 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Risk-reducing salpingo-oophorectomy (RRSO) is associated with 50% reduction of BRCA1/2-associated breast cancer (BC) risk, possibly through decreased growth activity. In this pilot study, tumor characteristics and growth rates of BRCA1/2-associated primary BCs (PBCs) detected after RRSO were compared with those of PBCs originating without RRSO. From a cohort of 271 women with BRCA1/2-associated screen detected BC, we selected 20 patients with PBC detected ≥12 months after RRSO (RRSO group). Controls were 36 BRCA1/2 mutation carriers with PBC detected without RRSO (non-RRSO group) matched for age at diagnosis (± 2.5 y) and for BRCA1 or BRCA2 mutation. Pathology samples were revised for histological subtype, tumor differentiation grade, mitotic activity index (MAI), estrogen receptor (ER), progesterone receptor (PR), and HER2 status. Tumor growth rates, expressed as tumor volume doubling times (DT), were calculated from revised magnetic resonance and mammographic images. Median age at PBC diagnosis was 52 y (range 35–67). PBCs after RRSO had lower MAIs (12 vs. 22 mitotic counts/2 mm, P = 0.02), were smaller (11 vs. 17 mm, P = 0.01), and tend to be PR-positive more often than PBCs without RRSO (38% vs. 13%, P = 0.07). Differentiation grade, ER, and HER2 status were not different. Median DT was 124 d (range 89–193) in the RRSO group and 93 days (range 54–253) in the non-RRSO group (P = 0.47). BC occurring after RRSO in BRCA mutation carriers features a lower MAI, suggesting a less aggressive biological phenotype. When confirmed in larger series, this may have consequences for BC screening protocols after RRSO.
Background
BRCA1/2 mutation carriers face increased lifetime risks by the age of 70 y of developing breast cancer (BC; 55–85%), contralateral breast cancer (CBC; 20–60%) and ovarian cancer (18–54% for BRCA1 and 3–23% for BRCA2 mutation carriers).Citation1-Citation5 In view of the increased ovarian cancer risk, and the unavailability of an adequate screening tool, the majority of BRCA1/2 mutation carriers opt for risk-reducing salpingo-oophorectomy (RRSO), mostly before 50 y of age.Citation6,Citation7 RRSO significantly reduces the risk of ovarian/fallopian tube cancer by more than 95%,Citation6,Citation8,Citation9 while it is also associated with a primary BC (PBC) risk-reduction of about 50%, being most pronounced when performed at premenopausal age.Citation7,Citation10
BRCA1/2-associated BCs are often diagnosed at young age and are more often poorly differentiated than sporadic BCs (grade 3 in 50–75% vs. 35%, respectively).Citation11,Citation12 The BRCA1 BC phenotype is mainly estrogen receptor (ER) and progesterone receptor (PR) negative, and does not express HER2, resulting in approximately 60% of the BCs being triple negative.Citation12 The BRCA2 BC phenotype is quite similar to sporadic BCs regarding ER, PR, and HER2 status.Citation12 Furthermore, shorter tumor volume doubling times (DT), expressing faster tumor growth, have been described for both BRCA1- and BRCA2-associated tumors as compared with non-BRCA1/2-associated tumors in patients of similar age.Citation13 At increasing age, BRCA1/2-associated tumors have longer DTs,Citation13 a more favorable differentiation grade and are more often ER positive possibly due to changes in ovarian hormone production.Citation12,Citation14,Citation15 In view of the mentioned observations and the reduced BC risk after RRSO, we hypothesized that PBCs developing after RRSO-induced menopause might show altered characteristics and decreased tumor growth. The latter is also an observation at our institute, although an earlier study on tumor growth did not find a correlation of menopausal status with tumor growth.Citation13 To our knowledge, no detailed data are available on this topic. The finding of a lowered growth rate of BCs occurring after RRSO might have consequences for BC screening protocols for the subgroup of BRCA1/2 mutation carriers who underwent RRSO.
We performed a pilot study in a matched cohort of BRCA mutation carriers, and compared tumor characteristics and tumor growth rates of PBCs developing after RRSO with PBCs originating without RRSO.
