Abstract
Drug-induced multidrug resistance (MDR) has been linked to overexpression of drug transporting proteins in head and neck squamous cell carcinoma (HNSCC) in vitro. The aim of this work was to reassess these findings in a murine xenograft model.
NOD-SCID mice xenotransplanted with 106 HNO97 cells were treated for four consecutive weeks with weekly paclitaxel, biweekly cisplatin (both intraperitoneal), or 5-fluorouracil (5-FU, administered by osmotic pump). Tumor volume and body weight were weekly documented. Expression of drug transporters and Ki-67 marker were examined using quantitative real-time polymerase chain reaction and/or immunohistochemistry.
Both paclitaxel and cisplatin significantly reduced tumor volumes after 2–3 weeks. 5-FU-treated animals had significantly lower body weights after 2 or 4 weeks of chemotherapy. None of the drugs affected expression of drug transporters at the mRNA level. However, P-glycoprotein (Pgp) protein expression was increased by paclitaxel (P < 0.01). Ki-67 expression did not change during treatment irrespective of the drug applied.
Paclitaxel and cisplatin are effectively tumor volume reducing drugs in a murine xenograft model of HNSCC. Paclitaxel enhanced Pgp expression at the protein level, but not at the mRNA level suggesting transcriptional induction to be of minor relevance. In contrast, posttranscriptional mechanisms or Darwinian selection of intrinsically drug transporter overexpressing MDR cells might lead to iatrogenic chemotherapy resistance in HNSCC.
Introduction
Multidrug resistance (MDR) acquired during previous cycles of chemotherapy seems to be of relevance for the chemotherapy of head and neck squamous cell carcinoma (HNSCC), because studies enrolling such patients for second line treatment demonstrated considerably lower response rates than studies excluding pre-treated patients.Citation1 The classical MDR phenotype is mediated by ATP-binding cassette (ABC)-transporters extruding anticancer agents or their metabolites from cells thus mediating drug resistance.Citation2 Paclitaxel, cisplatin, and 5-fluorouracil (5-FU) are standard anti-HNSCC drugsCitation3 whose efficacies are limited by several ABC-transporters at least in vitro.Citation4-Citation9 In addition, efficacy of cisplatin is also influenced by transporters involved in copper homeostasis. Human copper transporter 1 (hCTR1/SLC31A1) mediates the cellular uptake of copper, cisplatin, and oxaliplatin.Citation10 The P-type ATPase ATP7b is also associated with transport and resistance to platinum-containing drugs.Citation11 In HNSCC, these drug transporters are expressed and their expression levels are increased in vitro upon exposure to paclitaxel, cisplatin, or 5-FU.Citation12 However, there is very little clinical data reporting post-chemotherapeutic expression levels of MDR proteins. Moreover, the potential mechanistic tropism of this condition is unclear. However, it is well known that the expression of ABC-transporters is regulated by xenobiotic sensing nuclear factors such as pregnane-x-receptor (PXR, NR1I2)Citation13 affecting the transcription of such target genes thus mediating drug–drug interactions and chemotherapy resistance.Citation14-Citation18
To fill the gap between in vitro studies and ex vivo tumor evaluations and in order to reassess in vitro findings, we evaluated anti-HNSCC efficacies and drug transporter and PXR inducing properties of paclitaxel, cisplatin, or 5-FU in a murine xenotransplantation model through recording of tumor volume and measuring respective expression levels by quantitative real-time polymerase chain reaction and immunohistochemistry.
Results
Evaluation of antiproliferative efficacies
Tumor volumes of mock-treated animals nearly doubled during study phase. 5-FU-treated mice also demonstrated increases of tumor volumes with no statistically significant differences to mock-treated animals at any time point evaluated ().
Figure 1. Antiproliferative effects of cytostatics. Tumor volumes were normalized to each individual mouse and are reported relatively to initial tumor volume set to 1. (A) 5-FU vs control. Data are expressed as mean ± SEM for n = 9–10. Statistical significance was evaluated using non-parametric Mann–Whitney test comparing tumor volumes at respective time points. (B) Paclitaxel or cisplatin vs control. Data are expressed as mean ± SEM for n = 6–13. Statistical significance was evaluated by non-parametric ANOVA with Kruskal–Wallis post-hoc test comparing tumor volumes at respective time points.
