Abstract
Current pemetrexed/platinum chemotherapy does not produce a satisfactory therapeutic response in advanced lung cancer patients. The aim of this study was to determine whether the administration of gefitinib, a tyrosine kinase inhibitor (TKI), intercalated with pemetrexed/platinum could improve the efficacy in chemotherapy-naïve patients with advanced non-squamous NSCLC without subsequent gefitinib maintenance therapy. Treatment-naïve patients with stage IIIB or IV NSCLC were randomly assigned to receive pemetrexed (500 mg/m2 d1) and either cisplatin (75 mg/m2 d1) or carboplatin (AUC = 5 d1) plus gefitinib (250 mg/d on days 3 to 16 of a 3-week cycle) (PC-G) or pemetrexed–platinum (PC) alone. Randomization was stratified according to the tobacco smoking status and EGFR mutational status of the patients. The primary endpoint was the non-progression rate (NPR) at 12 weeks. Secondary endpoints included progression-free survival (PFS), overall response rate (ORR), overall survival (OS), and biosafety. The NPR at 12 weeks was 84.5% for the PC-G treatment arm and 83.1% for the PC treatment arm (P = 0.87). Median PFS was 7.9 months for the PC-G arm and 7.0 months for the PC arm (P = 0.57). The ORR was 50.0% for the PC-G arm and 47.4% for the PC arm (P = 0.78). Median survival was 25.4 mo for the PC-G arm and 20.8 mo for the PC arm (P = 0.54). The incidence of adverse events was similar between the two treatment arms, except for a higher incidence of skin rash with PC-G. Predefined subgroup analyses demonstrated that PC-G significantly increased the PFS compared with the PC regimen in patients with EGFR mutations (P = 0.017). Although gefitinib intercalated with pemetrexed/platinum chemotherapy did not improve the NPR at 12 weeks compared with chemotherapy, an improvement in the PFS for the intercalated treatment arm was seen in the subgroup of patients with EGFR mutations.
Introduction
Non-small cell lung cancer (NSCLC) is one of the malignancies with the highest mortality rate worldwide. In many patients, the diagnosis is first established at an advanced stage of the disease.Citation1,Citation2 Current treatment for advanced lung cancer does not produce a satisfactory therapeutic response because only limited improvement in survival has been achieved over the past 30 y.Citation3 Much work has been dedicated to improving treatment by combining tyrosine kinase inhibitors of the epidermal growth factor receptor (EGFR-TKIs) with standard platinum-based doublet chemotherapy using a concurrent, intercalated, or maintenance approach.Citation4,Citation5
Randomized studies using a concurrent combination of EGFR-TKI and chemotherapy demonstrated no advantage in unselected NSCLC patients.Citation4,Citation5 Two factors that may have contributed to this lack of effect are (1) the potential antagonism of such drug combinations: preclinical studies have suggested that EGFR-TKIs can induce G1-phase cell cycle arrest, which could have spared the tumor cells from the cytotoxic activity of cell cycle phase-dependent chemotherapeutic agents,Citation6 and (2) the study subjects were not selected with regard to biomarkers: recent work has suggested that EGFR-TKIs produce a better clinical outcome in the NSCLC patients with a EGFR mutation than in those without a mutation. A phase II study (FAST-ACT I) on the sequential intercalated combination of erlotinib following chemotherapy with gemcitabine/platinum has shown improvement of PFS and well-tolerated toxicity when compared with gemcitabine/platinum alone as a first-line therapy.Citation7 Subsequently, the result of the phase III FAST-ACT II study confirmed that such an intercalated combination therapy could significantly improve the PFS, even the OS in the unselected patients with NSCLC population, especially in the patients with EGFR mutations.Citation8 However, in these two trials, erlotinib was administered sequentially during chemotherapy and maintained after the end of chemotherapy, which made it difficult to determine whether the survival benefit was due to the sequential therapy during chemotherapy, the subsequent maintenance treatment, or both. Therefore, it was necessary to design a study to investigate specifically the effect of sequential intercalated EGFR-TKI therapy on chemotherapy without any confounding effects from maintenance treatment.