Results
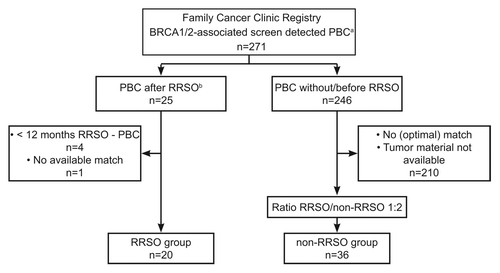
From a cohort of 271 patients with screen detected BRCA1/2-associated BC retrieved from the FCC database 21 female BRCA1/2 mutation carriers were identified with a PBC detected at least 12 mo after RRSO. One woman with BC after RRSO was excluded from further analysis because no match was available (). Of 246 proven or obligate mutation carriers with BC without RRSO, 36 appropriate matches (including two obligate mutation carriers) were found for the non-RRSO group (). For four RRSO women only one appropriate match was found.
Figure 1. Patient inclusion from the Rotterdam Family Cancer Clinic. aPBC, primary breast cancer; bRRSO, risk-reducing salpingo-oophorectomy.
Patient characteristics and demographics are listed in . As year of diagnosis was not a matching criterion, median year of PBC diagnosis in the RRSO group was 2009 vs. 2001 in the non-RRSO group (P = 0.001). RRSO was performed at a median age of 50 y, and four women (20%) were postmenopausal at RRSO. Nine women (45%) of this group had used hormone replacement therapy (HRT) between RRSO and PBC diagnosis. In the non-RRSO group, 18 women (50%) were postmenopausal at PBC diagnosis, none of them having used HRT. More PBCs were detected by MRI in the RRSO group (14 out of 20, 70% by MRI) than in the non-RRSO-group (8 out of 36, 22% by MRI, P = 0.001) as compared with mammography (). Both groups were comparable regarding age at PBC (due to matching), parity and other hormonal factors.
Table 1. Patient characteristics
Radiological tumor measurements and growth analysis of invasive carcinomas
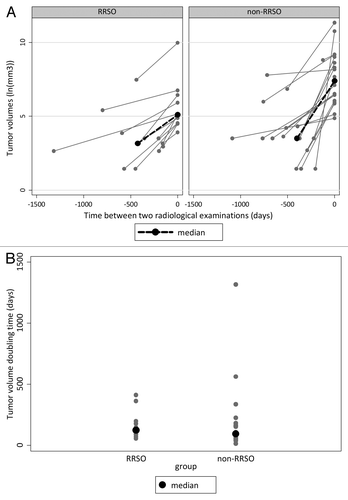
Tumor volume doubling times (DTs) of invasive BCs, as an expression of tumor growth rate, could be calculated for 12 of 17 tumors (71%) in the RRSO group and for 18 of 34 tumors (53%) in the non-RRSO group (). In total, 13 tumors (43%) were only visible on the imaging examination at diagnosis (5 on MRI, 8 on mammography), concerning 10 patients of the non-RRSO group. Twelve tumors (40%) were on revision visible on two consecutive examinations, and 5 tumors (17%) were visible on three or more consecutive examinations performed over a time period of 0.5–3.5 y (4 in the RRSO group, 1 in the non-RRSO group). Median DT of the PBCs was 124 d (IQR 89–193) in the RRSO group and 93 d (IQR 54–253) in the non-RRSO group (P = 0.47) (; ).
Table 2. Radiological tumor growth analysis of invasive carcinomas
Figure 2. (A) Tumor volumes over time and (B) tumor volume doubling times (DTs) for primary breast cancers occurring after risk-reducing salpingo-oophorectomy (RRSO) and without RRSO (non-RRSO). ln, natural logarithm; tumor volumes: V = 4/3π*1/2a*1/2b*1/2c, where a, b, and c are perpendicular tumor diameters on MRI or mammography; tumor volume doubling time (DT): DT = (ln2)/β; β = slope between natural logarithms of tumor volumes.
