Abstract
Tumor endothelial marker 1 (TEM1, endosialin) is a tumor vascular marker with significant diagnostic and therapeutic potential. However, in vivo small animal models to test affinity reagents specifically targeted to human (h)TEM1 are limited. We describe a new mouse tumor model where tumor vascular endothelial cells express hTEM1 protein.
Methods: Immortalized murine endothelial cells MS1 were engineered to express hTEM1 and firefly luciferase and were inoculated in nude mice either alone, to form hemangioma-like endothelial grafts, or admixed with ID8 ovarian tumor cells, to form chimeric endothelial-tumor cell grafts. MORAb-004, a monoclonal humanized IgG1 antibody specifically recognizing human TEM1 was evaluated for targeted theranostic applications, i.e., for its ability to affect vascular grafts expressing hTEM1 as well as being a tool for molecular positron emission tomography (PET) imaging.
Results: Naked MORAb-004 treatment of mice bearing angioma grafts or chimeric endothelial-tumor grafts significantly suppressed the ability of hTEM1-positive endothelial cells, but not control endothelial cells, to form grafts and dramatically suppressed local angiogenesis. In addition, highly efficient radioiodination of MORAb-004 did not impair its affinity for hTEM1, and [124I]-MORAb-004-PET enabled non-invasive visualization of tumors enriched with hTEM1-positive, but not hTEM1 negative vasculature with high degree of specificity and sensitivity.
Conclusion: The development of a new robust endothelial graft model expressing human tumor vascular proteins will help accelerate the development of novel theranostics targeting the tumor vasculature, which exhibit affinity specifically to human targets but not their murine counterparts. Our results also demonstrate the theranostic potential of MORAb-004 as PET imaging tracer and naked antibody therapy for TEM1-positive tumor.
Targeting drugs and imaging probes to tumor vasculature is an attractive alternative to targeting tumor cells,Citation1-Citation6 due the high target accessibility to the circulation and possibility of direct blockage in tumor blood supply.Citation7,Citation8 Numerous molecules enriched or selectively expressed in tumor vasculature have been identified.Citation4,Citation9 Monoclonal antibodies (mAb) to these determinants may deliver drugs and imaging probes to diverse and often more than one types of tumors.Citation10,Citation11
Antibody-based single photon emission CT (SPECT) and positron emission tomography (PET) are clinically used imaging modalities that provide real-time noninvasive detection of pathologies. Several radiolabeled mAbs are currently approved by FDA for diagnostic imaging of cancer by SPECT.Citation12 PET is even more sensitive modality allowing higher resolution (achieving ~1 mm) and accurate quantitative analysis.Citation12 Combining PET with CT provides exact mapping of isotope signal in the anatomical structures. A recent study in patients with renal tumors showed that pre-operative immuno-PET imaging could accurately differentiate between renal cell carcinoma and benign pathology.Citation13 Thus, PET imaging of tumor markers holds promise of non-invasive diagnosis, staging, and longitudinal monitoring of tumor response to therapy, supporting personalized treatment.
Tumor endothelial marker 1 (TEM1/endosialin/CD248) is an 80.9 kDa cell surface protein implicated in development, vascular cell adhesion and migration,Citation14,Citation15 neoangiogenesis,Citation16,Citation17 and tumor progression.Citation18 TEM1 has been found in the vasculature of breast, colon, brain cancer, sarcomas, and other tumors,Citation4,Citation16,Citation17,Citation19-Citation23 while its level in normal blood vessels of adult tissues is below detection limit.Citation16,Citation17 TEM1 is expressed by endothelial progenitor cells,Citation24 tumor endothelial cells, pericytes, and tumor-associated fibroblasts.Citation25-Citation27 In breast cancer, TEM1 overexpression correlates with lymph node metastasis, recurrence, and death.Citation28 Tem1−/− mice show a dramatic reduction in tumor growth, invasiveness, and metastasis, but are otherwise healthy and exhibit normal wound healing.Citation29 We have reported increased TEM1 expression in human ovarian cancer endothelial cells and vasculature-associated leukocytes.Citation30 Therefore, TEM1 is an attractive marker for targeted tumor imaging and therapy.
MORAb-004 is derived from antibody FB5 by immunizing mice with human fetal fibroblastsCitation31 and later humanized by grafting six complementarity determining regions (CDRs) on to a human IgG1k frame work.Citation32 It is in several phase I/II studies of tumor types including sarcomas, melanoma, and colon rectal cancer. Development of antibody-based targeting is complicated by interspecies differences. For example, MORAb-004 binds to human and monkey TEM1 homologs. Lack of binding to murine TEM-1 necessitates development of mouse models featuring human TEM-1 for pre-clinical studies of targeting and efficacy of MORAb-004 in this species. Here we developed of a new mouse model that features expression of human (h)TEM1 on murine tumor vascular endothelial cells by creating chimeric hTEM1-positive grafts and used this model for pre-clinical characterization of TEM-1 imaging using immuno-PET.
Results
Development of a mouse endothelial cells expressing hTEM1
We first engineered mouse endothelial MS1 cells to express firefly luciferase (fLuc), to enable optical imaging of tumors (Fig. S1). Transfected MS1 cells were sorted based on DsRed fluorescent marker to 99.9% purity and expanded. Luciferase activity of MS1/fLuc was confirmed in vitro (). Next, to develop murine endothelial cells expressing both fLuc and human TEM1 (hTEM1), we created a lentiviral expression vector with dual expression cassettes carrying hTEM1 and EmGFP.Citation33 MS1/fLuc cells were transduced with pKH-hTEM1/EmGFP followed by cell sorting based on EmGFP fluorescent marker. Expression of hTEM1 was validated by qPCR () and FACS analysis using MORAb-004 (). MS1/fLuc and MS1-hTEM1/fLuc had similar growth rates in vitro (Fig. S2), and luciferase activity was similar and detectable with as few as 100 cells or less in vitro (). Murine endothelial H5V cells engineered to express fLuc and hTEM1 as an alternative to MS1 cell system (), formed aggressive angiosarcoma tumor in nu/nu mice. Since mice succumbed quickly to these tumors, H5V cell system has not been used in this study.
