Abstract
Efforts to enhance the antileukemic properties of arsenic trioxide are clinically relevant and may lead to the development of new therapeutic approaches for the management of certain hematological malignancies. We provide evidence that concomitant treatment of acute myeloid leukemia (AML) cells or chronic myeloid leukemia (CML) cells with resveratrol potentiates arsenic trioxide-dependent induction of apoptosis. Importantly, clonogenic assays in methylcellulose demonstrate potent suppressive effects of the combination of these agents on primitive leukemic progenitors derived from patients with AML or CML. Taken together, these findings suggest that combinations of arsenic trioxide with resveratrol may provide an approach for targeting of early leukemic precursors and, possibly, leukemia initiating stem cells.
Introduction
Arsenic trioxide (As2O3) exhibits potent antineoplastic activities,Citation1 including antileukemic effects that have led to the introduction of this agent in the treatment of patients with acute promyelocytic leukemia (APL).Citation2 Beyond APL, As2O3 has cytotoxic effects on other types of leukemias and solid tumors in preclinical models, but at concentrations that may not be clinically achievable because of toxicities.Citation1 Thus, approaches to promote generation of its effects at lower concentrations are needed in order to allow its use in non-APL leukemias and other malignancies.Citation1 Concomitant use of this agent with other drugs that may promote the induction of antineoplastic responses may provide a strategy for the development of combinations for the treatment of other hematological malignancies.
Resveratrol is a phytoalexin substance with previously established antioxidant and antineoplastic properties.Citation3 This agent has been shown to induce apoptosis in a variety of lymphoid and myeloid leukemia cell lines.Citation4,Citation5 The pro-apoptotic activity of resveratrol in leukemia cells appears to be regulated by the induction of the Fas ligand (FasL), through activation of the ASK1/JNK-dependent signaling pathway.Citation6 Other studies in chronic myeloid leukemia (CML) cells have shown that resveratrol sensitizes CML cells to cell cycle arrest and apoptotic cell death when combined with imatinib mesylate.Citation7 There has also been work demonstrating that resveratrol induces cell-cycle arrest and decreases the expression of survivin irrespective of p53 status.Citation8-Citation10 Finally, there is evidence that in acute myeloid leukemia (AML), sensitization occurs through reactive oxygen species-mediated activation of the apoptotic pathway.Citation11 Resveratrol has also been linked to activation of autophagy. Recent studies have shown the ability of resveratrol to trigger an autophagic cell death in CML cells, via AMPK and JNK.Citation12 Furthermore, resveratrol has been shown to enhance radiation sensitivity in a variety of other malignancies, including melanoma, prostate cancer, and non-small lung cancer.Citation13-Citation15
In the present study, we sought to determine the effects of resveratrol on arsenic trioxide -induced antileukemic responses. We were particularly interested in examining whether resveratrol can potentiate arsenic trioxide-induced autophagy or apoptosis, as our recent studies have established that in addition to apoptotic cell death, induction of autophagy is a key mechanism for the generation of arsenic-induced antileukemic effects on primary leukemic precursors.Citation16,Citation17 As expected, both agents were found to induce an autophagic state, but resveratrol does not further enhance induction of autophagy by As2O3. On the other hand, resveratrol potently enhances As2O3-dependent apoptotic cell death and promotes the suppressive effects of As2O3 on primary leukemic progenitors from patients with AML and CML, suggesting a developmental therapeutics approach to target early leukemic precursors and leukemia initiating stem cells.
Results
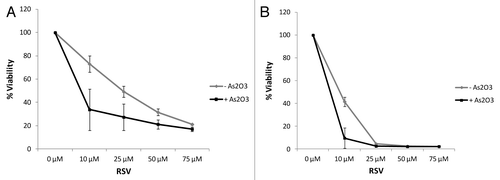
In initial studies, we examined the induction of autophagy by resveratrol in leukemia cells. Treatment with resveratrol resulted in upregulation of LC3-II in the U937 AML cell line and the K562 CML cell line (). Similarly, and as expected,Citation16,Citation17 treatment with As2O3 resulted in upregulation of expression of LC3II (). However, there was no synergy between the two agents in inducing expression of this marker of autophagy (). Treatment of leukemic cells with As2O3 resulted in induction of apoptosis, as reflected by annexin V staining (). Such induction of apoptosis was clearly enhanced by combinations of As2O3 with resveratrol (). Consistent results were also obtained in immunoblotting experiments by probing for the expression of the late apoptosis marker, poly (ADP-ribose) polymerase (PARP) and its cleaved isoform (). To further define the functional relevance of combined treatment of leukemic cells with As2O3 and resveratrol, we examined the effects of these agents on the BCR-ABL-transformed leukemic cell line KT1. Treatment with increasing doses of resveratrol and As2O3 resulted in decreased cell viability of KT1 cells with significant effects being recorded even at low resveratrol concentrations at either 48 h or 120 h ().