Histological tumor characteristics
Tumor characteristics are presented in . The RRSO group comprised three cases of DCIS (two BRCA1, one BRCA2) and 17 invasive PBCs (15 BRCA1, two BRCA2), concerning 15 ductal carcinomas, one lobular and one metaplastic carcinoma. The non-RRSO group comprised two cases of DCIS (one BRCA1, one BRCA2) and 34 invasive ductal carcinomas (30 BRCA1, four BRCA2), including one with metaplastic characteristics. Median tumor size of the invasive PBCs was 10.0 mm (interquartile range [IQR] 6.5–16.0) in the RRSO group, vs. 17.0 mm (IQR 10.0–25.0) in the non-RRSO group (P = 0.01). The majority of invasive PBCs in both groups was node negative (15/17 in the RRSO group and 25/34 in the non-RRSO group, P = 0.30).
Table 3. Histological tumor characteristics
MAI of the PBCs was significantly lower in the RRSO group than in the non-RRSO group, with a median of 12 mitoses/2 mm2 (IQR 1–20) and 22 mitoses/2 mm2 (IQR 14–28.5), respectively (P = 0.02). No differences were found in the amount of tubule formations, nuclear pleomorphism, overall Bloom and Richardson grade, ER status, or HER2 status. The proportion of PR positive PBCs (PR H-score ≥10) was higher in the RRSO group than in the non-RRSO group (38% vs. 13%) without reaching statistical significance (P = 0.07), while median PR H-score was significantly higher in the RRSO than in the non-RRSO group (3 vs. 0, P = 0.05). As a consequence, the percentage of triple negative PBCs was lower in the RRSO group than in the non-RRSO group (47% vs. 68%) without reaching statistical significance (P = 0.21).
Discussion
In this pilot study in an age-matched cohort consisting of BRCA1/2-associated BC patients, PBCs occurring after RRSO were featured by significantly lower mitotic counts, a trend for more PR positivity, and (non-significantly) more often ER positivity as compared with PBCs without RRSO. Tumor volume doubling time (DT) was non-significantly longer in the RRSO group. To our knowledge, this is the first report comparing tumor characteristics and growth patterns of PBCs occurring after RRSO to those without RRSO.
The significantly lower mitotic count in PBCs occurring after RRSO as compared with PBCs without RRSO suggests that estrogen depletion induced by RRSO decreases cell proliferation. As the majority of PBCs in this study was ER negative, the mechanism behind this observation remains unclear. Various authors confirm that the development of BRCA1-associated triple negative BCs is susceptible to estrogen depletion or inhibition as achieved by RRSO or tamoxifen.Citation16-Citation18 It has been hypothesized that the explanation lies in high ER expression of early stages of triple negative BC genesis.Citation19,Citation20 Estrogens may facilitate BRCA1-mutant cell proliferation and tumor development in premalignant mammary tissue until ER expression extinguishes in later stages, possibly after the loss of transcriptional ER-activation by the second BRCA1 allele.Citation21 By this mechanism, estrogen depletion by RRSO may inhibit tumor development of triple negative BC in a very early stage. Furthermore, there is some evidence suggesting that in a later stage of tumor development estrogen may induce changes even in ER-negative BCs by affecting the microenvironment of the tumor.Citation22
Interestingly, a recent study found that also RRSO performed after natural menopause was associated with BC risk-reduction.Citation23 The authors suggest that androgens, being produced by the ovaries after menopause, may affect cell proliferation either directly or indirectly through the aromatization to estrogens,Citation23 and possibly play a role in the risk-reduction of hormone receptor negative breast cancer.
As 85% of the study patients were BRCA1 mutation carriers, our findings are majorly driven by BRCA1. BRCA1-associated BCs are known to have higher mitotic counts than BRCA2-associated and sporadic BCs,Citation24 possibly because proteins associated with normally functioning BRCA1 genes inhibit cell proliferation.Citation25,Citation26 Separate analyses of BRCA1 carriers alone revealed comparable results as for the overall group (data not shown). To our knowledge, reduced cell proliferation in BRCA1-associated BCs after RRSO or menopause has not been described so far. Although tubule formation and nuclear pleomorphism, two other components of the Bloom and Richardson grade scoring system, and overall differentiation grade were not significantly different in PBCs after vs. without RRSO, there is evidence that MAI is the most important prognostic factor in early stage BCs.Citation27,Citation28 The finding of lower MAI in PBCs after RRSO therefore suggests a less aggressive biological growth pattern of this subgroup.