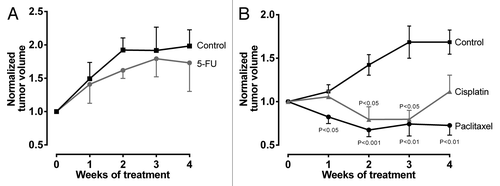
In the paclitaxel/cisplatin/control arm, the mock-treated animals again exhibited clear increases of tumor volume. In contrast, tumor growth and tumor volumes were significantly reduced by paclitaxel throughout the observation period and cisplatin after two and three weeks of chemotherapy, respectively ().
Effect of cytostatics on mRNA and protein expression levels
Expression levels of the transporters investigated was assessed at the mRNA level by quantitative real-time RT-PCR and at the protein level by immunohistochemistry.
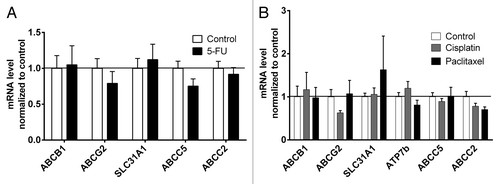
PXR and ATP7b (5-FU arm) were too low expressed to be properly quantified by qRT-PCR. 5-FU had no effect on mRNA expression levels. ABCG2 tended to be suppressed by cisplatin and ABCC2 tended to be downregulated by both cisplatin and paclitaxel. However, none of the drugs significantly altered mRNA expression levels ().
Figure 2. Effect of cytostatics on mRNA expression levels of drug transporters. (A) Tumor mRNA expressions of 5-FU treated animals were normalized to mock-treated controls. NR1I2 and ATP7b were too low expressed to be properly quantified. Data are expressed as mean ± SEM for n = 4–10. Statistical significance was evaluated by non-parametric Mann–Whitney test. (B) Tumor mRNA expressions of paclitaxel- or cisplatin-treated animals were normalized to mock-treated controls. NR1I2 was too low expressed to be properly quantified. Data are expressed as mean ± SEM for n = 5–8. Statistical significance was evaluated by non-parametric ANOVA with Kruskal–Wallis post-hoc test.
Protein expression levels were evaluated by a multiplication score reflecting both staining intensity and extent of stained cells. Consequently, increases in expression values () result from either enhanced intensity, enhanced number of expressing cells, or both. hCTR1 tended to be downregulated by 5-FU (), cisplatin, and paclitaxel (), but without statistical significance. Cisplatin and paclitaxel also non-significantly increased expression of MRP2 (). In contrast, paclitaxel did significantly enhance protein expression of Pgp (P < 0.01) ( and ).
Figure 3. Effect of cytostatics on protein expression levels of drug transporters and PXR. (A) Tumor protein expression levels of 5-FU treated animals compared with mock-treated controls. Data are expressed as mean ± SEM for n = 6–10. Statistical significance was evaluated by non-parametric Mann–Whitney test. (B) Tumor expression levels of paclitaxel- or cisplatin-treated animals compared with mock-treated controls. Data are expressed as mean ± SEM for n = 5–9. Statistical significance was evaluated by non-parametric ANOVA with Kruskal–Wallis post-hoc test. P ≤ 0.05 was considered significant.
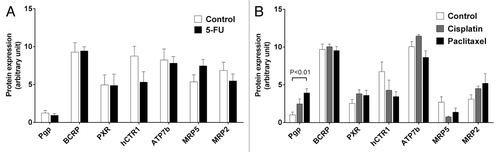
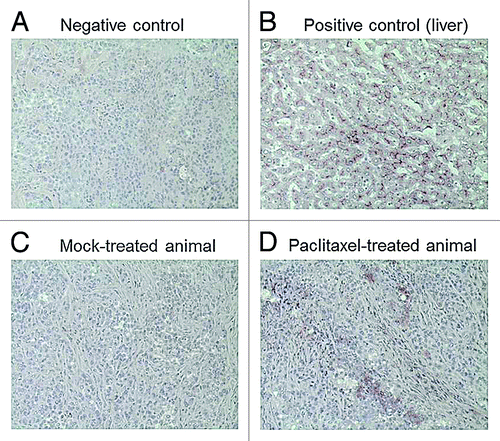
Figure 4. Representative images of tissue slices stained for Pgp expression. (A) Negative control (tumor slice treated with secondary antibody only); (B) Positive control (liver) showing typical Pgp expression at the canalicular membrane of hepatocytes; (C) Tumor Pgp expression in a mock-treated animal; (D) Tumor Pgp expression in a paclitaxel-treated animal.
Effects of cytostatics on body weight
5-FU treatment delayed the natural weight gain of the ten weeks old mice. Differences in body weight were statistically significant after two (P = 0.0052) and four (P = 0.0355) weeks of chemotherapy with 5-FU.