Figure 1. Induction of autophagy by resveratrol or As2O3 in leukemia cells. (A) U937 cells were treated with resveratrol (5 μM) (R5) or (10 μM) (R10), for 24 h, as indicated. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-LC3 or anti-GAPDH antibodies, as indicated. (B) K562 cells were treated with the indicated concentrations of resveratrol for the indicated times. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-LC3 or anti-GAPDH antibodies, as indicated. (C) K562 cells treated with As2O3 at the indicated concentrations for 24 h, in the presence or absence of resveratrol (25 μM), as indicated. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-LC3 or anti-GAPDH antibodies, as indicated.
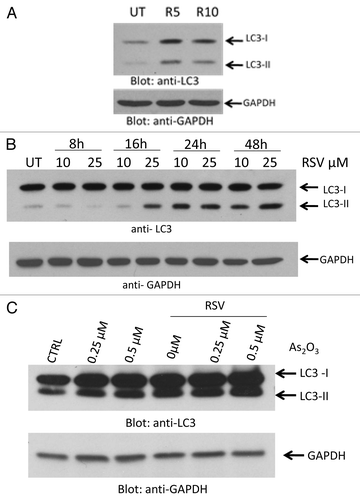
Figure 2. Combination treatment As2O3 and resveratrol induces apoptosis in a dose-dependent manner. (A) KT1 cells were treated with the indicated concentrations of As2O3 and resveratrol for 48 h and subjected to flow cytometric analysis for annexin V staining. Means ± SE of 2 independent experiments are shown. (B) KT1 cells treated with As2O3 and resveratrol for 48 h at the indicated concentrations. Total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-PARP or anti-HSP90 antibodies, as indicated.
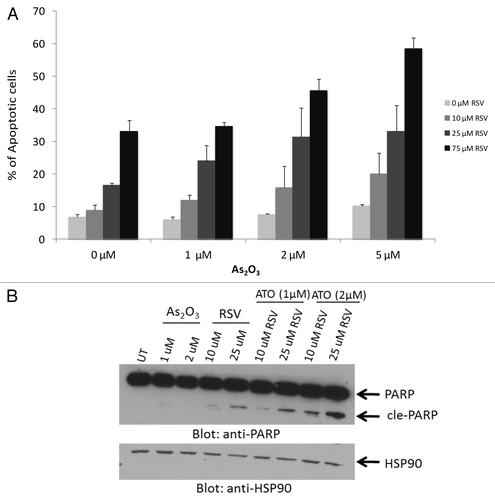
Figure 3. Combination treatment of As2O3 and resveratrol reduces cell viability in a dose-dependent manner. KT1 cells were incubated in the presence or absence of As2O3 (2 μM) and the indicated concentrations of resveratrol for either 48 h (A) or 120 h (B). Cell viability was determined by an MTT assay. Data are expressed as a percentage of viability of control untreated samples. Means ± SE of 2 independent experiments are shown.
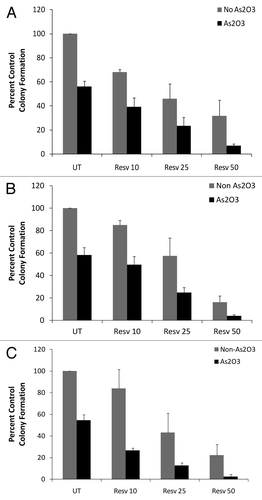
In subsequent experiments, we determined whether co-treatment of leukemia cells with As2O3 and increasing concentrations of resveratrol amplified the effects of these drugs on primitive leukemic precursors, compared with each drug alone. We exposed the BCR-ABL expressing leukemia cell lines K562 () and KT1 (), and the acute myeloid leukemia cell line U937 (), to As2O3 and, concomitantly, increasing concentrations of resveratrol. Effects on leukemic precursors were assessed by clonogenic assays in methylcellulose. Escalating doses of resveratrol resulted in enhanced antileukemic responses, as assessed by leukemic CFU-L colony formation derived from K562, KT1 or U937 cells ().