Still, 43% of the tumors in the RRSO group had high mitotic counts (≥13 mitoses/2 mm2). An explanation may be that the time period of 12 mo between RRSO and BC diagnosis considered in this study was relatively short, and that some tumors already had developed before RRSO. Of interest, PBCs with a high MAI were detected at a median of 24 mo after RRSO, while this was 69 mo for tumors with a lower MAI (0–12 mitoses/2 mm2; data not shown). This supports previous data suggesting that the maximum level of risk reduction by RRSO is effective more than 12 mo post-RRSO, although some risk reducing effect is already present one year after RRSO.Citation7
We observed a trend for more PR positivity in the RRSO group, but without significant difference in ER status (). Consequently, fewer tumors in the RRSO group (47%) were triple negative, as compared with 68% in the non-RRSO group. The latter percentage is in accordance with data from the literature for BRCA1-associated BC,Citation12 and mirrors the fact that the majority of our patients were BRCA1 mutation carriers. Earlier studies found that the proportion of ER and PR positive tumors in BRCA1-associated BC increases with increasing age at diagnosis, but is still lower than the percentage of ER-positivity in sporadic tumors irrespective of age.Citation12,Citation14,Citation15 In these studies however, menopausal status and history of RRSO were not taken into account. As patients in the current study were matched for age, the increased expression of PR in PBCs in the RRSO group, in our opinion, suggests transcriptional activation by ER and therefore can be a sign of increasing ER-functionality.Citation29-Citation31 Therefore, in a larger series we expect not only increase of PR positivity, but also of ER positivity in PBCs after RRSO. To our knowledge, only one study reported on BC characteristics after RRSO,Citation32 but due to a different study design and patient cohort, the outcomes of both studies are not comparable.
Tumor size at surgery as reported in pathology reports was significantly smaller in the RRSO group than in the non-RRSO group. This is most likely a reflection of the differences in screening regimens between the two groups. In the RRSO group all women knew their BRCA mutation status prior to RRSO and consequently were screened by means of annual MRI and mammography, according to Dutch guidelines. The non-RRSO group was more heterogeneous with respect to radiological screening, since 19 of the 36 women had not been genetically tested until PBC diagnosis. First, time intervals between screening examinations were longer in the non-RRSO group. Second, due to our matching criteria and evolving approaches over time regarding RRSO, year of diagnosis ranged from 1999 to 2012 in the RRSO group as compared with 1987 to 2011 in the non-RRSO group with consequently varying quality of radiological screening examinations. These differences between the two groups probably resulted in earlier detection and in smaller tumor sizes at diagnosis in the RRSO group (). In smaller tumors, mitotic counts may be lower, as has been reported for screen-detected sporadic BCs.Citation33 Therefore, the reduced mitotic activity we found in BCs developing after RRSO may partly have been a consequence of the smaller tumor size in this group.
Median tumor volume DT was longer in the RRSO group than in the non-RRSO group, but this difference was not statistically significant. As pointed out before, in the RRSO group women were more often screened with MRI, resulting in more precise tumor volume assessment. Because of the better imaging quality of MRI over mammography and of digital mammography in recent years as compared with previous analog mammography, tumors in the RRSO-group may have been longer visible in retrospect, resulting in lower DTs, suggesting slower growth. Further, in both groups large (interquartile) ranges for DTs were found (), suggesting that the formula used for DT was imprecise. Possibly the assumptions of presumed obloid tumor shape and the exponential growth are a too simplified approach of real tumor volume and growth. In combination with small groups, this might be the reason no statistical significant DT difference was found.
Unfortunately, due to the small numbers it was not possible to take menopausal status and HRT use into account regarding differences in histological characteristics and tumor volume DT. Of the non-RRSO group, 51% was naturally postmenopausal at PBC diagnosis and growth in these tumors may already have been restrained due to declined or absent production of ovarian hormones. Moreover, 45% of women in the RRSO group used HRT before PBC. Based on our hypothesis of tumor growth stimulation by estrogens, we expect that the differences between the groups in MAI, ER, and PR status and DT will increase when comparing HRT-naïve patients in the RRSO group with premenopausal PBC patients in the non-RRSO group.