For patients with non-squamous advanced (stage IIIB/IV) NCSLC, administration of pemetrexed plus cisplatin has demonstrated superior survival and modest toxicity compared with the combination cisplatin/gemcitabine, which has been regarded as the standard regimen for advanced NSCLC.Citation9,Citation10 However, early studies were always conducted using doublet regimens that did not include pemetrexed as the control therapy arm, which may have led to an overestimation of the benefit of the sequential EGFR-TKI therapy.Citation11,Citation12 In addition, sequential administration of pemetrexed followed by EGFR-TKI has proven to be promising.
This present randomized, phase II study was therefore conducted to evaluate the efficacy and toxicity of gefitinib intercalated with pemetrexed–platinum without gefitinib maintenance (PC-G) compared with pemetrexed–platinum (PC) alone as the first-line therapy in chemotherapy-naïve patients with advanced non-squamous NSCLC.
Results
Patients
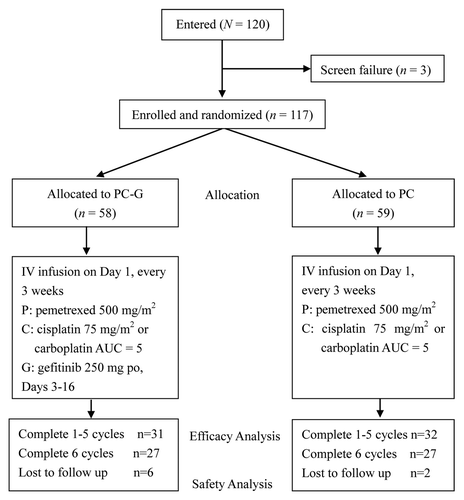
A total of 120 patients were screened to participate in the study, and 117 were randomized into the two treatment groups (58 for the PC-G arm; 59 for the PC alone) between January 2010 and May 2012 (). Baseline characteristics of the study patients were similar between the two groups ().
Table 1. Baseline patient characteristics by treatment group
Efficacy
The non-progression rate (NPR) at 12 wk was found to be 84.5% (49/58) for the PC-G arm and 83.1% (49/59) for the PC arm (P = 0.87). The objective response rate (ORR) was 50.0% (27/54) for the PC-G arm and 47.4% (27/57) for the PC arm (95% CI −1.6% to 2.1%; P = 0.78). Median PFS was 7.9 mo for the PC-G arm and 7.0 mo for the PC arm (HR: 0.88, 95% CI 0.56–1.37; P = 0.57). By the end of the study, 36.2% (21/58) of the patients in the PC-G group and 44.1% (26/59) in the PC group had died. The median OS was 25.4 mo for the PC-G arm compared with 20.8 mo for the PC arm (HR 0.84, 95% CI 0.47–1.48; P = 0.54) ().
Figure 2. Kaplan–Meier plots of progression-free survival (PFS) in both treatment groups. (A) PFS for all NSCLC patients; (B) PFS for never smokers; (C) PFS for current or ex-smokers; (D) PFS for patients with EGFR mutant; (E) PFS for patients with wild-type EGFR; (F) PFS for patients with unknown EGFR status; (G) Kaplan–Meier plots of overall survival (OS) in both treatment groups. Abbreviations: PC, pemetrexed plus either cisplatin or carboplatin; G, gefitinib; HR, hazard ratio.
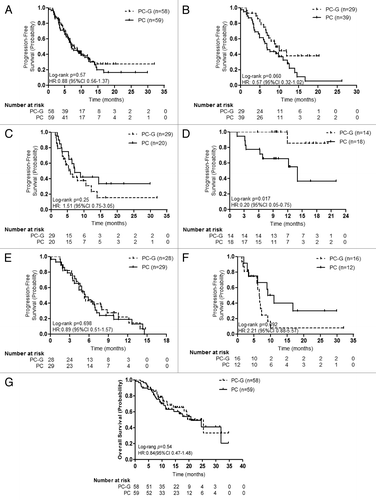
Predefined subgroup analysis showed that PC-G produced favorable HR (0.20, 95% CI 0.05–0.75; P = 0.017) for patients with EGFR mutations (exons 19/21) compared with PC with regard to PFS. The median PFS was 14.0 mo for the PC arm. However, for the PC-G arm, the median PFS was not reached before the conclusion of the study. For patients with wild-type EGFR (0.89, 95% CI 0.51–1.57; P = 0.698) and those with unknown EGFR genotype (2.21, 95% CI 0.88–5.57; P = 0.092), no significant difference was found between the two treatment groups. Additionally, the PC-G regimen showed a trend for better PFS compared with the PC regimen in the never-smokers (HR: 0.57, 95% CI 0.32–1.02; P = 0.06). In patients with EGFR mutations (exons 19/21), the ORR was 76.9% for the PC-G arm and 50% for the PC arm (P = 0.13). In contrast, in patients with wild-type EGFR, the ORR was 51.9% and 50%, respectively (P = 0.89). In patients with unknown EGFR genotype, the ORR was 21.4% and 36.4%, respectively (P = 0.41) ().