Paclitaxel had no effect on body weight, whereas cisplatin tended to reduce it throughout the observation period. However, the effects did not reach statistical significance (P > 0.05).
Effects of cytostatics on Ki-67 staining
Drug treatments did not change the percentage of Ki-67 positively stained nuclei. Mean values (5-FU, 12.6%; control, 5.2%; paclitaxel, 14.0%; cisplatin, 5.8%; control, 12.5) did not differ significantly.
Discussion
Acquired chemotherapy resistance is an unsolved problem in the treatment of HNSCC. For instance, patients with previous chemotherapy demonstrated lower response rates to second line treatment than patients who were not pre-treated.Citation1 The relevance of drug transporters for such clinical chemotherapy resistance in HNSCC is largely unknown. Some data from in vitro studies demonstrate exposure to typical anti-HNSCC drugs to influence both drug transporter expression and subsequent drug sensitivity of HNSCC cell lines. In detail, especially paclitaxel exhibited high potency to induce drug transporters and to cause high resistance to paclitaxel or cisplatin (cross-resistance).Citation12 This recent data was in line with findings in other cancer entities or cell models.Citation4,Citation19-Citation21 Now, the results of this in vivo approach partly confirm these earlier findings on paclitaxel’s potency to induce expression of drug transporters, especially that of Pgp.Citation19,Citation20 In contrast to 5-FU or cisplatin, paclitaxel was the only cytostatic leading to a significant induction of Pgp expression. However, this enhancement was only detectable at the protein level ( and ) and not at the mRNA level (). In theory, increased expression of MDR transporters after cytotoxic drug exposure can be caused by two mechanisms. First, Darwinian selection eradicating sensitive cells and concurrently promoting ABC-transporter overexpressing drug resistant cells, that are clonally selected when exposed to cytostatic drugs.Citation22-Citation24 Genetic instability favors this mechanism,Citation25,Citation26 that has also been described in HNSCC.Citation27,Citation28 Together, Darwinian selection is a probable scenario also in our HNSCC study since paclitaxel exposure sustainably reduced tumor volume () indicating cytoreduction of sensitive cells, while Pgp overexpressing cells withstood the drug exposure. Second, transcriptional mechanisms leading to enhanced expression of transporter genes. For such transcriptional induction, nuclear receptors (e.g., PXR) need to be expressed and functionally active.Citation13 Under these prerequisites, paclitaxel is known to bind to PXR, to activate it, and to finally lead to enhanced transcription of its main target genes such as ABCB1 (Pgp). When PXR is however absent (e.g., via RNA interference mediated knock-down), induction is shut down or at least diminished.Citation29,Citation30 In consequence, the clear-cut missing alterations of ABCB1 mRNA expression levels in our mouse model might be related to functionality of PXR, because the PXR protein itself is obviously expressed (). Indeed, during the course of this study we simultaneously characterized PXR functionality in vitro in a subset of HNSCC cell lines through PXR reporter-gene assays. Surprisingly, 6 out of 8 cell lines exhibited non-functional PXR. The cell line HNO97 was also demonstrated to express malfunctioned PXR, because even strong inducers such as rifampicin failed to activate it.Citation31 Consequently, it is quite comprehensible why in this current study with HNO97 cells, drug transporters were universally not induced at the transcriptional mRNA level, because PXR is obviously dysfunctional in this cell line. Thus, alternative mechanisms such as posttranscriptional regulation or selection processes might have led to the punctual overexpression of Pgp, the most prominent and most important MDR mediating ABC-transporter.Citation2
Despite our disease-oriented mouse model is expected to reflect clinical manifestation of HNSCC more precisely than in vitro approaches,Citation32 this study also has some limitations. Ki-67 expressions did not change upon drug exposure indicating absent inhibition of tumor cell proliferation. However, induction of apoptosis could alternatively have led to the observed significant reductions of tumor volumes by paclitaxel and cisplatin. Moreover, xenograft models are partly disadvantageous for representation of human cancer disease, because cell lines differ from natural tumor and have adapted to cell culture conditions over years or even decades. Alternatively, genetically modified mouse models (knockout of p53 and/or BRCA1) spontaneously developing tumors are state-of-the-art approaches to study drug resistance.Citation33 However, some of these obvious downsides do not apply to our approach. First, we intended to investigate induction of human drug transporters. Murine transporters are unequally to their human counterparts and are regulated in a different manner. Consequently, xenotransplantation of human cells is mandatory for our purpose. Second, the HNSCC cell line used (HNO97) has been demonstrated to reliably represent HNSCC with documented original tumor characteristics.Citation34 Taken together, we are convinced that this approach is suitable for the investigation of drug effects on expression of human drug transporters in HNSCC, despite assignability to HNSCC in general is probably invalid, because only one cell line has been investigated. Moreover, other results (potent inductions at mRNA and protein level) could also have been achieved with one of the few exceptional cell lines exhibiting proper PXR function.Citation31
In conclusion, this study used a murine xenotransplantation model for HNSCC and demonstrates that paclitaxel and cisplatin are effectively tumor volume reducing cytostatics. Paclitaxel additionally enhanced Pgp protein expression. At the mRNA level, however, none of the drugs had inductive effects suggesting transcriptional mechanisms to be of minor relevance. In contrast, fluortranscriptional mechanisms and Darwinian selection of intrinsically transporter overexpressing MDR cells might be an additional mechanism leading to iatrogenic chemotherapy resistance in HNSCC.