Strikingly, in five patients the tumor was visible on three or more screening examinations over a time period of 0.5–3.5 y before BC diagnosis. All women were BRCA1 mutation carriers and screened by MRI, while four of them had undergone RRSO. In all five cases the lesion was noticed earlier, but classified as “probably benign”, while additional ultrasonography showed no signs of malignancy. Our observations support the fact that radiologists must be aware that in BRCA1 mutation carriers, and especially after RRSO, BC can present during screening as small benign looking lesions.
An important strength of our pilot study concerns the matched design, chosen to adjust for age at PBC and type of mutation (BRCA1 or BRCA2). Furthermore, pathology samples were revised by a breast pathologist, and all imaging examinations were revised by a breast radiologist, both unaware of RRSO status and therefore not biased regarding results.
However, we are aware of some relevant limitations. First, only 20 patients were eligible for the RRSO group due to the relatively low number of PBCs detected after RRSO. Women who consult our cancer center nowadays are encouraged to undergo RRSO as of 40 y of age, and some women already have suffered from BC by that time. Strict inclusion criteria and the matched design restricted further enlargement of the non-RRSO group. While some women in the RRSO group were relatively old at the time of PBC diagnosis (>60 y), only few BRCA mutation carriers were identified with a first BC occurring at older age without prior RRSO. Groups were too small to perform multivariable analysis to correct for other variables possibly of influence on tumor biology, such as HRT use, menopausal status and tumor size at detection. Second, the group consisted mostly of BRCA1 mutation carriers, as this is most frequently seen in the Netherlands. The number of BRCA2 mutation carriers was too small to perform a subgroup analysis.
In conclusion, the lower MAI and the increased proportion of PR positive BRCA1/2-associated BCs developing after RRSO suggest a less aggressive biological phenotype compared with PBCs occurring without RRSO. This was not confirmed by significantly longer DTs, probably due to small numbers. Our findings in BRCA1/2-associated PBCs occurring after RRSO are the first of this kind, but confirmation is warranted in larger sample sizes, since these findings may have consequences for less intensive breast cancer screening protocols after RRSO in mutation carriers, with possibly less outpatient clinic visits, less distress for the patient, and lower costs.
Methods
Patients
Since the start of the Rotterdam Family Cancer Clinic (FCC, approximately 1991), women at increased risk of hereditary breast and/or ovarian cancer are prospectively followed. From this cohort, we identified BRCA1/2 mutation carriers with a PBC detected at least 12 mo after RRSO (RRSO group, cases). Patients were matched for age at PBC (±2.5 y) and type of mutation (BRCA1 or BRCA2), with an intended ratio of 1:2, to obligate or proven BRCA1/2 mutation carriers with a PBC developing without RRSO (non-RRSO group, controls) ().
Further eligibility criteria included (a) PBC detected at screening or presenting as interval carcinoma between two screening examinations (previous examination within 2 y before diagnosis), and (b) availability of tumor material for pathology revision. Exclusion criteria were risk-reducing mastectomy prior to PBC, neoadjuvant chemotherapy and/or a history of ovarian cancer. Detailed data on hormonal status and reproductive factors including menarche, number of pregnancies and childbearing, breast feeding, use of oral contraceptives, and age at RRSO and/or menopause were collected from medical records.
Written informed consent was obtained according to research protocols approved by the Medical Ethical Committee.
Radiological tumor measurements and growth rate assessment
Radiological images of serial screening examinations (magnetic resonance imaging [MRI] or mammography; previous and at PBC diagnosis) of selected patients were collected. Eligibility criteria for this research question included (a) invasive carcinoma and (b) the availability of at least two well interpretable imaging examinations of the same screening modality (MRI, preferably, or mammography), one made at diagnosis and one within two years prior to PBC diagnosis.