Table 2. Efficacy analysis
Safety
The cohort of patients for the safety study included all randomized patients who received at least one dose of the study medications (n = 117). Fifty-five patients in each treatment arm (95% for PC-G, 93% for PC) had at least one AE that was considered to be possibly related to the study treatment (). Most of the reported AEs were grade 1 or 2 in severity. Grade 3 treatment-related AE was reported in 22% of the PC-G-treated patients and 15% of the PC-treated patients (Chi-square test, P = 0.32).
Table 3. Incidence and severity of treatment related adverse events
The most common adverse event in the PC-G group was skin rash, which was noticed in 47% (27/58) of the patients (any grade). More severe skin rash (grade 3 or higher) was observed in 16% (9/58) of the patients. The incidence of hematologic adverse events was similar in both treatment arms. Anemia was the most commonly reported hematologic toxicity. The incidence of anemia was similar in the two treatment arms (52% vs. 46%). Stomatitis was the most common gastrointestinal reaction in the PC-G arm, while constipation was common in the PC arm. No treatment-related death was reported in either arm of the study.
Subsequent systemic anticancer therapy
After progression of the disease, 45 (77.6%) patients in the PC-G arm and 46 (78.0%) patients in the PC arm received anti-cancer treatment following the study period. Docetaxel and erlotinib were the most frequently used drugs as second-line or higher-line treatments ().
Table 4. Post-study anti-cancer therapy
Discussion
To our knowledge, this study is the first randomized, phase II trial to evaluate whether gefitinib intercalated with chemotherapy can provide improved benefits to unselected, chemotherapy-naïve patients with advanced non-squamous NSCLC who did not receive gefitinib maintenance therapy. Previous studies have shown that NPR, used as a study endpoint, can predict clinical benefit in cancer patients.Citation13-Citation15 When designing this study, we presumed that the NPR could be improved from 64% in the PC group to 84% after 12 wk (almost 4 cycles) of PC-G treatment. Our results showed that the intercalated addition of gefitinib did not improve the treatment outcomes not only with regard to NPR but also with regard to ORR, PFS, or OS.