Materials and Methods
Materials
Culture media, medium supplements, PBS, antibiotics, and 5-FU, were purchased from Sigma-Aldrich. Fetal calf serum (FCS) was purchased from PAA. Cisplatin and paclitaxel were from the university hospital’s pharmacy. The antibodies against PXR (G-11) and hCTR1 (G15) and the ImmunoHistoMount mounting medium were obtained from Santa Cruz Biotechnology. The antibody against breast cancer resistance protein (BCRP) was from Alexis Biochemicals. Anti-P-glycoprotein (Pgp) antibody (C219) was from Calbiochem. Anti-multidrug resistance associated protein 2 (MRP2) antibody (M2III-6) was from Thermo. Anti-ATP7b antibody was from Acris. Anti-MRP5 antibody was from GeneTex. Anti-Ki-76 antibody and BD Matrigel were from Beckton Dickinson. The Vectastain Elite ABC Kits including the anti-mouse, anti-goat, anti-rat, or anti-rabbit IgG peroxidase linked secondary antibodies and the AEC Substrate Kit were from Vector Laboratories. Mayer’s Haemalaun solution was purchased from Merck. The RNeasy Kit was from Qiagen and the RevertAid™ H Minus First Strand cDNA Synthesis Kit was from Fermentas, and the qPCR SYBR Green Mix was purchased from Abgene. Osmotic pumps (model 1004) and respective filling tubes were from Charles River.
Murine xenograft model for HNSCC
The HNSCC cell line used (HNO97) was authenticated and has been derived from an intraoperatively obtained sample, established, and characterized as reported previously.Citation35 Suitability of this cell line for representation of HNSCC was recently confirmed by comprehensive tumor cell biological characterization.Citation34 HNO97 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat inactivated FCS, 100 U/ml penicillin, and 100 µg/ml streptomycin sulfate.
Six-week-old, female, NOD-SCID mice were purchased from Charles River and were handled under governmental and institutional rules and regulations after approval by the respective authorities. Animals were housed under specific pathogen-free conditions with a 12 h light/dark cycle and received food and tap water ad libitum.
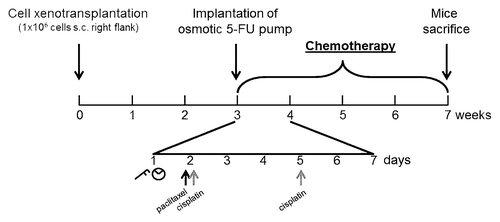
Briefly, 1 × 106 cells (suspended in 200 µl BD Matrigel) were subcutaneously injected at the right flank of the mice under general anesthesia using ketamine (75 mg/kg) and xylazine (25 mg/kg). Three weeks after xenotransplantation, when tumors had reached a volume of approximately 300 mm3, mice were randomized into treatment groups ().
Figure 5. Experimental schedule: Three weeks after subcutaneous (s.c.) xenotransplatation of 106 HNO97 cells, chemotherapy was initiated, that lasted for four consecutive weeks. One representative week is shown in detail: Paclitaxel (black arrows) or cisplatin (gray arrows) were administered once or twice a week, respectively. Tumor volume (caliper symbol) and body weight (scale symbol) were evaluated once a week. 5-FU administration was mediated through an osmotic pump non-recurringly implanted into the peritoneal cave at the beginning of chemotherapeutic study phase.