Images were revised by a breast radiologist (I Obdeijn) being unaware of RRSO status of the patients, regarding visibility of the lesion and perpendicular tumor diameters. If the tumor was visible on ≥2 comparable, consecutive examinations, the first and the last examination were used for tumor volume calculations. If the tumor was clearly visible on MRI, three perpendicular tumor diameters were measured. On mammography, three perpendicular diameters were measured if possible, but if only two diameters could be measured, the smaller of the two diameters was used as third diameter. Tumor volumes were calculated by using a formula for obloid spheroids: .Citation13,Citation34 Because of the assumed exponential growth pattern of small tumors,Citation35 an exponential formula was used to calculate tumor volume doubling time:
with β being the slope of the straight line between the logarithms of the tumor volumes vs. time.Citation13,Citation34 If the tumor was only visible on MRI or mammography at diagnosis, the tumor volume of the preceding examination was set corresponding with the assumed lower detection limit of that imaging examination, being 2 mm for MRI, corresponding with a volume of 0.004 cm3, and 4 mm for mammography, corresponding with a volume of 0.033 cm3.Citation13
Histological tumor characteristics
Pathology slides were revised by a breast pathologist (CHM van Deurzen) unaware of the RRSO status of patients. Items scored concerned: tumor subtype according to the World Health Organization classification, grade according to the modified Bloom and Richardson score (based on tubule formation, nuclear pleomorphism and mitotic activity index [MAI]),Citation36 and ER, PR, and HER2 status. For categorization of MAI, thresholds of the modified Bloom and Richardson grade were used resulting in three categories (low 0–7 mitoses/2 mm2, moderate 8–12 mitoses/2 mm2 and high ≥ 13 mitoses/2 mm2).Citation37 For ER and PR, histoscores (H-scores) were calculated as the sum of the percentages of immunoreactive staining of tumor cells, multiplied by ordinal values corresponding to the intensity levels of the staining: H-score (0–300) = % weakly immunoreactive cells × 1 + % moderately immunoreactive cells × 2 + % intensely immunoreactive cells × 3. An H-score of ≥10 was considered positive, since 10% of immunoreactive staining of tumor cells, independent of intensity, is the cut-off point for ER/PR positivity according to Dutch national guidelines.Citation37 Patients with carcinoma in situ without an invasive component were also included. Data on tumor size and nodal status were obtained from the database and/or pathology reports.
Statistical analysis
Differences between the RRSO and non-RRSO groups were tested by using Chi-square and Fisher exact tests for categorical variables, and by using Mann–Whitney U tests for continuous variables. The SPSS computer package (version 20.0) was used for statistical analyses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003; 302:643 - 6; http://dx.doi.org/10.1126/science.1088759; PMID: 14576434
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401 - 8; http://dx.doi.org/10.1056/NEJM199705153362001; PMID: 9145676
- van der Kolk DM, de Bock GH, Leegte BK, Schaapveld M, Mourits MJ, de Vries J, van der Hout AH, Oosterwijk JC. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat 2010; 124:643 - 51; http://dx.doi.org/10.1007/s10549-010-0805-3; PMID: 20204502
- Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, Froster UG, Schlehe B, Bechtold A, Arnold N, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 2009; 27:5887 - 92; http://dx.doi.org/10.1200/JCO.2008.19.9430; PMID: 19858402
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003; 72:1117 - 30; http://dx.doi.org/10.1086/375033; PMID: 12677558
- Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, Evans G, Isaacs C, Daly MB, Matloff E, et al, Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346:1616 - 22; http://dx.doi.org/10.1056/NEJMoa012158; PMID: 12023993
- Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, Weber B, Rebbeck T, Neuhausen SL, Ghadirian P, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol 2005; 23:7491 - 6; http://dx.doi.org/10.1200/JCO.2004.00.7138; PMID: 16234515
- Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, Murphy J, Ghadirian P, Friedman E, Foulkes WD, et al, Hereditary Ovarian Cancer Clinical Study Group. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 2006; 296:185 - 92; http://dx.doi.org/10.1001/jama.296.2.185; PMID: 16835424
- Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, Ellis NA, Boyd J, Borgen PI, Barakat RR, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2002; 346:1609 - 15; http://dx.doi.org/10.1056/NEJMoa020119; PMID: 12023992
- Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon-Albright L, Isaacs C, Olopade O, Garber JE, Godwin AK, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst 1999; 91:1475 - 9; http://dx.doi.org/10.1093/jnci/91.17.1475; PMID: 10469748
- Consortium BCL, Breast Cancer Linkage Consortium. Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases. Lancet 1997; 349:1505 - 10; http://dx.doi.org/10.1016/S0140-6736(96)10109-4; PMID: 9167459
- Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Spurdle A, Robson M, Sherman M, et al, HEBON, EMBRACE, GEMO Study Collaborators, kConFab Investigators, SWE-BRCA Collaborators, Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 2012; 21:134 - 47; http://dx.doi.org/10.1158/1055-9965.EPI-11-0775; PMID: 22144499
- Tilanus-Linthorst MM, Obdeijn IM, Hop WC, Causer PA, Leach MO, Warner E, Pointon L, Hill K, Klijn JG, Warren RM, et al. BRCA1 mutation and young age predict fast breast cancer growth in the Dutch, United Kingdom, and Canadian magnetic resonance imaging screening trials. Clin Cancer Res 2007; 13:7357 - 62; http://dx.doi.org/10.1158/1078-0432.CCR-07-0689; PMID: 18094417
- Eerola H, Heikkilä P, Tamminen A, Aittomäki K, Blomqvist C, Nevanlinna H. Relationship of patients’ age to histopathological features of breast tumours in BRCA1 and BRCA2 and mutation-negative breast cancer families. Breast Cancer Res 2005; 7:R465 - 9; http://dx.doi.org/10.1186/bcr1025; PMID: 15987451
- Foulkes WD, Metcalfe K, Sun P, Hanna WM, Lynch HT, Ghadirian P, Tung N, Olopade OI, Weber BL, McLennan J, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res 2004; 10:2029 - 34; http://dx.doi.org/10.1158/1078-0432.CCR-03-1061; PMID: 15041722
- Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010; 304:967 - 75; http://dx.doi.org/10.1001/jama.2010.1237; PMID: 20810374
- Phillips KA, Milne RL, Rookus MA, Daly MB, Antoniou AC, Peock S, Frost D, Easton DF, Ellis S, Friedlander ML, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol 2013; 31:3091 - 9; http://dx.doi.org/10.1200/JCO.2012.47.8313; PMID: 23918944
- Gronwald J, Tung N, Foulkes WD, Offit K, Gershoni R, Daly M, Kim-Sing C, Olsson H, Ainsworth P, Eisen A, et al, Hereditary Breast Cancer Clinical Study Group. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer 2006; 118:2281 - 4; http://dx.doi.org/10.1002/ijc.21536; PMID: 16331614
- Li W, Xiao C, Vonderhaar BK, Deng CX. A role of estrogen/ERalpha signaling in BRCA1-associated tissue-specific tumor formation. Oncogene 2007; 26:7204 - 12; http://dx.doi.org/10.1038/sj.onc.1210527; PMID: 17496925
- Jones LP, Tilli MT, Assefnia S, Torre K, Halama ED, Parrish A, Rosen EM, Furth PA. Activation of estrogen signaling pathways collaborates with loss of Brca1 to promote development of ERalpha-negative and ERalpha-positive mammary preneoplasia and cancer. Oncogene 2008; 27:794 - 802; http://dx.doi.org/10.1038/sj.onc.1210674; PMID: 17653086
- Cerne JZ, Zong L, Jelinek J, Hilsenbeck SG, Wang T, Oesterreich S, McGuire SE. BRCA1 promoter methylation status does not predict response to tamoxifen in sporadic breast cancer patients. Breast Cancer Res Treat 2012; 135:135 - 43; http://dx.doi.org/10.1007/s10549-012-2117-2; PMID: 22706629