EGFR-TKI has been used in combination with standard platinum-based doublet chemotherapy in the concurrent, intercalated, or maintenance regimens. Studies on the concurrent administration of chemotherapy and EGFR-TKI confirmed a lack of efficacy. Four large-scale phase III clinical trials, including TRIBUTE, TALENT, INTACT-I, and INTACT-II, have demonstrated that platinum-based doublets in combination with TKI did not bring survival benefit to patients with advanced NSCLC.Citation4,Citation5,Citation11,Citation12 The second approach is to administer EGFR-TKI as maintenance therapy when tumor response is achieved or during stable disease following cytotoxic chemotherapy. Two phase III studies, SATURNCitation16-Citation20 and INFORM,Citation21,Citation22 have shown improvement in PFS, but the biomarker-based selection of patients for maintenance therapy is disputable. The third choice is the intercalated administration. Concurrent administration may not be effective because of TKI-induced, G1-phase cell-cycle arrest.Citation23 When EGFR-TKI and chemotherapy are given in a sequentially intercalated way, thus achieving pharmacodynamic separation of the two agents, the inhibitory drug interaction could be avoided.Citation24 Mok and colleagues tested this concept in a randomized phase II study (FAST-ACT-I).Citation7 A total of 154 treatment-naïve Asian patients were randomized to receive gemcitabine plus cisplatin or carboplatin on day 1 and gemcitabine on day 8 followed by erlotinib from days 15 to 28 or the same chemotherapy followed by placebo on a 4-wk cycle. The primary endpoint was the NPR at 8 wk. The results indicated that the PFS was significantly longer in the intercalated administration arm compared with the control arm (HR 0.47; P = 0.018). The OS was similar because the placebo control arm was unblinded at disease progression and offered the option of erlotinib as a post-study treatment. Thereafter, these authors reported the findings of the phase 3 FAST-ACT-II trial using the same treatment design in The Lancet Oncology.Citation8 The results demonstrated that the intercalated regimen improved the PFS (7.6 vs. 6.0 mo, P < 0.0001) and OS (18.3 vs. 15.2 mo, P = 0.042) in an unselected population of East Asian patients. However, in the FAST-ACT-I and FAST-ACT-II trials, the TKI was administered not only intercalated sequentially during chemotherapy but also as maintenance therapy after the end of chemotherapy, which limits the ability to demonstrate whether the survival benefit was due to the intercalated therapy during chemotherapy or the subsequent maintenance treatment because TKI maintenance therapy has been shown to be beneficial in the SATURN, INFORM, and other studies.Citation16,Citation21,Citation25,Citation26 Our phase II randomized, controlled study partially answered this question. Without confounding effect from gefitinib maintenance therapy, the intercalated regimen of chemotherapy plus TKI provided no added benefit to unselected, chemotherapy-naïve patients with advanced non-squamous NSCLC compared with chemotherapy alone.
In the subgroup of patients with EGFR mutation in our study, an improvement in PFS was observed for the intercalated administration arm (HR, 0.20; median, not reached vs. 14 mo), which was similar to that in FAST-ACT-II. In a predefined subgroup analysis of approximately two-thirds of the patient cohort of FAST-ACT-II, only patients with EGFR-activating mutations benefited significantly from the intercalated regimen (median PFS 16.8 vs. 6.9 mo, P < 0.0001; median overall survival 31.4 vs. 20.6 mo, P = 0.0092). These findings demonstrated that the EGFR mutational status may serve as a biomarker to identify patients who can benefit the most from the intercalated combination therapy. Unfortunately, although more than 70% of the patients had tissue samples available for translational biomarker research, tumors positive for EGFR mutations were identified in less than 30% of the patients. Thus, the exploratory subgroup analysis should not be over interpreted. A larger randomized phase II or phase III study with PFS as the endpoint in the group of patients with EGFR mutations is warranted to define the value of the intercalated combination. It should also be noted that there was a trend toward worse PFS and OS among patients with EGFR unknown status when treated with PC-G vs. PC in our study, even though the difference was not statistically significant. This further emphasizes the need to test the mutational status at the time of the diagnosis.
The JMDB studyCitation27-Citation29 indicated that for patients with non-squamous NSCLC and for patients of Asian descent, the PC regimen had significantly better efficacy than GC, which was used as the control arm in FAST-ACT-I and FAST-ACT-II. Our study chose the pemetrexed-containing regimen as the control arm to avoid overestimating the benefit from the intercalated EGFR-TKI and strengthen the quality of our results. Furthermore, the preclinical study showed that concurrent or sequential administration of pemetrexed with EGFR-TKI has synergistic anti-tumor activity for both TKI-sensitive and TKI-resistant cell lines. This synergism may be related to the activation of the EGFR/PI3K/AKT pathways induced by pemetrexed. These pathways can be inhibited by the subsequent administration of TKI to maximize the inhibition of tumor growth.