Chemotherapeutic treatments
Paclitaxel and cisplatin dosing was based on previous studies on HNSCC xenograft models. Paclitaxel (10 µg/g) was injected intraperitoneally once weekly,Citation36 whereas cisplatin (1 µg/g) was administered twice a week.Citation37 Because of its short elimination half-time and to mimic clinical infusion administration, 5-FU was administered using an osmotic pump (0.11 µl/h flow) implanted into the peritoneal cave ().Citation38 This pump delivered 1.75 mg of 5-FU in total over four weeks of treatment.
Placebo treatments consisted of either vehicle injections (control for paclitaxel or cisplatin, respectively) or vehicle-delivering pumps (control for 5-FU).
Evaluation of antiproliferative efficacies
Cytostatic efficacies were evaluated weekly by measurement of tumor volume calculated by the following equation: tumor volume = (a × b2)/2, where a represents the length of the tumor and b the width.
Ki-67 staining index was evaluated through immunohistochemistry. Histological sections were automatically imaged in a 40× magnification (resolution: 0.23 μm/pixel) using the Hamamatsu NanoZoomer 2.0-HT Scan System (Hamamatsu Photonics). For the scanning of the object slides, the slide scanner automatically detects the region of interest (ROI) that contains the tissue and also determines automatically a valid focal plane for scanning. The image processing algorithms have been developed using TissuemorphDP™ version 4.5.1 (Visiopharm). Software was developed on a Personal Computer: 64 Bit, Intel Quad-Core i7 @ 3.40 GHz and 8GB RAM with Microsoft Windows 7 operating system. The first image processing step involves a segmentation of all nuclei in previously manual selected ROIs. ROIs were selected by including all tissue areas of the whole slide by omitting fragmented and folded areas. The segmentation was done by a watershed segmentationCitation39,Citation40 on the IHS-S color band. Then the positive nuclei were detected within a HDAB-DAB color band, provided by a color deconvolution algorithm.Citation41 As a post-processing step, areas that were too small where removed by an area-filter. Percent of positively strained nuclei were considered the relevant output variable.
Quantification of mRNA expressions by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
After four weeks of chemotherapy, mice were sacrificed through spinal cord dislocation and tumors were immediately removed and frozen in ice-cold isopentane and stored at −80 °C until further analysis. RNA was isolated from tumor specimen using RNeasy-Kit and cDNA was synthesized with the RevertAid™ H Minus First Strand cDNA Synthesis Kit according to the manufacturer’s instructions. Expression of mRNA was quantified by qRT-PCR with a LightCycler® 480 (Roche Applied Science) using the SYBR Green format with the Absolute QPCR SYBR Green Mix. Primer sequences were published previously.Citation12 The following genes were quantified: NR1I2 (encoding for PXR), ABCB1 (encoding for Pgp), ABCC2 (encoding for MRP2), ABCC5 (encoding for MRP5), ABCG2 (encoding BCRP), SLC31A1 (encoding hCTR1), and ATP7b (also called Wilson disease protein). The most suitable housekeeping gene for normalization was identified using geNorm (version 3.4, Center for Medical Genetics).Citation42 Among the housekeeping genes tested (β2-microglobulin; glucose-6-phosphate dehydrogenase, G6PDH; glucuronidase β; ribosomal protein L13 (RPL13); hypoxanthine-phosphoribosyltransferase 1, HPRT; 60S (human) acidic ribosomal protein P1 (HUPO), G6PDH/HUPO proved to be the most stable ones for the 5-FU/control arm and G6PDH/HPRT for the paclitaxel/cisplatin/control data set. Data were evaluated by calibrator-normalized relative quantification with efficiency correction using LightCycler® 480 software as published previously.Citation43 Results are expressed as the ratio of target gene/housekeeping genes divided by the corresponding ratio of the calibrator (cDNA of the HNO97 cell line). All samples were amplified in duplicate.
