Abstract
Tyrosine kinase inhibitors are de facto the most commonly used targeted therapies for upfront treatment of metastatic renal cell carcinoma (mRCC). After the first era in which targeted agents were compared with placebo and interferon-α, a new phase has started in recent years characterized by head-to-head trials comparing targeted agents in superiority or non-inferiority trials. Recently, the results a head-to-head phase III trial comparing axitinib to sorafenib as upfront therapy in patients affected by mRCC have been reported. We discuss several critical aspects of this study and the results of our metaanalysis on the activity of axitinib over sorafenib in a larger population with the intent to confirm the superiority of axitinib. Despite this, the definition of primary endpoint remains a central factor in determining the final results of a trial.
In recent years, two pathways have been emphasized as the main targets of therapy in metastatic renal cell carcinoma (mRCC): the vascular endothelial growth factor (VEGF) with its receptor (VEGFR) and the mammalian target of rapamicin (mTOR). Since 2006, five VEGF/VEGFR inhibitors (sorafenib, sunitinib, pazopanib, axitinib, and bevacizumab) and two mTOR inhibitors (temsirolimus and everolimus), have been approved for the treatment of mRCC, superseding cytokine-based therapy. Initially, targeted agents were compared with placebo and interferon-α, but more recently, a new phase has been characterized by head-to-head trials in which therapies are compared with each other in superiority and non-inferiority trials.
Recently, Hutson et al. reported the results of the head-to-head phase III AGILE trial that compared axitinib to sorafenib as upfront therapy in patients affected by mRCC.Citation1 In this study, treatment-naïve patients with measurable, clear-cell mRCC were randomized 2:1 to receive axitinib 5 mg BID or sorafenib 400 mg BID. The study aimed to show a reduction in the risk of progression by 44% (hazard ratio [HR] = 0.56, overall one-sided α = 0.025) in patients treated with axitinib compared with sorafenib, corresponding to a 78% improvement of progression-free survival (PFS) from 5.5 to 9.8 mo.
A total of 192 patients were treated with axitinib and 96 with sorafenib; the median PFS was 10.1 mo (95% CI 7.2–12.1) with axitinib and 6.5 mo (4.7–8.3) with sorafenib, but this difference was not statistically significant because the final HR was greater than the expected one (HR = 0.77, 95% CI 0.56–1.05; one-sided P = 0.038). In a pre-specified analysis, the PFS was confirmed to be better for axitinib compared with sorafenib (HR = 0.64, 95% CI 0.42–0.99; one-sided P = 0.022) in patients with an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0, while this was equal in patient with ECOG-PS of 1 (HR = 0.93, 95% CI 0.59–1.48; one-sided P = 0.38). Moreover, the study reported a higher overall response rate in favor of axitinib (32% vs. 15%; one-sided P = 0.0006) and an expected toxicity profile for both therapies.
Despite the increase in overall response rate (ORR) and PFS reached by axitinib, the median PFS of 10.1 mo was comparable to current first-line agents such as sunitinib (11.0 mo) and pazopanib (11.1 mo). This study remained formally negative because it did not reach the preplanned HR. The authors tried to explain these negative data by highlighting the good performance of sorafenib and worse performance of axitinib compared with the expected outcomes. In fact, the phase III AXIS trial testing second-line axitinib over sorafenib reported in the sub-group of treatment-naïve patients a significant difference in terms of PFS of 12.1 vs. 6.5 mo for axitinib and sorafenib, respectively (P < 0.0001).Citation2
Considering all these data, we tried to speculate on the methodological aspects leading to these negative results. We supported the authors’ conclusions, affirming that the primary endpoint to reduce the risk of progression by 44% might be considered broadly ambitious. Subsequently, we analyzed some aspects that might have contributed to this negative result. First, the control arm, sorafenib, cannot be considered as the standard first-line therapy in mRCC. In fact, when this study started enrollment (June 2010), the results of the major first-line trials in mRCC had only been largely presented at international conferences and published on PubMed, e.g., sunitinib (2006), bevacizumab + interferon-α (AVOREN trial, 2007), and pazopanib (2010).