In another subgroup analysis, the PFS of never-smokers appears to be longer than that of current or ex-smokers (P = 0.06). This may be because the prevalence of EGFR mutations was 20.4% (10/49) among the smokers but was 32.4% (22/68) among the never-smokers (P = 0.027). The prevalence of EGFR activating mutation in the smokers is acceptable. In NSCLC of any histology, pooled frequencies of EGFR mutations ranged from 8.4% to 35.9% for ever/heavy smokers and from 37.6% to 62.5% for never/light smokers, depending on ethnicity.Citation30 Not only in the Asian but in the western population this different prevalence of EGFR mutation between the smokers and never-smokers does exist. Taking account of the relatively small sample size, further study is required to clarify whether never-smoker status can serve as a biomarker for screening Chinese patients who can benefit the most from intercalated treatment if tumor tissues are not available. For western population, never-smoker status may be more meaningful for predicting the candidates of such an intercalated treatment due to a larger difference in prevalence of EGFR mutations between smokers and never-smokers.
Just as in the FAST-ACT I and II trials, intercalated dosing of TKI with chemotherapy was well tolerated, without additional hematologic toxicity in our study. Dose reduction and dose interruption were uncommon with gefitinib. Skin rash was observed in 47% of the patients in the PC-G arm, and 16% of these skin rash events were grade 3 or higher. However, skin rash could be managed with the best supportive treatment. Diarrhea was more common in the PC-G arm (20% vs. 8% in the PC arm), but almost all these events were grade 1 or 2. No treatment-related death was reported in either group.
Conclusions
This study demonstrates for the first time that intercalated administration of TKI with standard platinum-containing doublet chemotherapy did not improve the clinical outcome of unselected, chemotherapy-naïve patients with advanced non-squamous NSCLC. Additional studies are warranted to evaluate the clinical value of such intercalated combination in patients with EGFR mutations.
Patients and Methods
Study design
This single-center, randomized, controlled, open-label, phase II study was conducted between December 2009 and May 2012 at Fudan University Shanghai Cancer Center in accordance with the Declaration of Helsinki, the international Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local regulations. The study was approved by the ethics committee of Fudan University Shanghai Cancer Center, and written informed consent was obtained from all study patients. This study is registered with ClinicalTrials.gov as number NCT01769066.
Eligibility criteria
Eligible patients were at least 18 y of age, with histologically or cytologically confirmed advanced or recurrent stage IIIB or IV non-squamous NSCLC (Union for International Cancer Control classification, version 6) and an Eastern Cooperation Oncology Group (ECOG) performance status of 0 to 1. These patients also had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST 1.0), adequate hematological, hepatic, and renal functions, and a life expectancy of more than 12 wk. Patients were ineligible to participate if they had received previous systemic anticancer therapy or had severe drug allergy, active infection or another serious disease or condition, uncontrolled brain metastases, uncontrolled pleural effusion and/or pericardial effusion, or second malignancy, pregnancy, or lactation.
Procedures
Patients were randomly assigned in a 1:1 ratio to receive gefitinib (250 mg/day, orally) on day 3 to 16 of a 3-wk cycle in combination with pemetrexed (500 mg/m2 i.v. infusion on day 1) and either cisplatin (75 mg/m2 i.v. infusion on day 1) or carboplatin (AUC = 5, i.v. infusion on day 1) or pemetrexed–platinum alone. Treatment was repeated every 3 wk and continued until disease progression, unacceptable toxicity, or completion of a maximum of 6 cycles. Maintenance therapy was not allowed. The choice of second or higher line anti-cancer therapy was at the investigator’s discretion. Randomization of the study patients was stratified according to the tobacco-smoking status (current or ex-smoker/never-smoker) and EGFR genotype (mutation, wild-type, or unknown). The EGFR mutational status was determined using direct sequencing of exons 18 to 21. The EGFR mutation was defined by the presence of deletions on exon 19 or L858R mutations on exon 21. Patients with other types of mutations or deletions were classified as wild type for convenience of the analysis. Patients were followed up until their death or the study closure.
Baseline characteristics were recorded at the study enrollment. Radiological assessment was conducted at baseline and every 6 wk thereafter. Tumor response (according to RECIST 1.0) was evaluated by CT or magnetic resonance imaging scan of the patients’ chest and abdomen every 6 wk during chemotherapy and after completion of chemotherapy. Brain imaging and bone scans were performed before enrollment. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria (NCI-CTC 3.0) for Adverse Events.