Quantification of protein expressions by immunohistochemistry
Stored tumor specimens were cut into 5 µm slices with a cryostat (Leica CM 1850 UV from Leica Biosystems) and were evaluated for drug transporter expression by immunohistochemistry according to manufacturer’s instructions. All steps were performed in a humidity chamber at room temperature. In brief, after thawing and drying of object slides, tumor specimens were exposed for 1 h to 40 µl of diluted antigen-specific antibodies. After washing 3 × 5 min with PBS, 40 µl of diluted secondary-antibodies were applied for 30 min. After washing 3 × 5 min with PBS, avidin–biotin complex solution was pipetted onto object slides and incubated for 30 min. After washing off the avidin–biotin complex solution, the 3-amino-9-ethylcarbazole (AEC) solution was added and slides were carefully observed for staining reaction. Reaction was then stopped through washing with aqua bidest. Nuclei were subsequently stained with Haemalaun solution. Tumor slides were finally embedded using mounting solution. Five 10× magnification images of every tumor and stained antigen, respectively, were taken (microscope Olympus BX50, Olympus; software Cell Imaging, Olympus) and were independently assessed by two blinded investigators (D.T., J.W.). Each investigator ranked a value for the expression intensity from 0 (no expression) to 3 (very strong expression) and a value describing the extent of tumor staining (1, 0–24%; 2, 25–49%; 3, 50–74%; 4, 75–100%). These values were multiplied. The final score of each tumor was then calculated as the mean of these two independent evaluations. Consequently, lowest score was 0, highest score was 12. Tissues slides of healthy liver and intestine were used as positive controls and were provided by the tissue bank of the National Center of Tumor Diseases (NCT) at the institute of pathology at the University of Heidelberg.
Statistical analysis
Differences in tumor volumes during chemotherapy with 5-FU vs. control were evaluated using non-parametric Mann–Whitney test comparing tumor volumes at respective time points. Effects of paclitaxel or cisplatin vs. control were evaluated by non-parametric ANOVA with Kruskal–Wallis post-hoc test also comparing tumor volumes at respective time points.
Expression levels of drug transporters and Ki-67 were evaluated at the end of chemotherapy. Differential expression levels (mRNA, protein, Ki-67 staining) and differences in body weights were respectively evaluated by non-parametric Mann–Whitney test (5-FU vs control) or non-parametric ANOVA with Kruskal–Wallis post-hoc test (paclitaxel vs cisplatin vs control). P ≤ 0.05 was considered significant.
| Abbreviations: | ||
| MDR | = | multidrug resistance |
| HNSCC | = | head and neck squamous cell carcinoma |
| 5-FU | = | 5-fluorouracil |
| Pgp | = | P-glycoprotein |
| PXR | = | pregnane-x-receptor |
| ABC-transporter | = | adenosine-triphosphate binding cassette transporter |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This project was funded by grant WE 4135/3-1 und HE 2357/2-1 from the German Research Foundation. The authors thank Corina Mueller, Stephanie Rosenzweig, Natalia Heinzelbecker, and Melanie Greibich for excellent technical assistance.
References
- Fanucchi M, Khuri FR. Chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck. Semin Oncol 2004; 31:809 - 15; http://dx.doi.org/10.1053/j.seminoncol.2004.09.014; PMID: 15599859
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2:48 - 58; http://dx.doi.org/10.1038/nrc706; PMID: 11902585
- Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2006; 24:2644 - 52; http://dx.doi.org/10.1200/JCO.2005.05.3348; PMID: 16763278
- Kamazawa S, Kigawa J, Minagawa Y, Itamochi H, Shimada M, Takahashi M, Sato S, Akeshima R, Terakawa N. Cellular efflux pump and interaction between cisplatin and paclitaxel in ovarian cancer cells. Oncology 2000; 59:329 - 35; http://dx.doi.org/10.1159/000012191; PMID: 11096346
- Lagas JS, Vlaming ML, van Tellingen O, Wagenaar E, Jansen RS, Rosing H, Beijnen JH, Schinkel AH. Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res 2006; 12:6125 - 32; http://dx.doi.org/10.1158/1078-0432.CCR-06-1352; PMID: 17062689
- Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther 2004; 3:833 - 8; PMID: 15252144
- Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W 3rd, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther 2005; 4:855 - 63; http://dx.doi.org/10.1158/1535-7163.MCT-04-0291; PMID: 15897250
- Guminski AD, Balleine RL, Chiew YE, Webster LR, Tapner M, Farrell GC, Harnett PR, Defazio A. MRP2 (ABCC2) and cisplatin sensitivity in hepatocytes and human ovarian carcinoma. Gynecol Oncol 2006; 100:239 - 46; http://dx.doi.org/10.1016/j.ygyno.2005.08.046; PMID: 16213010
- Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, Willey JC. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer 2005; 4:18; http://dx.doi.org/10.1186/1476-4598-4-18; PMID: 15882455
- Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 2004; 3:1543 - 9; PMID: 15634647
- Nakayama K, Kanzaki A, Ogawa K, Miyazaki K, Neamati N, Takebayashi Y. Copper-transporting P-type adenosine triphophatase (ATP7B) as a cisplatin-based chemoresistance marker in ovarian carcinoma: comparative analysis with expression of MDR1, MRP, LRP and BCRP. Int J Cancer 2002; 101:488 - 95; http://dx.doi.org/10.1002/ijc.10608; PMID: 12216079
- Theile D, Ketabi-Kiyanvash N, Herold-Mende C, Dyckhoff G, Efferth T, Bertholet V, Haefeli WE, Weiss J. Evaluation of drug transporters’ significance for multidrug resistance in head and neck squamous cell carcinoma. Head Neck 2011; 33:959 - 68; http://dx.doi.org/10.1002/hed.21559; PMID: 20737486
- Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene 2003; 22:7496 - 511; http://dx.doi.org/10.1038/sj.onc.1206950; PMID: 14576854
- Blumberg B, Sabbagh W Jr., Juguilon H, Bolado J Jr., van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 1998; 12:3195 - 205; http://dx.doi.org/10.1101/gad.12.20.3195; PMID: 9784494
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med 2001; 7:584 - 90; http://dx.doi.org/10.1038/87912; PMID: 11329060
- Beijnen JH, Schellens JH. Drug interactions in oncology. Lancet Oncol 2004; 5:489 - 96; http://dx.doi.org/10.1016/S1470-2045(04)01528-1; PMID: 15288238
- Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR. Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos 2002; 30:608 - 12; http://dx.doi.org/10.1124/dmd.30.5.608; PMID: 11950795
- Huang R, Murry DJ, Kolwankar D, Hall SD, Foster DR. Vincristine transcriptional regulation of efflux drug transporters in carcinoma cell lines. Biochem Pharmacol 2006; 71:1695 - 704; http://dx.doi.org/10.1016/j.bcp.2006.03.009; PMID: 16620787
- Hille S, Rein DT, Riffelmann M, Neumann R, Sartorius J, Pfützner A, Kurbacher CM, Schöndorf T, Breidenbach M. Anticancer drugs induce mdr1 gene expression in recurrent ovarian cancer. Anticancer Drugs 2006; 17:1041 - 4; http://dx.doi.org/10.1097/01.cad.0000231480.07654.b5; PMID: 17001177
- Schöndorf T, Neumann R, Benz C, Becker M, Riffelmann M, Göhring UJ, Sartorius J, von König CH, Breidenbach M, Valter MM, et al. Cisplatin, doxorubicin and paclitaxel induce mdr1 gene transcription in ovarian cancer cell lines. Recent Results Cancer Res 2003; 161:111 - 6; http://dx.doi.org/10.1007/978-3-642-19022-3_10; PMID: 12528803
- Su GM, Davey MW, Davey RA. Induction of broad drug resistance in small cell lung cancer cells and its reversal by paclitaxel. Int J Cancer 1998; 76:702 - 8; http://dx.doi.org/10.1002/(SICI)1097-0215(19980529)76:5<702::AID-IJC15>3.0.CO;2-5; PMID: 9610729
- Muller C, Laval F, Soues S, Birck C, Charcosset JY. High cell density-dependent resistance and P-glycoprotein-mediated multidrug resistance in mitoxantrone-selected Chinese hamster cells. Biochem Pharmacol 1992; 43:2091 - 102; http://dx.doi.org/10.1016/0006-2952(92)90166-G; PMID: 1376119
- Slapak CA, Daniel JC, Levy SB. Sequential emergence of distinct resistance phenotypes in murine erythroleukemia cells under adriamycin selection: decreased anthracycline uptake precedes increased P-glycoprotein expression. Cancer Res 1990; 50:7895 - 901; PMID: 1979251
- Slapak CA, Fracasso PM, Martell RL, Toppmeyer DL, Lecerf JM, Levy SB. Overexpression of the multidrug resistance-associated protein (MRP) gene in vincristine but not doxorubicin-selected multidrug-resistant murine erythroleukemia cells. Cancer Res 1994; 54:5607 - 13; PMID: 7923205
- Diaz LA Jr., Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012; 486:537 - 40; PMID: 22722843