Second, we do not completely agree with the sentence affirming that sorafenib performed better than in the historical data because the value of 5.5 mo expected in the study design was equal to the PFS reached in the TARGET trialCitation3 and close to the 5.7 mo reached in the phase II first-line trial.Citation4 This evaluation did not account for study biases, such as the 30% of patients treated with both IFN-α and IL-2 in the first study and the unfavorable selection of patients that characterized the second one. Furthermore, in the AXIS trial, sorafenib reported a median PFS of 6.5 mo with the upper bound of the 95% confidence interval reaching 8.3 mo.Citation2
Despite the negative data reported by Hutson, we tried to determine if a better outcome for axitinib could be reached by considering a larger population. To demonstrate this hypothesis, we metaanalyzed the HRs for PFS of the two studies that compared axitinib to sorafenib: the AGILE and AXIS trials.Citation1,Citation2 Regarding the AXIS trial, only 251 patients untreated with a previous targeted therapy were considered; in this group, axitinib decreased the risk of progression over sorafenib by 64% (HR = 0.464, 95% CI: 0.318–0.676; P < 0.0001). The metaanalysis was performed on a total population of 539 patients; among these, 318 received axitinib and 221 received sorafenib as their first targeted therapy. RevMan 5.2 software was used for the analysis.Citation5 The cumulative HR confirmed the superiority of axitinib, reporting a significant reduction in the risk of progression by 40% (HR = 0.60, random effect, 95% CI: 0.37–0.99; P = 0.04), as shown in .
Figure 1. Forest plot of cumulative HR for PFS in patients treated with axitinib or sorafenib as first targeted agent.
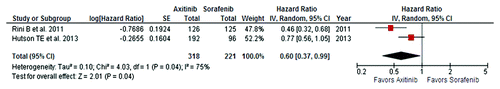
This analysis has several limitations. Among these, the most important is the previous treatment with cytokine therapy in patients enrolled in the AXIS trial. Conversely, the presence of cytokine-pretreated patients was largely reported in other two phase III trials that validated the efficacy of pazopanib and tivozanib as up-front targeted agents in mRCC.Citation6,Citation7
Finally, we can confirm the superior activity of axitinib over sorafenib in untreated patients with the largest reduction in the risk of progression reached in a head-to-head trial comparing two targeted agents in mRCC. Despite this positive result, the use of a larger population did not allow us to reach the pre-planned HR of 0.56 expected in the AGILE trial, confirming that a more feasible end-point added to a major effort to enroll patients may change the final result. At the moment, we cannot use axitinib as up-front therapy in mRCC, and we support the idea that ambitious end-points poorly guided by clinical evidence may be responsible for the lack of a promising shot in the war against cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, Rosbrook B, Chen C, Kim S, Vogelzang NJ. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013; 14:1287 - 94; http://dx.doi.org/10.1016/S1470-2045(13)70465-0; PMID: 24206640
- Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378:1931 - 9; http://dx.doi.org/10.1016/S0140-6736(11)61613-9; PMID: 22056247
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al, TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356:125 - 34; http://dx.doi.org/10.1056/NEJMoa060655; PMID: 17215530
- Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27:1280 - 9; http://dx.doi.org/10.1200/JCO.2008.19.3342; PMID: 19171708
- Manager R. (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012. Available at: http://ims.cochrane.org/revman/download
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010; 28:1061 - 8; http://dx.doi.org/10.1200/JCO.2009.23.9764; PMID: 20100962
- Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, Tomczak P, Lyulko O, Alyasova A, Harza M, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013; 31:3791 - 9; http://dx.doi.org/10.1200/JCO.2012.47.4940; PMID: 24019545