Figure 4. Enhanced antileukemic responses by combinations of As2O3 and resveratrol. (A–C) Primitive leukemic progenitor colony formation derived from K562 (A), U937 (B), or KT1 (C) cells was assessed in clonogenic assays in methylcellulose, following incubation of cells with As2O3 (0.5 μM) and the indicated concentrations of resveratrol. Data are expressed as a percentage of CFU-L of control untreated cells. Means ± SE of 3 (A) and 2 (B and C) independent experiments for each panel are shown.
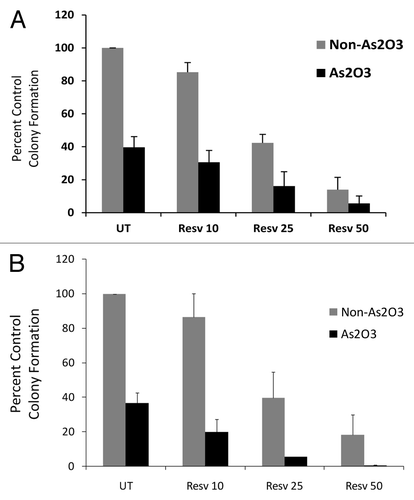
As these findings suggested,enhanced antileukemic responses by combinations of resveratrol with As2O3, we sought to determine whether such effects occur on primary leukemic precursors from patients with CML or AML. As shown in , when the effects of combining As2O3 with escalating concentrations of resveratrol were determined on primary leukemic cells from CML patients, we found consistent enhancing effects (). Similar results were obtained when responses were assessed on leukemic precursors from AML patients (). Thus, combinations of resveratrol with As2O3 result in more potent effects than each agent alone, raising the possibility of combining these agents as a therapeutic approach to target leukemic precursors in myeloid leukemias.
Figure 5. Resveratrol enhances As2O3 responses in primary leukemia progenitors. (A) Effects of combinations of As2O3 (0.5 μM) and the indicated concentrations of resveratrol on leukemic CFU-GM from CML patients. Data are expressed as a percentage of CFU-GM of control untreated cells. Means ± SE of 6 independent experiments are shown. (B) Effects of combinations of As2O3 (0.5 μM) and the indicated concentrations of resveratrol on CFU-L from different AML patients. Data are expressed as a percentage of CFU-L of control untreated cells. Means ± SE of 3 independent experiments are shown.
Discussion
Beyond its clinical activity in APL, As2O3 has clinical potential for the treatment of other neoplastic disorders, as shown by extensive work using in vitro and in vivo preclinical models for different malignancies.Citation1 However, the ability of arsenic compounds to generate such responses is frequently limited in non-APL malignant cells by the emergence of leukemic cell resistance mechanisms and/or the simultaneous activation of negative feedback regulatory pathways that limit induction of arsenic biological responses. Extensive work in the past has provided evidence that such negative feedback regulatory cascades include the p38 MAP kinaseCitation18,Citation19 and its upstreamCitation20 and downstreamCitation21,Citation22 effectors, as well as the MEK-ERK pathway and its effector kinase RSK1.Citation23,Citation24 Other work has also implicated AKT/mTOR signaling pathways as negative feedback regulators activated in response to treatment of leukemia cells with arsenic trioxideCitation25,Citation26 underscoring the complexity of leukemic cell resistance mechanisms. The simultaneous engagement of multiple feedback loops poses therapeutic challenges and makes selective targeting of leukemic cells difficult and limiting. Thus efforts to develop combinations of arsenic trioxide with other agents that promote its antileukemic effects via unique mechanisms of action are important, especially when the demonstrated capacity of arsenic to eliminate leukemic initiating stem cells under certain circumstancesCitation27 is taken into account.