Figure 1. Characterization of MS1-TEM1/fLuc and MS1/fLuc endothelial cells. Murine endothelial cells MS1 expressing firefly Luciferase (fLuc) and DsRed (MS1/fLuc) were infected with lentivirus carrying human (h)TEM1 and GFP-Emerald (MS1-hTEM1/fLuc). (A) Quantitative PCR analysis for hTEM1 in H5V and MS1 cells following lentiviral transfection of hTEM1 or control expression cassette. (B) FACS analysis of MS1 cell lines using biotinylated hTEM1-specific antibody MORAb-004 (Ctl: control MS1 cells transduced with empty expression cassette; TEM1: MS1 cells transduced with hTEM1; Ab: MORAb-004). (C and D) MS1-hTEM1/fLuc cells showed a similar luminescent intensity to MS1/fLuc control cells in vitro. Representative image of in vitro bioluminescence study is shown in (C), and summarized in (D). Linearity is observed between plated cell number and bioluminescence. (E and F) MS1/fLuc murine cell injection in vivo formed hemangiomas four weeks following injection of 5 × 102–5 × 105 cells subcutaneously (s.c.) in the flank of nu/nu mice. Representative image of in vivo bioluminescence study is shown in (E) and summarized in (F). In vivo optical imaging can readily detect as few as 5 × 102 cells.
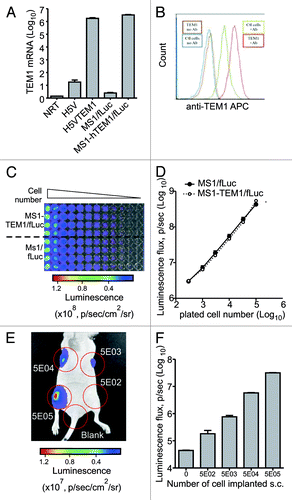
When injected s.c. in the flanks of nu/nu mice, MS1/fLuc and MS1-hTEM1/fLuc cells within two weeks established detectable hemangioma grafts with similar bioluminescence intensity, which persisted for up to 15 wk (data not shown). Next, we evaluated the lower limit of detection of luciferase-positive MS1/fLuc cells that had to be injected in order to detect an endothelial graft using bioluminescence imaging in vivo. We injected a total of 10 × 106 MS1 cells, of which a variable proportion of cells were fLuc-positive MS1 cells (5 × 102 to 5 × 105). There was a linear relationship between the number of MS1/fLuc cells injected and the luminescence intensity detected, with endothelial grafts detectable two weeks after injection of as few as 5 × 102 MS1/fLuc cells ().
Development of a tumor vascular model expressing hTEM1
To test an in vivo model of tumor vasculature expressing hTEM1, mouse ovarian ID8 tumor cells (2 × 106) were injected s.c. in the mouse flank, alone or admixed with MS1-hTEM1/fLuc or control MS1/fLuc cells (10 × 106) to create mixed or chimeric tumor grafts consisting of both exogenous tumor and endothelial cells. Mice were subjected to optical imaging 14–21 d after graft implantation. Tumors established only with ID8 cells were palpable, but exhibited no luminescence. In contrast, chimeric tumors formed from ID8 and MS1-hTEM1/fLuc or MS1/fLuc cells were readily detectable by bioluminescence, and with similar intensity (). All three tumor grafts recruited vasculature that was grossly visible (). Analysis of tissue sections () and hemoglobin content of the implants () revealed that tumors supplemented with MS1-hTEM1/fLuc or MS1/fLuc cells showed enhanced vascularization relative to tumors formed with ID8 cells only. There was no appreciable difference in vascularization between tumors enriched with MS1-hTEM1/fLuc and tumors enriched with MS1/fLuc cells ().
Figure 2. Characterization of the in vivo chimeric vascular-tumor graft model. Chimeric grafts were produced in nu/nu mice by s.c. injection of 106 ID8 cells alone or by implantation of 5 × 106 MS1-TEM1/fLuc or control MS1/fLuc cells mixed with 5 × 105 ID8 tumor cells in 100 μL of serum-free cell culture media. (A) Optical imaging detects chimeric ID8 tumors enriched with MS1/fLuc and MS1-hTEM1/fLuc endothelial cells, while as expected, ID8 tumors lacking MS1/fLuc cells could not be visualized. (B) Representative images of gross vascularization of ID8 tumors alone or those tumors enriched with MS1/fLuc or MS1-hTEM/fLuc endothelial cells. (C) Hemoglobin content of ID8 tumors with or without additional MS1 endothelial cells (n = 7). *P < 0.05; ns, no significance by Student t test. (D) Immunostaining (IHC) with biotinylated TEM1 mAb MORAb-004-biotin shows TEM1-staining of MS1-TEM1/fLuc cells forming capillaries (arrowhead, D).
