org/10.1007/978-1-4419-8871-3_15; PMID: 15015564
- Onishi M, Ichikawa T, Kurozumi K, Date I. Angiogenesis and invasion in glioma. Brain Tumor Pathol 2011; 28:13 - 24; http://dx.doi.org/10.1007/s10014-010-0007-z; PMID: 21221826
- Plate KH, Scholz A, Dumont DJ. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol 2012; 124:763 - 75; http://dx.doi.org/10.1007/s00401-012-1066-5; PMID: 23143192
- Chi AS, Sorensen AG, Jain RK, Batchelor TT. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist 2009; 14:621 - 36; http://dx.doi.org/10.1634/theoncologist.2008-0272; PMID: 19487335
- Dietrich J, Norden AD, Wen PY. Emerging antiangiogenic treatments for gliomas - efficacy and safety issues. Curr Opin Neurol 2008; 21:736 - 44; http://dx.doi.org/10.1097/WCO.0b013e3283131370; PMID: 19060566
- Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Medabalmi P, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg 2009; 110:173 - 80; http://dx.doi.org/10.3171/2008.4.17492; PMID: 18834263
- Takano S, Yamashita T, Ohneda O. Molecular therapeutic targets for glioma angiogenesis. J Oncol 2010; 2010:351908; http://dx.doi.org/10.1155/2010/351908; PMID: 20414463
- Khasraw M, Simeonovic M, Grommes C. Bevacizumab for the treatment of high-grade glioma. Expert Opin Biol Ther 2012; 12:1101 - 11; http://dx.doi.org/10.1517/14712598.2012.694422; PMID: 22663137
- Hu JL, Xue YC, Xie MY, Zhang R, Otani T, Minami Y, Yamada Y, Marunaka T. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J Antibiot (Tokyo) 1988; 41:1575 - 9; http://dx.doi.org/10.7164/antibiotics.41.1575; PMID: 3198491
- Chen J, Ouyang ZG, Zhang SH, Zhen YS. Down-regulation of the nuclear factor-kappaB by lidamycin in association with inducing apoptosis in human pancreatic cancer cells and inhibiting xenograft growth. Oncol Rep 2007; 17:1445 - 51; PMID: 17487403
- Zhen H, Xue Y, Zhen Y. [Inhibition of angiogenesis by antitumor antibiotic C1027 and its effect on tumor metastasis]. Zhonghua Yi Xue Za Zhi 1997; 77:657 - 60; PMID: 9772532
- Zhen YS, Ming XY, Yu B, Otani T, Saito H, Yamada Y. A new macromolecular antitumor antibiotic, C-1027. III. Antitumor activity. J Antibiot (Tokyo) 1989; 42:1294 - 8; http://dx.doi.org/10.7164/antibiotics.42.1294; PMID: 2759910
- Liu YP, Li QS, Huang YR, Zhou MJ, Liu CX. Pharmacokinetics of C-1027 in mice as determined by TCA-RA method. World J Gastroenterol 2005; 11:717 - 20; PMID: 15655829
- Ru Q, Shang BY, Miao QF, Li L, Wu SY, Gao RJ, Zhen YS. A cell penetrating peptide-integrated and enediyne-energized fusion protein shows potent antitumor activity. Eur J Pharm Sci 2012; 47:781 - 9; http://dx.doi.org/10.1016/j.ejps.2012.09.002; PMID: 22982402
- Tatar Z, Thivat E, Planchat E, Gimbergues P, Gadea E, Abrial C, Durando X. Temozolomide and unusual indications: review of literature. Cancer Treat Rev 2013; 39:125 - 35; http://dx.doi.org/10.1016/j.ctrv.2012.06.002; PMID: 22818211
- Grossman R, Tyler B, Hwang L, Zadnik P, Lal B, Javaherian K, Brem H. Improvement in the standard treatment for experimental glioma by fusing antibody Fc domain to endostatin. J Neurosurg 2011; 115:1139 - 46; http://dx.doi.org/10.3171/2011.8.JNS11125; PMID: 21923243