There is emerging evidence that the flavonoid resveratrol exhibits antileukemic effects. Previous studies have demonstrated that resveratrol induces autophagic cell death in BCR-ABL transformed leukemic cells,Citation7 while it also causes cell cycle arrest in promyelocytic leukemia cells.Citation10 We have previously shown that arsenic trioxide is a potent anti-leukemic agent and that, beyond apoptosis, autophagy plays a major role in the generation of arsenic responses in vitro in AMLCitation16 and CMLCitation17 cells. Remarkably, resveratrol has been reported to reduce cardiotoxicity and nephrotoxicity caused by As2O3 treatment,Citation28 while in a leukemia setting, there is emerging evidence suggesting that resveratrol can be used in As2O3-treated APL patients post-therapy to decrease arsenic-induced side effects such as hepatotoxicity.Citation29 There is also some evidence that resveratrol can enhance the anti-leukemic effects of imatinib mesylate on CML cells.Citation7
In the present study, we provide evidence that resveratrol sensitizes AML and CML leukemia cell lines to the effects of As2O3 in vitro. We also show that resveratrol enhances the inhibitory effects of As2O3 on primary AML or CML primitive leukemic progenitors in vitro. Our studies demonstrate that such effects of resveratrol likely reflect potentiation of the pro-apoptotic properties of arsenic trioxide and do not appear to reflect modulation of arsenic-dependent autophagic cell death. It should be noted that previous studiesCitation16 have firmly established that induction of both apoptosis and autophagy are required for the generation of the suppressive effects of As2O3 on primitive leukemic precursors. This was shown by experiments demonstrating that pharmacological inhibitors of autophagy or apoptosis can partially reverse the inhibitory effects of arsenic on leukemic progenitors in clonogenic assays in methylcellulose.Citation16 Although an enhancing effect of resveratrol on arsenic-dependent induction of an autophagic state could not be demonstrated in the current study, this could be attributed to an interplay between the various cell death pathways occurring simultaneously.Citation30 Future studies should clarify this issue and define specific components of pro-apoptotic or autophagic machineries that are modified and may account for the enhancing effects of resveratrol. Nevertheless, our findings demonstrate the potential of combination approaches to enhance the effects of arsenic on primitive AML and/or CML progenitors and further efforts in that direction are warranted.
Material and Methods
Cells and reagents
The K562, KT1, and U937 human leukemia cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. Arsenic trioxide (As2O3) and resveratrol were purchased from Sigma. Antibodies against PARP and LC3 were obtained from Cell Signaling Technology, Inc. Antibodies against c-Abl and p62/SQSMT1 were obtained from Santa Cruz Biotechnology Inc., and antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Millipore.
Cell lysis and immunoblotting
Cells were treated with the designated doses of As2O3 for the indicated times and subsequently lysed in the phosphorylation lysis buffer as previously described.Citation18,Citation19 Immunoblotting using an enhanced chemiluminescence (ECL) method was performed as previously described.Citation31,Citation32
Cell proliferation assays
Cells were treated with the indicated doses of As2O3, in the presence or absence of resveratrol for the indicated times. Cell proliferation assays using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) method were performed as in our previous studies.Citation33
Evaluation of apoptosis
Apoptosis was evaluated by flow cytometry for annexin V/PI staining as in our previous studiesCitation25 using FITC Annexin V Apoptosis Detection Kit from BD PharMingen. For assessment of apoptosis assays, cells were treated with As2O3 (1 to 5 μM) with the indicated concentrations of resveratrol for 48 h, prior to flow cytometric analysis.
Clonogenic assays in methylcellulose to assess leukemic progenitor colony formation
Peripheral blood or bone marrows from patients with CML or AML were collected after obtaining consent approved by the Institutional Review Board of Northwestern University. The effects of arsenic trioxide on the growth of leukemic progenitors (CFU-L) were assessed by clonogenic assays in methylcellulose, as in previous studies.Citation34,Citation35 As2O3 was used at final concentrations of 0.5 μM and for resveratrol at concentrations of 10, 25, and 50 μM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Institutes of Health grants CA121192, CA155566, CA77816, and CA174205; and by grant I01CX000916 from the Department of Veterans Affairs. E.B. was supported by NIH training grant T32CA070085.