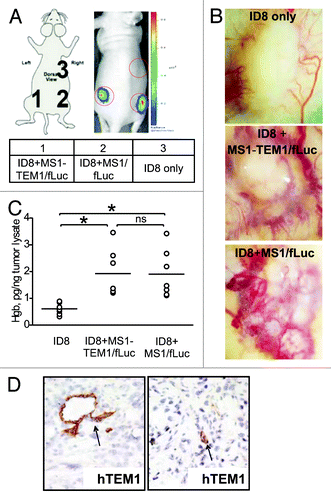
Immunohistochemistry (IHC) using MORAb-004 mAb revealed the expression of hTEM1 in tumors located within endothelial cells of tumor capillaries (). Since the proportion of tumor vessels positive for hTEM1 varied (data not shown), we optimized the ratio of endothelial MS1-hTEM1/fLuc endothelial cells to ID8 tumor cells in vivo in order to maximize the number of hTEM1-positive cells in vessels. We found that a MS1-hTEM1/fLuc:ID8 cell ratio between 10:1 and 20:1 was optimal for adequate tumor growth without excessive dilution of MS1 cells, resulting in a sufficient number of vessels expressing hTEM1 for robust and reproducible luminescent intensity of tumors.
TEM1 tumor-specific targeted therapy using MORAb-004
MORAb-004 is a humanized monoclonal IgG1 to the fibronectin-binding domain of hTEM1, neutralizing the adhesive functions of TEM1 and inhibiting migration of human endothelial cells in vitro.Citation15 However, the lack of mouse models restricts testing the effects of MORAb-004 in vivo. Having a xenograft hTEM-1 tumor model developed, we tested effect of MORAb-004 on development of hTEM1-positive vascular grafts in mice. We first employed the angioma graft model, where mice were first inoculated with MS1-hTEM1/fLuc and MS1/fLuc cells in opposite flanks. Mice received i.p. injections of 100 μg (5 mg/kg) naked MORAb-004 mAb or control IgG1 in an equivalent volume of PBS (100 μL) starting on the day of tumor inoculation and every 48 h thereafter, for 2 wk. No apparent signs of toxicity, such as weight loss, fur changes or reduced mobility, were observed in response to treatment. Control IgG1 had no effect on the formation of hemangioma grafts by either hTEM1-positive or hTEM1-negative MS1 cells. In contrast, MORAb-004 induced near-complete suppression of hemangioma graft formation by hTEM1-positive endothelial cells (n = 8 per group). No effect was observed on the contralateral graft developed with hTEM1-negative MS1/fLuc cells ().
Figure 3. TEM1-specific targeting in vivo by MORAb-004. (A) Subcutaneous (s.c.) MS1 angioma grafts were produced in nu/nu mice by a subcutaneous injection of 5 × 106 MS1-hTEM1/fLuc or control MS1/fLuc cells in 100 μL of serum-free cell culture media. MORAb-004 (5 mg/kg) was injected intraperitoneally (i.p.) at day 0 and every 48 h thereafter for 2 wk. Optical bioluminescence imaging was performed every other day to monitor the survival of MS1 endothelial cells expressing fLuc (n = 8). Representative images from one animal with both MS1-hTEM1/fLuc (arrow) and control MS1/fLuc cells (red arrowhead) at indicated time points are shown in (B); (C) Admixed ID8 + MS1 s.c. grafts were produced in nu/nu mice. MORAb-004 (5 mg/kg) was injected i.p. at day 0 and every 48 h, and optical bioluminescence imaging were performed every other day to monitor the survival of MS1/fLuc endothelial cells (n = 8). After 2 wk, animals bearing chimeric ID8 + MS1 tumor grafts were euthanized and a photograph of the graft was taken ex vivo (D).
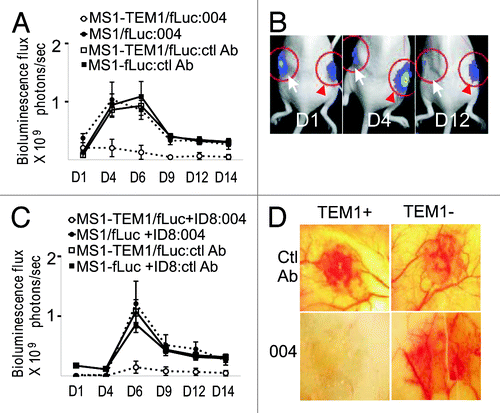
We further examined whether MORAb-004 inhibits formation of hTEM1-positive vessels in ID8 tumors. Mice were inoculated with ID8 cells admixed with MS1-hTEM1/fLuc or MS1/fLuc cells into opposite flanks and then treated with MORAb-004 (5 mg/kg) three times per week starting on the day of tumor inoculation as above in the angioma therapy study (n = 8 per group). MORAb-004 induced a near complete suppression of establishment of hTEM1-positive MS1/fLuc vessels within tumor grafts, while it did not affect hTEM1-negative MS1/fLuc tumors, as assessed by bioluminescence imaging (). Control IgG1 had no effect on the establishment of hTEM1-positive MS1/fLuc cells or hTEM1-negative MS1 cells within tumor grafts (). Thus, MORAb-004 suppresses the formation of hTEM1-expressing tumor neovasculature in vivo.
Because human IgG1 can trigger antibody-dependent cell-mediated cytotoxicity (ADCC) in the mouse through the activation of murine Fc receptor (FcR),Citation34 we assessed whether MORAb-004 (a humanized IgG1) could induce killing of cell targets expressing human TEM1 by murine macrophages. We found that MORAb-004 binds to murine monocytes via FcR, and this binding could be blocked by anti-murine CD16/CD32 antibody (Fig. S4A). Furthermore, addition of MORAb-004 led to increased killing of MS1-hTEM1/fLuc cells when co-cultured with splenocytes from nude mice. Splenocyte activation, as assessed by elevated secretion of monocyte chemotactic protein-1 (MCP-1) levels, increased in a specific manner when MORAb-004 bound hTEM1 on MS1-hTEM1/fLuc cells (Fig. S4B and C).