- Zhang YH, Yue ZJ, Zhang H, Tang GS, Wang Y, Liu JM. Temozolomide/PLGA microparticles plus vatalanib inhibits tumor growth and angiogenesis in an orthotopic glioma model. Eur J Pharm Biopharm 2010; 76:371 - 5; http://dx.doi.org/10.1016/j.ejpb.2010.08.011; PMID: 20816959
- Verhoeff JJ, Lavini C, van Linde ME, Stalpers LJ, Majoie CB, Reijneveld JC, van Furth WR, Richel DJ. Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol 2010; 21:1723 - 7; http://dx.doi.org/10.1093/annonc/mdp591; PMID: 20064829
- Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol 2012; 181:1126 - 41; http://dx.doi.org/10.1016/j.ajpath.2012.06.030; PMID: 22858156
- Kennedy DR, Gawron LS, Ju J, Liu W, Shen B, Beerman TA. Single chemical modifications of the C-1027 enediyne core, a radiomimetic antitumor drug, affect both drug potency and the role of ataxia-telangiectasia mutated in cellular responses to DNA double-strand breaks. Cancer Res 2007; 67:773 - 81; http://dx.doi.org/10.1158/0008-5472.CAN-06-2893; PMID: 17234789
- Kennedy DR, Ju J, Shen B, Beerman TA. Designer enediynes generate DNA breaks, interstrand cross-links, or both, with concomitant changes in the regulation of DNA damage responses. Proc Natl Acad Sci U S A 2007; 104:17632 - 7; http://dx.doi.org/10.1073/pnas.0708274104; PMID: 17978180
- Liu W, Christenson SD, Standage S, Shen B. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science 2002; 297:1170 - 3; http://dx.doi.org/10.1126/science.1072110; PMID: 12183628
- Kesari S, Schiff D, Doherty L, Gigas DC, Batchelor TT, Muzikansky A, O’Neill A, Drappatz J, Chen-Plotkin AS, Ramakrishna N, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol 2007; 9:354 - 63; http://dx.doi.org/10.1215/15228517-2007-006; PMID: 17452651
- Kim JT, Kim JS, Ko KW, Kong DS, Kang CM, Kim MH, Son MJ, Song HS, Shin HJ, Lee DS, et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol Rep 2006; 16:33 - 9; PMID: 16786120
- Weller M, Gorlia T, Cairncross JG, van den Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, Macdonald DR, Forsyth P, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 2011; 77:1156 - 64; http://dx.doi.org/10.1212/WNL.0b013e31822f02e1; PMID: 21880994
- Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2011; 29:142 - 8; http://dx.doi.org/10.1200/JCO.2010.30.2729; PMID: 21135282
- Sandström M, Johansson M, Bergström P, Bergenheim AT, Henriksson R. Effects of the VEGFR inhibitor ZD6474 in combination with radiotherapy and temozolomide in an orthotopic glioma model. J Neurooncol 2008; 88:1 - 9; http://dx.doi.org/10.1007/s11060-008-9527-3; PMID: 18228115
- Cantarella G, Risuglia N, Dell’eva R, Lempereur L, Albini A, Pennisi G, Scoto GM, Noonan DN, Bernardini R. TRAIL inhibits angiogenesis stimulated by VEGF expression in human glioblastoma cells. Br J Cancer 2006; 94:1428 - 35; http://dx.doi.org/10.1038/sj.bjc.6603092; PMID: 16622457
- Paku S. Current concepts of tumor-induced angiogenesis. Pathol Oncol Res 1998; 4:62 - 75; http://dx.doi.org/10.1007/BF02904699; PMID: 9555124
- Pluda JM. Tumor-associated angiogenesis: mechanisms, clinical implications, and therapeutic strategies. Semin Oncol 1997; 24:203 - 18; PMID: 9129690
- Munaut C, Noël A, Hougrand O, Foidart JM, Boniver J, Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer 2003; 106:848 - 55; http://dx.doi.org/10.1002/ijc.11313; PMID: 12918061