References
- Platanias LC. Biological responses to arsenic compounds. J Biol Chem 2009; 284:18583 - 7; http://dx.doi.org/10.1074/jbc.R900003200; PMID: 19363033
- Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 2010; 116:3751 - 7; http://dx.doi.org/10.1182/blood-2010-02-269621; PMID: 20705755
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275:218 - 20; http://dx.doi.org/10.1126/science.275.5297.218; PMID: 8985016
- Dörrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res 2001; 61:4731 - 9; PMID: 11406544
- Sommers RK, Brady WA, Moore WH Jr.. Dichotic ear preferences of stuttering children and adults. Percept Mot Skills 1975; 41:931 - 8; http://dx.doi.org/10.2466/pms.1975.41.3.931; PMID: 1215135
- Su JL, Lin MT, Hong CC, Chang CC, Shiah SG, Wu CW, Chen ST, Chau YP, Kuo ML. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis 2005; 26:1 - 10; http://dx.doi.org/10.1093/carcin/bgh220; PMID: 15217905
- Puissant A, Grosso S, Jacquel A, Belhacene N, Colosetti P, Cassuto JP, Auberger P. Imatinib mesylate-resistant human chronic myelogenous leukemia cell lines exhibit high sensitivity to the phytoalexin resveratrol. FASEB J 2008; 22:1894 - 904; http://dx.doi.org/10.1096/fj.07-101394; PMID: 18245170
- Fulda S. Regulation of cell death and survival by resveratrol: implications for cancer therapy. Anticancer Agents Med Chem 2012; 12:874 - 9; http://dx.doi.org/10.2174/187152012802650129; PMID: 22292764
- Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene 2004; 23:6702 - 11; http://dx.doi.org/10.1038/sj.onc.1207630; PMID: 15273734
- Duraj J, Bodo J, Sulikova M, Rauko P, Sedlak J. Diverse resveratrol sensitization to apoptosis induced by anticancer drugs in sensitive and resistant leukemia cells. Neoplasma 2006; 53:384 - 92; PMID: 17013532
- Taranto D, Suozzo R, de Sio I, Romano M, Caporaso N, Del Vecchio Blanco C, Coltorti M. Effect of metoclopramide on transmural oesophageal variceal pressure and portal blood flow in cirrhotic patients. Digestion 1990; 47:56 - 60; http://dx.doi.org/10.1159/000200477; PMID: 2292350
- Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, Auberger P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res 2010; 70:1042 - 52; http://dx.doi.org/10.1158/0008-5472.CAN-09-3537; PMID: 20103647
- Fang Y, DeMarco VG, Nicholl MB. Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci 2012; 103:1090 - 8; http://dx.doi.org/10.1111/j.1349-7006.2012.02272.x; PMID: 22417066
- Fang Y, Bradley MJ, Cook KM, Herrick EJ, Nicholl MB. A potential role for resveratrol as a radiation sensitizer for melanoma treatment. J Surg Res 2013; 183:645 - 53; http://dx.doi.org/10.1016/j.jss.2013.02.037; PMID: 23522452
- Liao HF, Kuo CD, Yang YC, Lin CP, Tai HC, Chen YY, Chen YJ. Resveratrol enhances radiosensitivity of human non-small cell lung cancer NCI-H838 cells accompanied by inhibition of nuclear factor-kappa B activation. J Radiat Res 2005; 46:387 - 93; http://dx.doi.org/10.1269/jrr.46.387; PMID: 16394628
- Goussetis DJ, Altman JK, Glaser H, McNeer JL, Tallman MS, Platanias LC. Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. J Biol Chem 2010; 285:29989 - 97; http://dx.doi.org/10.1074/jbc.M109.090530; PMID: 20656687
- Goussetis DJ, Gounaris E, Wu EJ, Vakana E, Sharma B, Bogyo M, Altman JK, Platanias LC. Autophagic degradation of the BCR-ABL oncoprotein and generation of antileukemic responses by arsenic trioxide. Blood 2012; 120:3555 - 62; http://dx.doi.org/10.1182/blood-2012-01-402578; PMID: 22898604
- Verma A, Mohindru M, Deb DK, Sassano A, Kambhampati S, Ravandi F, Minucci S, Kalvakolanu DV, Platanias LC. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to arsenic trioxide. J Biol Chem 2002; 277:44988 - 95; http://dx.doi.org/10.1074/jbc.M207176200; PMID: 12239215
- Giafis N, Katsoulidis E, Sassano A, Tallman MS, Higgins LS, Nebreda AR, Davis RJ, Platanias LC. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res 2006; 66:6763 - 71; http://dx.doi.org/10.1158/0008-5472.CAN-05-3699; PMID: 16818652