Characterization of radiolabeled MORAb-004 binding to hTEM1 in vitro
MORAb-004 binds to human TEM1 with high affinityCitation15 and biotinylated MORAb-004 discriminates hTEM1-positive MS1/fLuc cells from hTEM1-negative MS1/fLuc cells (see ). ELISA with MS1-hTEM1/fLuc and SKOV3-hTEM1 cells confirmed binding specificity to hTEM1 regardless of the cell line of origin, revealing an IC50 of 1.54 ± 0.05 nM and 1.65 ± 0.10 nM, respectively (Fig. S3). Radioiodination with 125I/124I produced labeled MORAb-004 with the yields of 65−95%, radiochemical purity >98% and specific radioactivity of 5−10 mCi/mg and 15−20 mCi/mg for [125I]-MORAb-004 and [124I]-MORAb-004, respectively. The apparent binding affinity Kd of [125I]-MORAb-004 to MS1-hTEM1/fLuc was 0.52 ± 0.02 nM with up to 1.3 × 106 mAb binding sites, Bmax, available per cell (). There was no binding to control MS1/fLuc cells. Similar Kd values were obtained with SKOV3-hTEM1 cells (Fig. S3). A competitive (radioimmunoassay) RIA with intact MORAb-004 demonstrated that the apparent Kd of non-labeled MORAb-004 was 0.75 ± 0.06 nM, suggesting that the affinity of [125I]-MORAb-004 was not adversely affected by radioiodination (). The immunoreactivity of MORAb-004 following radiolabeling with 124I was also determined by the Lindmo assay,Citation35 where the immunoreactivity was 90−97% immediately following labeling, and was maintained up to 2 wk post-labeling (). Taken together, these data demonstrate that a high radiolabeling yield, coupled with sub-nanomolar affinity to hTEM1, [124I]-MORAb-004 appears-suitable for further in vivo immuno-PET validation.
Figure 4. In vitro binding of [125/124I]-MORAb-004 to live cells expressing human TEM1. The binding of radioiodinated MORAb-004 mAb ([125I]-MORAb-004) to hTEM1 was assessed in live cells expressing hTEM1 (MS1-hTEM1/fLuc) compared with control cells not expressing hTEM1 (MS1/fLuc). (A) Cell surface binding of radioiodinated hTEM1 mAb [125I]-MORAb-004 was determined by live-cell radioimmunoassay (RIA). Adherent cells were incubated with increasing concentrations of [125I]-MORAb-004 for 2 h at 4 °C, and binding data were plotted as [125I]-mAb molecules bound per cell ([125I]-mAb/cell) (with Scatchard binding plot as inset). The binding affinity (Kd) of [125I]-MORAb-004 and corresponding Bmax per cell were calculated based on data points fitting as described in Materials and Methods. (B) The competitive inhibition of TEM1 binding was determined by co-incubation of [125I]-MORAb-004 with increasing concentrations of unlabeled MORAb-004 with cells for 2 h at 4 °C. Binding data were plotted as [125I]-mAb molecules bound per cell (mAb/cell), and the IC50 for MORAb-004 was determined by fitting this data to a four-parameter fit as described in Methods. (C) Immunoreactivity (IR) of [124I]-MORAb-004, was determined by Lindmo assay, with serial dilutions of cell suspension incubated with 10 ng of [124I]-MORAb-004 for 1 h at 37 °C. IR, by this method, was >97%. The radiolabeling efficiency for [124I]-MORAb-004 and [125I]-MORAb-004 was calculated as is totally activity bound/total activity in reaction. Radiochemical purity was determined by a trichloroacetic acid precipitation assay. Using a Nanodrop spectrophotometer (Thermofisher) to determine mAb concentrations post-labeling, the [125/124I]-mAb specific activities were reporter as mCi/mg.
![Figure 4. In vitro binding of [125/124I]-MORAb-004 to live cells expressing human TEM1. The binding of radioiodinated MORAb-004 mAb ([125I]-MORAb-004) to hTEM1 was assessed in live cells expressing hTEM1 (MS1-hTEM1/fLuc) compared with control cells not expressing hTEM1 (MS1/fLuc). (A) Cell surface binding of radioiodinated hTEM1 mAb [125I]-MORAb-004 was determined by live-cell radioimmunoassay (RIA). Adherent cells were incubated with increasing concentrations of [125I]-MORAb-004 for 2 h at 4 °C, and binding data were plotted as [125I]-mAb molecules bound per cell ([125I]-mAb/cell) (with Scatchard binding plot as inset). The binding affinity (Kd) of [125I]-MORAb-004 and corresponding Bmax per cell were calculated based on data points fitting as described in Materials and Methods. (B) The competitive inhibition of TEM1 binding was determined by co-incubation of [125I]-MORAb-004 with increasing concentrations of unlabeled MORAb-004 with cells for 2 h at 4 °C. Binding data were plotted as [125I]-mAb molecules bound per cell (mAb/cell), and the IC50 for MORAb-004 was determined by fitting this data to a four-parameter fit as described in Methods. (C) Immunoreactivity (IR) of [124I]-MORAb-004, was determined by Lindmo assay, with serial dilutions of cell suspension incubated with 10 ng of [124I]-MORAb-004 for 1 h at 37 °C. IR, by this method, was >97%. The radiolabeling efficiency for [124I]-MORAb-004 and [125I]-MORAb-004 was calculated as is totally activity bound/total activity in reaction. Radiochemical purity was determined by a trichloroacetic acid precipitation assay. Using a Nanodrop spectrophotometer (Thermofisher) to determine mAb concentrations post-labeling, the [125/124I]-mAb specific activities were reporter as mCi/mg.](/cms/asset/6a8a9b63-f762-4e24-8ac0-bae5ff2dc56c/kcbt_a_10927825_f0004.gif)
In vivo biodistribution and immuno-PET with radioiodinated MORAb-004
We next addressed targeting of hTEM1-expressing tumor vasculature by [124I]-MORAb-004. Biodistribution studies with [125I]-MORAb-004 at 1, 4, and 24 h post injection were performed in a graft model with ID8 plus MS1-hTEM1/fLuc and ID8 plus MS1/fLuc tumors. As supported by ROI analysis of PET data, uptake by hTEM1-positive tumor was high at 4 h post injection and was maintained at 24 h (). Blood uptake at 1 h post injection was fairly high at 27.7% ID/g as expected for IgG mAbs, and slowly decreased over time to 7.9% ID/g at 24 h. hTEM1-positive tumor to blood or muscle ratios increased over time, whereas hTEM1-positive to negative tumors were quite high at 6.9 at 4 h post injection and reached 7.3 at 24 h. Thyroid uptake slowly increased to 2% ID/organ suggesting slow dehalogenation of the radioiodine.