- Blagosklonny MV. STI-571 must select for drug-resistant cells but ‘no cell breathes fire out of its nostrils like a dragon’. Leukemia 2002; 16:570 - 2; http://dx.doi.org/10.1038/sj.leu.2402409; PMID: 11960334
- Zuo C, Zhang H, Spencer HJ, Vural E, Suen JY, Schichman SA, Smoller BR, Kokoska MS, Fan CY. Increased microsatellite instability and epigenetic inactivation of the hMLH1 gene in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 2009; 141:484 - 90; http://dx.doi.org/10.1016/j.otohns.2009.07.007; PMID: 19786217
- Bennett KL, Romigh T, Eng C. AP-2alpha induces epigenetic silencing of tumor suppressive genes and microsatellite instability in head and neck squamous cell carcinoma. PLoS One 2009; 4:e6931; http://dx.doi.org/10.1371/journal.pone.0006931; PMID: 19742317
- Masuyama H, Suwaki N, Tateishi Y, Nakatsukasa H, Segawa T, Hiramatsu Y. The pregnane X receptor regulates gene expression in a ligand- and promoter-selective fashion. Mol Endocrinol 2005; 19:1170 - 80; http://dx.doi.org/10.1210/me.2004-0434; PMID: 15650019
- Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Down-regulation of pregnane X receptor contributes to cell growth inhibition and apoptosis by anticancer agents in endometrial cancer cells. Mol Pharmacol 2007; 72:1045 - 53; http://dx.doi.org/10.1124/mol.107.037937; PMID: 17636047
- Rigalli JP, Reuter T, Herold-Mende C, Dyckhoff G, Haefeli WE, Weiss J, Theile D. Minor role of pregnane-x-receptor for acquired multidrug resistance in head and neck squamous cell carcinoma in vitro. Cancer Chemother Pharmacol 2013; 71:1335 - 43; http://dx.doi.org/10.1007/s00280-013-2133-x; PMID: 23479137
- Braakhuis BJ, van Dongen GA, Bagnay M, van Walsum M, Snow GB. Preclinical chemotherapy on human head and neck cancer xenografts grown in athymic nude mice. Head Neck 1989; 11:511 - 5; http://dx.doi.org/10.1002/hed.2880110607; PMID: 2584006
- Rottenberg S, Borst P. Drug resistance in the mouse cancer clinic. Drug Resist Updat 2012; 15:81 - 9; http://dx.doi.org/10.1016/j.drup.2012.01.001; PMID: 22335919
- Freier K, Hofele C, Knoepfle K, Gross M, Devens F, Dyckhoff G, Plinkert P, Lichter P, Herold-Mende C. Cytogenetic characterization of head and neck squamous cell carcinoma cell lines as model systems for the functional analyses of tumor-associated genes. J Oral Pathol Med 2010; 39:382 - 9; PMID: 20149059
- Ninck S, Reisser C, Dyckhoff G, Helmke B, Bauer H, Herold-Mende C. Expression profiles of angiogenic growth factors in squamous cell carcinomas of the head and neck. Int J Cancer 2003; 106:34 - 44; http://dx.doi.org/10.1002/ijc.11188; PMID: 12794754
- Fujita K, Sano D, Kimura M, Yamashita Y, Kawakami M, Ishiguro Y, Nishimura G, Matsuda H, Tsukuda M. Anti-tumor effects of bevacizumab in combination with paclitaxel on head and neck squamous cell carcinoma. Oncol Rep 2007; 18:47 - 51; PMID: 17549344
- Nozawa H, Tadakuma T, Ono T, Sato M, Hiroi S, Masumoto K, Sato Y. Small interfering RNA targeting epidermal growth factor receptor enhances chemosensitivity to cisplatin, 5-fluorouracil and docetaxel in head and neck squamous cell carcinoma. Cancer Sci 2006; 97:1115 - 24; http://dx.doi.org/10.1111/j.1349-7006.2006.00287.x; PMID: 16984384
- Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, McCarty MF, Bucana CD, Mazar AP, Ellis LM. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer 2003; 104:496 - 503; http://dx.doi.org/10.1002/ijc.10958; PMID: 12584749
- Jung C, Kim C. Segmenting clustered nuclei using H-minima transform-based marker extraction and contour parameterization. IEEE Trans Biomed Eng 2010; 57:2600 - 4; http://dx.doi.org/10.1109/TBME.2010.2060336; PMID: 20656653
- Beucher S. The watershed transformation applied to image segmentation. Scanning Microsc Int 1992; 6:299 - 314
- Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 2001; 23:291 - 9; PMID: 11531144
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3:RESEARCH0034; http://dx.doi.org/10.1186/gb-2002-3-7-research0034; PMID: 12184808
- Albermann N, Schmitz-Winnenthal FH, Z’graggen K, Volk C, Hoffmann MM, Haefeli WE, Weiss J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol 2005; 70:949 - 58; http://dx.doi.org/10.1016/j.bcp.2005.06.018; PMID: 16054595