- Péqueux C, Raymond-Letron I, Blacher S, Boudou F, Adlanmerini M, Fouque MJ, Rochaix P, Noël A, Foidart JM, Krust A, et al. Stromal estrogen receptor-α promotes tumor growth by normalizing an increased angiogenesis. Cancer Res 2012; 72:3010 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-11-3768; PMID: 22523036
- Kotsopoulos J, Lubinski J, Lynch HT, Kim-Sing C, Neuhausen S, Demsky R, Foulkes WD, Ghadirian P, Tung N, Ainsworth P, et al. Oophorectomy after menopause and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev 2012; 21:1089 - 96; http://dx.doi.org/10.1158/1055-9965.EPI-12-0201; PMID: 22564871
- Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Vijver MJ, Farid LM, Venter D, Antoniou A, Storfer-Isser A, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst 1998; 90:1138 - 45; http://dx.doi.org/10.1093/jnci/90.15.1138; PMID: 9701363
- Holt JT, Thompson ME, Szabo C, Robinson-Benion C, Arteaga CL, King MC, Jensen RA. Growth retardation and tumour inhibition by BRCA1. Nat Genet 1996; 12:298 - 302; http://dx.doi.org/10.1038/ng0396-298; PMID: 8589721
- Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet 1995; 9:444 - 50; http://dx.doi.org/10.1038/ng0495-444; PMID: 7795653
- Baak JP, van Diest PJ, Voorhorst FJ, van der Wall E, Beex LV, Vermorken JB, Janssen EA. Prospective multicenter validation of the independent prognostic value of the mitotic activity index in lymph node-negative breast cancer patients younger than 55 years. J Clin Oncol 2005; 23:5993 - 6001; http://dx.doi.org/10.1200/JCO.2005.05.511; PMID: 16135467
- Baak JP, Gudlaugsson E, Skaland I, Guo LH, Klos J, Lende TH, Søiland H, Janssen EA, Zur Hausen A. Proliferation is the strongest prognosticator in node-negative breast cancer: significance, error sources, alternatives and comparison with molecular prognostic markers. Breast Cancer Res Treat 2009; 115:241 - 54; http://dx.doi.org/10.1007/s10549-008-0126-y; PMID: 18665447
- Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Easton DF. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol 2002; 20:2310 - 8; http://dx.doi.org/10.1200/JCO.2002.09.023; PMID: 11981002
- Osin P, Gusterson BA, Philp E, Waller J, Bartek J, Peto J, Crook T. Predicted anti-oestrogen resistance in BRCA-associated familial breast cancers. Eur J Cancer 1998; 34:1683 - 6; http://dx.doi.org/10.1016/S0959-8049(98)00248-2; PMID: 9893652
- Johnston SR, Saccani-Jotti G, Smith IE, Salter J, Newby J, Coppen M, Ebbs SR, Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res 1995; 55:3331 - 8; PMID: 7614468
- Fakkert IE, Mourits MJ, Jansen L, van der Kolk DM, Meijer K, Oosterwijk JC, van der Vegt B, Greuter MJ, de Bock GH. Breast Cancer Incidence After Risk-Reducing Salpingo-Oophorectomy in BRCA1 and BRCA2 Mutation Carriers. Cancer Prev Res (Phila) 2012; 5:1291 - 7; http://dx.doi.org/10.1158/1940-6207.CAPR-12-0190; PMID: 23009828
- Groenendijk RP, Bult P, Tewarie L, Peer PG, van der Sluis RF, Ruers TJ, Wobbes T. Screen-detected breast cancers have a lower mitotic activity index. Br J Cancer 2000; 82:381 - 4; PMID: 10646892
- Peer PG, van Dijck JA, Hendriks JH, Holland R, Verbeek AL. Age-dependent growth rate of primary breast cancer. Cancer 1993; 71:3547 - 51; http://dx.doi.org/10.1002/1097-0142(19930601)71:11<3547::AID-CNCR2820711114>3.0.CO;2-C; PMID: 8490903
- Retsky MW, Swartzendruber DE, Wardwell RH, Bame PD. Is Gompertzian or exponential kinetics a valid description of individual human cancer growth?. Med Hypotheses 1990; 33:95 - 106; http://dx.doi.org/10.1016/0306-9877(90)90186-I; PMID: 2259298
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19:403 - 10; http://dx.doi.org/10.1111/j.1365-2559.1991.tb00229.x; PMID: 1757079
- NABON. Breast Cancer Guideline [Internet]. Integraal Kankercentrum Nederland, 2012.