Table 1. Biodistribution of radioiodinated [125I]-MORAb-004 in ID8+MS1-hTEM1/fLuc and ID8+MS1/fLuc tumor bearing mice at 1, 4, and 24 h p.i.
Small animal PET imaging of mice 18 h following intravenous administration [124I]-MORAb-004 revealeduptake in mice with tumors harboring hTEM1-positive vasculature, with negligible uptake in tumors whose vasculature did not express hTEM1 (). There was also negligible circulating activity in the blood pool, and abdomen. At 6 d, overall tumor activity was reduced but the hTEM1-positive tumor remained positive by PET as compared with surrounding tissue ().
Figure 5. In vivo ImmunoPET imaging of tumor-bearing mice with [124I]-MORAb-004. (A) Nude mice bearing tumors developed with ID8 cells and MS1-hTEM1/fLuc cells (arrow) or control tumors developed with ID8 cells and MS1/fLuc cells (red arrowhead) were subject to bioluminescence imaging two weeks following s.c. cell inoculation. (B and C) Small animal PET imaging of the same mice were conducted (B) 18 h, and (C) 6 d post-injection of [124I]-MORAb-004 (~80 μCi, 5 μg per mouse). Mice were pre-fed KI for 24 h prior to PET. Serial posterior transverse (top row) and coronal (bottom row) PET images are shown (0.5 mm thick slices). A strong 124I-PET signal is present in the hTEM1-postive tumor (arrow), with clear delineation. No uptake could be appreciated in the hTEM1-negative tumor. (D and E) Estimated activities and tissue uptake ratios as measured by PET from ROIs drawn on tumors, liver, and soft tissue at 18 h (D) and 6 d (E) post-injection of [124I]-MORAb-004.
![Figure 5. In vivo ImmunoPET imaging of tumor-bearing mice with [124I]-MORAb-004. (A) Nude mice bearing tumors developed with ID8 cells and MS1-hTEM1/fLuc cells (arrow) or control tumors developed with ID8 cells and MS1/fLuc cells (red arrowhead) were subject to bioluminescence imaging two weeks following s.c. cell inoculation. (B and C) Small animal PET imaging of the same mice were conducted (B) 18 h, and (C) 6 d post-injection of [124I]-MORAb-004 (~80 μCi, 5 μg per mouse). Mice were pre-fed KI for 24 h prior to PET. Serial posterior transverse (top row) and coronal (bottom row) PET images are shown (0.5 mm thick slices). A strong 124I-PET signal is present in the hTEM1-postive tumor (arrow), with clear delineation. No uptake could be appreciated in the hTEM1-negative tumor. (D and E) Estimated activities and tissue uptake ratios as measured by PET from ROIs drawn on tumors, liver, and soft tissue at 18 h (D) and 6 d (E) post-injection of [124I]-MORAb-004.](/cms/asset/8a73b36d-4dac-40f3-a4f3-7c88bb14d070/kcbt_a_10927825_f0005.gif)
Region-of-interest (ROI) analysis of the PET imaging data showed uptake values ranging from 11.6% to 19.3% ID/mL for hTEM1-positive tumor tissue at 18 h (). Control MS1/fLuc and ID8 tumor uptake was 1.6−2.4% ID/mL. hTEM1-positive tumor to hTEM-negative tumor (target to non-target) ratios ranged from 7.4 to 8 at 18 h. The fold-increase in uptake between hTEM1-positive to negative tumor grafts at 6 d remained high at 3.2 and 3 (), although it was clear that there was washout over the longitudinal study. A longitudinal imaging study with mice that were not pre-blocked with KI did reveal prominent thyroid uptake of radioiodine suggesting that dehalogenation is occurring over time (Video S1).
Discussion
TEM1 plays an important role in tumor neovascularization,Citation29 site of its specific expression in the adult vasculatureCitation4,Citation16,Citation17,Citation19-Citation23 and thus represents a promising target for tumor therapy and imaging. However, interspecies differences impede development of antibody based TEM-1 targeting. For example, although human and mouse TEM1 share 87.8% homology (UniGene by NCBI), MORAb-004 only recognizes human and monkey antigen but has no cross-reactivity with mouse TEM1.Citation15,Citation32 MORAb-004 is currently in several phase I/II clinical trials in cancer patients; However, MORAb-004 has not been tested in vivo in the mouse to date.