- McNeer JL, Goussetis DJ, Sassano A, Dolniak B, Kroczynska B, Glaser H, Altman JK, Platanias LC. Arsenic trioxide-dependent activation of thousand-and-one amino acid kinase 2 and transforming growth factor-beta-activated kinase 1. Mol Pharmacol 2010; 77:828 - 35; http://dx.doi.org/10.1124/mol.109.061507; PMID: 20159944
- Kannan-Thulasiraman P, Katsoulidis E, Tallman MS, Arthur JS, Platanias LC. Activation of the mitogen- and stress-activated kinase 1 by arsenic trioxide. J Biol Chem 2006; 281:22446 - 52; http://dx.doi.org/10.1074/jbc.M603111200; PMID: 16762916
- Dolniak B, Katsoulidis E, Carayol N, Altman JK, Redig AJ, Tallman MS, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Platanias LC. Regulation of arsenic trioxide-induced cellular responses by Mnk1 and Mnk2. J Biol Chem 2008; 283:12034 - 42; http://dx.doi.org/10.1074/jbc.M708816200; PMID: 18299328
- Lunghi P, Giuliani N, Mazzera L, Lombardi G, Ricca M, Corradi A, Cantoni AM, Salvatore L, Riccioni R, Costanzo A, et al. Targeting MEK/MAPK signal transduction module potentiates ATO-induced apoptosis in multiple myeloma cells through multiple signaling pathways. Blood 2008; 112:2450 - 62; http://dx.doi.org/10.1182/blood-2007-10-114348; PMID: 18583568
- Galvin JP, Altman JK, Szilard A, Goussetis DJ, Vakana E, Sassano A, Platanias LC. Regulation of the kinase RSK1 by arsenic trioxide and generation of antileukemic responses. Cancer Biol Ther 2013; 14:411 - 6; http://dx.doi.org/10.4161/cbt.23760; PMID: 23377826
- Altman JK, Yoon P, Katsoulidis E, Kroczynska B, Sassano A, Redig AJ, Glaser H, Jordan A, Tallman MS, Hay N, et al. Regulatory effects of mammalian target of rapamycin-mediated signals in the generation of arsenic trioxide responses. J Biol Chem 2008; 283:1992 - 2001; http://dx.doi.org/10.1074/jbc.M705227200; PMID: 18048359
- Yoon P, Giafis N, Smith J, Mears H, Katsoulidis E, Sassano A, Altman J, Redig AJ, Tallman MS, Platanias LC. Activation of mammalian target of rapamycin and the p70 S6 kinase by arsenic trioxide in BCR-ABL-expressing cells. Mol Cancer Ther 2006; 5:2815 - 23; http://dx.doi.org/10.1158/1535-7163.MCT-06-0263; PMID: 17121928
- Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 2008; 453:1072 - 8; http://dx.doi.org/10.1038/nature07016; PMID: 18469801
- Yu M, Xue J, Li Y, Zhang W, Ma D, Liu L, Zhang Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol 2013; 87:1025 - 35; http://dx.doi.org/10.1007/s00204-013-1026-4; PMID: 23471352
- Zhang W, Xue J, Ge M, Yu M, Liu L, Zhang Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem Toxicol 2013; 51:87 - 92; http://dx.doi.org/10.1016/j.fct.2012.09.023; PMID: 23023136
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 2009; 16:966 - 75; http://dx.doi.org/10.1038/cdd.2009.33; PMID: 19325568
- Uddin S, Majchrzak B, Woodson J, Arunkumar P, Alsayed Y, Pine R, Young PR, Fish EN, Platanias LC. Activation of the p38 mitogen-activated protein kinase by type I interferons. J Biol Chem 1999; 274:30127 - 31; http://dx.doi.org/10.1074/jbc.274.42.30127; PMID: 10514501
- Uddin S, Sassano A, Deb DK, Verma A, Majchrzak B, Rahman A, Malik AB, Fish EN, Platanias LC. Protein kinase C-δ (PKC-δ ) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol Chem 2002; 277:14408 - 16; http://dx.doi.org/10.1074/jbc.M109671200; PMID: 11839738
- Vakana E, Altman JK, Glaser H, Donato NJ, Platanias LC. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood 2011; 118:6399 - 402; http://dx.doi.org/10.1182/blood-2011-01-332783; PMID: 22021366
- Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, Druker BJ, Donato NJ, Altman JK, Barr S, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A 2010; 107:12469 - 74; http://dx.doi.org/10.1073/pnas.1005114107; PMID: 20616057
- Altman JK, Sassano A, Kaur S, Glaser H, Kroczynska B, Redig AJ, Russo S, Barr S, Platanias LC. Dual mTORC2/mTORC1 targeting results in potent suppressive effects on acute myeloid leukemia (AML) progenitors. Clin Cancer Res 2011; 17:4378 - 88; http://dx.doi.org/10.1158/1078-0432.CCR-10-2285; PMID: 21415215