- Kurzen H, Schmitt S, Näher H, Möhler T. Inhibition of angiogenesis by non-toxic doses of temozolomide. Anticancer Drugs 2003; 14:515 - 22; http://dx.doi.org/10.1097/00001813-200308000-00003; PMID: 12960735
- Mathieu V, De Nève N, Le Mercier M, Dewelle J, Gaussin JF, Dehoux M, Kiss R, Lefranc F. Combining bevacizumab with temozolomide increases the antitumor efficacy of temozolomide in a human glioblastoma orthotopic xenograft model. Neoplasia 2008; 10:1383 - 92; PMID: 19048117
- Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods 2003; 130:53 - 63; http://dx.doi.org/10.1016/S0165-0270(03)00206-1; PMID: 14583404
- Yousif S, Marie-Claire C, Roux F, Scherrmann JM, Declèves X. Expression of drug transporters at the blood-brain barrier using an optimized isolated rat brain microvessel strategy. Brain Res 2007; 1134:1 - 11; http://dx.doi.org/10.1016/j.brainres.2006.11.089; PMID: 17196184
- Wang J, Yao L, Zhao S, Zhang X, Yin J, Zhang Y, Chen X, Gao M, Ling EA, Hao A, et al. Granulocyte-colony stimulating factor promotes proliferation, migration and invasion in glioma cells. Cancer Biol Ther 2012; 13:389 - 400; http://dx.doi.org/10.4161/cbt.19237; PMID: 22313638
- Ali MA, Choy H, Habib AA, Saha D. SNS-032 prevents tumor cell-induced angiogenesis by inhibiting vascular endothelial growth factor. Neoplasia 2007; 9:370 - 81; http://dx.doi.org/10.1593/neo.07136; PMID: 17534442
- Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res 2004; 10:5622 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-04-0308; PMID: 15328205
- Rosca EV, Koskimaki JE, Pandey NB, Wolff AC, Popel AS. Development of a biomimetic peptide derived from collagen IV with anti-angiogenic activity in breast cancer. Cancer Biol Ther 2011; 12:808 - 17; http://dx.doi.org/10.4161/cbt.12.9.17677; PMID: 21878750
- Zhang W, Lu Y, Xu B, Wu J, Zhang L, Gao M, Zheng S, Wang A, Zhang C, Chen L, et al. Acidic mucopolysaccharide from Holothuria leucospilota has antitumor effect by inhibiting angiogenesis and tumor cell invasion in vivo and in vitro. Cancer Biol Ther 2009; 8:1489 - 99; http://dx.doi.org/10.4161/cbt.8.15.8948; PMID: 19483477
- Gu JW, Young E, Patterson SG, Makey KL, Wells J, Huang M, Tucker KB, Miele L. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther 2011; 11:910 - 7; http://dx.doi.org/10.4161/cbt.11.10.15473; PMID: 21451264
- Tai S, Sun Y, Liu N, Ding B, Hsia E, Bhuta S, Thor RK, Damoiseaux R, Liang C, Huang J. Combination of Rad001 (everolimus) and propachlor synergistically induces apoptosis through enhanced autophagy in prostate cancer cells. Mol Cancer Ther 2012; 11:1320 - 31; http://dx.doi.org/10.1158/1535-7163.MCT-11-0954; PMID: 22491797
- Gong JH, Liu XJ, Li Y, Zhen YS. Pingyangmycin downregulates the expression of EGFR and enhances the effects of cetuximab on esophageal cancer cells and the xenograft in athymic mice. Cancer Chemother Pharmacol 2012; 69:1323 - 32; http://dx.doi.org/10.1007/s00280-012-1827-9; PMID: 22311160
- Matsuzaki T, Yashiro M, Kaizaki R, Yasuda K, Doi Y, Sawada T, Ohira M, Hirakawa K. Synergistic antiproliferative effect of mTOR inhibitors in combination with 5-fluorouracil in scirrhous gastric cancer. Cancer Sci 2009; 100:2402 - 10; http://dx.doi.org/10.1111/j.1349-7006.2009.01315.x; PMID: 19764996