To complement the hTEM1 knock-in mouse model (Morphotek, Inc.), we have developed a vascular graft mouse model with tumor endothelium that expresses human TEM1, using MS1 cells transfected with both hTEM1 and luciferase (hTEM1/fLuc), allowing for optical imaging of engraftment. Admixed at an optimal ratio with ID8 tumor cells, these “marker” transfected MS1 cells formed tumors with hTEM-1-positive vessels. Of note, ID8 tumors enriched with MS1 cells appeared more congested, and their vascular density was increased vs ID8 tumors lacking MS1 cells, implying that MS1 cells participated in tumor vasculature formation. Importantly, however, administration of MORAb-004 in therapeutic doses (5 mg/kg) effectively suppressed the engraftment of MS1-hTEM1 cells in vivo both in the context of chimeric MS1:ID8 tumors as well as in the MS1 angioma model. It is not known whether the therapeutic effect of MORAb-004 was due to functional inhibition of TEM1-mediated adhesive interactions, as previously shown for human endothelial cells.Citation15 However, human IgG can trigger antibody-dependent cell-mediated cytotoxicity (ADCC) through murine macrophages,Citation34 thus activation of ADCC could explain the therapeutic effect seen in our model. In humans, besides ADCC, an additional direct therapeutic effect may be involved, based on the demonstrated neutralizing properties of the antibody in vitro.Citation15
Although TEM1 is a quasi-universal tumor target present in the vasculature of many solid tumor types, a variable proportion of tumors—depending on histotype—show low or no TEM1 expression.Citation36 A non-invasive detection of TEM1 in tumors may facilitate the selection of patients for specific therapies and would allow monitoring of therapeutic effect in real-time using immuno-PET.Citation13,Citation37,Citation38 Using our new hTEM1 vascular chimeric tumor graft mouse model, we studied tumor targeting of 124I-labeled mAb MORAb-004. Both analysis of isotope in organs postmortem and PET/CT validated the specific uptake of MORAb-004 in tumors expressing hTEM1, detectable by PET imaging. Studies titrating number of hTEM-1 positive cells showed that this approach is very sensitive and two weeks after tumor injection can indeed detect tumors that originally contained only 5000 TEM1-positive cells.
These findings have significant implications for imaging TEM1-positive tumors and their eradication. In addition to effect(s) of mAb leading to inhibition of tumors described above, delivery of isotopes emitting local radiation directed to either vascular or parenchymal tumor cells can be employed. Seeing as many tumor types, especially sarcomas, have tumor stroma highly positive for TEM1, TEM1-targeted radioimmunotherapy could lead to widespread tumor destruction.
Although in our model TEM1 is exclusively expressed on tumor endothelial cells, in human tumors, in addition to endothelial progenitor cellsCitation24 and endothelial cells, TEM1 is also expressed by pericytes and tumor associated fibroblasts.Citation25-Citation27 However, IgG can readily cross the leaky basement membrane of tumor vasculature and extravasate in the perivascular stroma, suggesting that MORAb-004 could bind readily to both endothelial as well as pericyte epitopes, thus making our model applicable to human disease.
In summary, we have established a chimeric endothelial-tumor cell graft model in the mouse that enables us to study the targeted delivery and subsequent effects of human grade therapeutics that have specificity to human but not mouse tumor vascular epitopes. We used this model to validate the application of 124I-based immuno-PET to non-invasively image tumors in vivo whose vasculature expresses hTEM1, and to show the therapeutic value of targeting tumor vasculature through MORAb-004. This model has the potential to accelerate the pace of developing theranostic approaches targeting the tumor vasculature, an emerging and important area in targeted tumor therapeutics.
Materials and Methods
Antibodies
Humanized anti-hTEM1 mAb MORAb-004 and biotinylated MORAb-004 used for FACS analysis and western blotting was from Morphotek.
Antibody radioiodination
MORAb-004 was radioiodinated with [125I] or [124I] using Iodogen vials (Pierce Thermoscientific) as we describedCitation39 and purified using desalting column (Pierce Thermoscientific) with 75–99% radiochemical yield.
“Naked” mAb therapy in vivo
Mice bearing MS1-hTEM1/fLuc or MS1/fLuc angioma grafts, or mice with ID8 tumor grafts enriched with MS1-hTEM1/fLuc or MS1/fLuc cells/vasculature, were treated with MORAb-004 mAb or control IgG at 5 mg/kg. Antibodies were injected i.p. in an equivalent volume (100 μL) of PBS three times per week for two weeks, starting on the day of tumor inoculation. Mice were observed daily for signs of toxicity, such as weight loss or lethargy, in response to treatment. MS1-hTEM1/fLuc or MS1/fLuc cells were monitored by quantitative bioluminescence imaging. Tumor volume was determined three times per week by measurement with calipers.
Biodistribution studies
Biodistribution studies were performed in nu/nu (strain 088 Nude−/−, Charles River) mice bearing ID8 tumors enriched with MS1-hTEM1/fLuc cells or control MS1/fLuc cells. Approximately 5 μCi of [125I]-MORAb-004 (5 μg/mouse) in 150 μL saline was injected into the lateral tail vein. The animals were sacrificed at 4, 24, and 48 h post-injection (p.i.) by cardiac excision under isofluorane anesthesia. The radioactivity in organs was measured using a gamma counter (1470 Wallac Wizard, Perkin-Elmer). Results were expressed as the percentage of injected dose (ID) per gram of tissue (%ID/g). Each value represents the mean ± SD of 3 mice, unless otherwise noted.
Animal imaging studies
In vivo bioluminescence imaging was performed as described elsewhere.Citation37 Longitudinal mouse imaging study by 124I-PET (n = 2 for each independent study), was performed using a Philips Mosaic Animal PET (A-PET) imaging system with an imaging field of view of ~11.5 cm.Citation38 Mice were pre-dosed with potassium iodide (KI) for thyroid blocking. Please refer to the Supplemental Materials for constructs, cell lines, antibody characterization, animals, and statistical analyses.
Additional material
Download Zip (996.9 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH transformative R01CA156695; Department of Defense BC094849; and the Honorable Tina Brozman Foundation. C.L. is also supported by funding from Basser Research Center for BRCA1/2, UPenn.
References
- Parker BS, Argani P, Cook BP, Liangfeng H, Chartrand SD, Zhang M, Saha S, Bardelli A, Jiang Y, St Martin TB, et al. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res 2004; 64:7857 - 66; http://dx.doi.org/10.1158/0008-5472.CAN-04-1976; PMID: 15520192
- Lu C, Bonome T, Li Y, Kamat AA, Han LY, Schmandt R, Coleman RL, Gershenson DM, Jaffe RB, Birrer MJ, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res 2007; 67:1757 - 68; http://dx.doi.org/10.1158/0008-5472.CAN-06-3700; PMID: 17308118
- Buckanovich RJ, Sasaroli D, O’Brien-Jenkins A, Botbyl J, Hammond R, Katsaros D, Sandaltzopoulos R, Liotta LA, Gimotty PA, Coukos G. Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol 2007; 25:852 - 61; http://dx.doi.org/10.1200/JCO.2006.08.8583; PMID: 17327606
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, et al. Genes expressed in human tumor endothelium. Science 2000; 289:1197 - 202; http://dx.doi.org/10.1126/science.289.5482.1197; PMID: 10947988
- Bhati R, Patterson C, Livasy CA, Fan C, Ketelsen D, Hu Z, Reynolds E, Tanner C, Moore DT, Gabrielli F, et al. Molecular characterization of human breast tumor vascular cells. Am J Pathol 2008; 172:1381 - 90; http://dx.doi.org/10.2353/ajpath.2008.070988; PMID: 18403594
- Madden SL, Cook BP, Nacht M, Weber WD, Callahan MR, Jiang Y, Dufault MR, Zhang X, Zhang W, Walter-Yohrling J, et al. Vascular gene expression in nonneoplastic and malignant brain. Am J Pathol 2004; 165:601 - 8; http://dx.doi.org/10.1016/S0002-9440(10)63324-X; PMID: 15277233
- Ulahannan SV, Brahmer JR. Antiangiogenic agents in combination with chemotherapy in patients with advanced non-small cell lung cancer. Cancer Invest 2011; 29:325 - 37; http://dx.doi.org/10.3109/07357907.2011.554476; PMID: 21469981
- Mulder K, Scarfe A, Chua N, Spratlin J. The role of bevacizumab in colorectal cancer: understanding its benefits and limitations. Expert Opin Biol Ther 2011; 11:405 - 13; http://dx.doi.org/10.1517/14712598.2011.557657; PMID: 21281258
- Merritt WM, Sood AK. Markers of angiogenesis in ovarian cancer. Dis Markers 2007; 23:419 - 31; http://dx.doi.org/10.1155/2007/257602; PMID: 18057525
- Lanza GM, Winter PM, Caruthers SD, Hughes MS, Hu G, Schmieder AH, Wickline SA. Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis 2010; 13:189 - 202; http://dx.doi.org/10.1007/s10456-010-9166-0; PMID: 20411320
- Street JM, Dear JW. The application of mass-spectrometry-based protein biomarker discovery to theragnostics. Br J Clin Pharmacol 2010; 69:367 - 78; http://dx.doi.org/10.1111/j.1365-2125.2009.03610.x; PMID: 20406221
- van Dongen GA, Visser GW, Lub-de Hooge MN, de Vries EG, Perk LR. Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist 2007; 12:1379 - 89; http://dx.doi.org/10.1634/theoncologist.12-12-1379; PMID: 18165614
- Divgi CR, Pandit-Taskar N, Jungbluth AA, Reuter VE, Gönen M, Ruan S, Pierre C, Nagel A, Pryma DA, Humm J, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 2007; 8:304 - 10; http://dx.doi.org/10.1016/S1470-2045(07)70044-X; PMID: 17395103
- Becker R, Lenter MC, Vollkommer T, Boos AM, Pfaff D, Augustin HG, Christian S. Tumor stroma marker endosialin (Tem1) is a binding partner of metastasis-related protein Mac-2 BP/90K. FASEB J 2008; 22:3059 - 67; http://dx.doi.org/10.1096/fj.07-101386; PMID: 18490383
- Tomkowicz B, Rybinski K, Foley B, Ebel W, Kline B, Routhier E, Sass P, Nicolaides NC, Grasso L, Zhou Y. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci U S A 2007; 104:17965 - 70; http://dx.doi.org/10.1073/pnas.0705647104; PMID: 17986615
- Christian S, Ahorn H, Koehler A, Eisenhaber F, Rodi HP, Garin-Chesa P, Park JE, Rettig WJ, Lenter MC. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J Biol Chem 2001; 276:7408 - 14; http://dx.doi.org/10.1074/jbc.M009604200; PMID: 11084048
- Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci U S A 1992; 89:10832 - 6; http://dx.doi.org/10.1073/pnas.89.22.10832; PMID: 1438285
- Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D, Augustin HG. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol 2008; 172:486 - 94; http://dx.doi.org/10.2353/ajpath.2008.070623; PMID: 18187565
- Brady J, Neal J, Sadakar N, Gasque P. Human endosialin (tumor endothelial marker 1) is abundantly expressed in highly malignant and invasive brain tumors. J Neuropathol Exp Neurol 2004; 63:1274 - 83; PMID: 15624764
- Buckanovich RJ, Sasaroli D, O’brien-Jenkins A, Botbyl J, Conejo-Garcia JR, Benencia F, Liotta LA, Gimotty PA, Coukos G. Use of immuno-LCM to identify the in situ expression profile of cellular constituents of the tumor microenvironment. Cancer Biol Ther 2006; 5:635 - 42; http://dx.doi.org/10.4161/cbt.5.6.2676; PMID: 16627987
- Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer 2005; 5:436 - 46; http://dx.doi.org/10.1038/nrc1627; PMID: 15928674
- Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res 2001; 61:6649 - 55; PMID: 11559528
- Walter-Yohrling J, Morgenbesser S, Rouleau C, Bagley R, Callahan M, Weber W, Teicher BA. Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res 2004; 10:2179 - 89; http://dx.doi.org/10.1158/1078-0432.CCR-03-1013; PMID: 15041739
- Bagley RG, Rouleau C, St Martin T, Boutin P, Weber W, Ruzek M, Honma N, Nacht M, Shankara S, Kataoka S, et al. Human endothelial precursor cells express tumor endothelial marker 1/endosialin/CD248. Mol Cancer Ther 2008; 7:2536 - 46; http://dx.doi.org/10.1158/1535-7163.MCT-08-0050; PMID: 18723498
- Simonavicius N, Robertson D, Bax DA, Jones C, Huijbers IJ, Isacke CM. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod Pathol 2008; 21:308 - 15; http://dx.doi.org/10.1038/modpathol.3801006; PMID: 18192970
- Wesseling P, Schlingemann RO, Rietveld FJ, Link M, Burger PC, Ruiter DJ. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol 1995; 54:304 - 10; http://dx.doi.org/10.1097/00005072-199505000-00003; PMID: 7745429
- MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR, Panico M, Sutton-Smith M, Dell A, van der Geer P, et al. Endosialin (TEM1, CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett 2005; 579:2569 - 75; http://dx.doi.org/10.1016/j.febslet.2005.03.071; PMID: 15862292
- Davies G, Cunnick GH, Mansel RE, Mason MD, Jiang WG. Levels of expression of endothelial markers specific to tumour-associated endothelial cells and their correlation with prognosis in patients with breast cancer. Clin Exp Metastasis 2004; 21:31 - 7; http://dx.doi.org/10.1023/B:CLIN.0000017168.83616.d0; PMID: 15065600
- Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C, Vogelstein B, St Croix B, Kinzler KW, Huso DL. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci U S A 2006; 103:3351 - 6; http://dx.doi.org/10.1073/pnas.0511306103; PMID: 16492758
- Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood 2005; 105:679 - 81; http://dx.doi.org/10.1182/blood-2004-05-1906; PMID: 15358628
- Rettig WJ. Immunogenetics of cell surface antigens of human cancer. Curr Opin Immunol 1992; 4:630 - 40; http://dx.doi.org/10.1016/0952-7915(92)90039-H; PMID: 1418731
- Zhou Y, Grasso L, Tomkowicz B, Ebel W, Routhier E, Phillips MD, Sass P, Old L, Nicolaides NC. Targeting endosialin/TEM1 as a novel anti-angiogenic agent for the treatment of neovascular disease and cancer. AACR poster 2008;http://www.morphotek.com/CorporateSite/media/Posters/Preclinical-MORAb-004-Study.pdf.
- Hasegawa K, Pham L, O’Connor MK, Federspiel MJ, Russell SJ, Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res 2006; 12:1868 - 75; http://dx.doi.org/10.1158/1078-0432.CCR-05-1803; PMID: 16551872
- Steplewski Z, Sun LK, Shearman CW, Ghrayeb J, Daddona P, Koprowski H. Biological activity of human-mouse IgG1, IgG2, IgG3, and IgG4 chimeric monoclonal antibodies with antitumor specificity. Proc Natl Acad Sci U S A 1988; 85:4852 - 6; http://dx.doi.org/10.1073/pnas.85.13.4852; PMID: 3387441
- Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA Jr.. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984; 72:77 - 89; http://dx.doi.org/10.1016/0022-1759(84)90435-6; PMID: 6086763
- Rouleau C, Curiel M, Weber W, Smale R, Kurtzberg L, Mascarello J, Berger C, Wallar G, Bagley R, Honma N, et al. Endosialin protein expression and therapeutic target potential in human solid tumors: sarcoma versus carcinoma. Clin Cancer Res 2008; 14:7223 - 36; http://dx.doi.org/10.1158/1078-0432.CCR-08-0499; PMID: 19010839
- Pryma DA, O’Donoghue JA, Humm JL, Jungbluth AA, Old LJ, Larson SM, Divgi CR. Correlation of in vivo and in vitro measures of carbonic anhydrase IX antigen expression in renal masses using antibody 124I-cG250. J Nucl Med 2011; 52:535 - 40; http://dx.doi.org/10.2967/jnumed.110.083295; PMID: 21421715
- O’Donoghue JA, Smith-Jones PM, Humm JL, Ruan S, Pryma DA, Jungbluth AA, Divgi CR, Carrasquillo JA, Pandit-Taskar N, Fong Y, et al. 124I-huA33 antibody uptake is driven by A33 antigen concentration in tissues from colorectal cancer patients imaged by immuno-PET. J Nucl Med 2011; 52:1878 - 85; http://dx.doi.org/10.2967/jnumed.111.095596; PMID: 22068895
- Chacko AM, Nayak M, Greineder CF, Delisser HM, Muzykantov VR. Collaborative enhancement of antibody binding to distinct PECAM-1 epitopes modulates endothelial targeting. PLoS One 2012; 7:e34958; http://dx.doi.org/10.1371/journal.pone.0034958; PMID: 22514693