Statistical analysis
The primary endpoint was the NPR at 12 wk (complete response, partial response, or stable disease >12 wk). A two-group Z test with a two-sided level of significance level of 0.1 will have 80% power to detect the difference between the PC-G (NPR 0.84) and PC (NPR 0.64) groups when the sample size was 58 in each group. Secondary endpoints included ORR (complete response or partial response), PFS, and OS. ORR was compared between the two groups using Chi-square tests. Log-rank tests were used to compare the survival between the treatment groups. Cox proportional hazards model was used to calculate the HR for sequential intercalated combination of gefitinib and chemotherapy vs. chemotherapy alone. A two-sided significance level of 0.05 was used for all analyses, which were performed using SPSS (version 19) software.
| Abbreviations: | ||
| CI | = | confidence interval |
| ECOG | = | Eastern Cooperative Oncology Group |
| EGFR | = | epidermal growth factor receptor |
| HR | = | hazard ratio |
| NPR | = | non-progression rate |
| NSCLC | = | non-small cell lung cancer |
| ORR | = | overall response rate |
| OS | = | overall survival |
| PC | = | pemetrexed–platinum |
| PC-G | = | pemetrexed–platinum plus gefitinib |
| PFS | = | progression-free survival |
| TKI | = | tyrosine kinase inhibitor |
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Drs Liu Fang and Xu Qinghua of Fudan University Shanghai Cancer Center-Institut Merieux Laboratory for their outstanding contribution in the translational research such as data analysis and biomarkers analysis.
References
- Are C, Rajaram S, Are M, Raj H, Anderson BO, Chaluvarya Swamy R, Vijayakumar M, Song T, Pandey M, Edney JA, et al. A review of global cancer burden: trends, challenges, strategies, and a role for surgeons. J Surg Oncol 2013; 107:221 - 6; http://dx.doi.org/10.1002/jso.23248; PMID: 22926725
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893 - 917; http://dx.doi.org/10.1002/ijc.25516; PMID: 21351269
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol 2004; 22:777 - 84; http://dx.doi.org/10.1200/JCO.2004.08.001; PMID: 14990632
- Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol 2004; 22:785 - 94; http://dx.doi.org/10.1200/JCO.2004.07.215; PMID: 14990633
- Davies AM, Ho C, Lara PN Jr., Mack P, Gumerlock PH, Gandara DR. Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin Lung Cancer 2006; 7:385 - 8; http://dx.doi.org/10.3816/CLC.2006.n.021; PMID: 16800963
- Mok TS, Wu YL, Yu CJ, Zhou C, Chen YM, Zhang L, Ignacio J, Liao M, Srimuninnimit V, Boyer MJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009; 27:5080 - 7; http://dx.doi.org/10.1200/JCO.2008.21.5541; PMID: 19738125
- Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, Srimuninnimit V, Sriuranpong V, Sandoval-Tan J, Zhu Y, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013; 14:777 - 86; http://dx.doi.org/10.1016/S1470-2045(13)70254-7; PMID: 23782814
- Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26:3543 - 51; http://dx.doi.org/10.1200/JCO.2007.15.0375; PMID: 18506025
- Grønberg BH, Bremnes RM, Fløtten O, Amundsen T, Brunsvig PF, Hjelde HH, Kaasa S, von Plessen C, Stornes F, Tollåli T, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2009; 27:3217 - 24; http://dx.doi.org/10.1200/JCO.2008.20.9114; PMID: 19433683
- Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, et al, TRIBUTE Investigator Group. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005; 23:5892 - 9; http://dx.doi.org/10.1200/JCO.2005.02.840; PMID: 16043829
- Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007; 25:1545 - 52; http://dx.doi.org/10.1200/JCO.2005.05.1474; PMID: 17442998
- Lara PN Jr., Redman MW, Kelly K, Edelman MJ, Williamson SK, Crowley JJ, Gandara DR, Southwest Oncology Group. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol 2008; 26:463 - 7; http://dx.doi.org/10.1200/JCO.2007.13.0344; PMID: 18202421
- Giaccone G, Gallegos Ruiz M, Le Chevalier T, Thatcher N, Smit E, Rodriguez JA, Janne P, Oulid-Aissa D, Soria JC. Erlotinib for frontline treatment of advanced non-small cell lung cancer: a phase II study. Clin Cancer Res 2006; 12:6049 - 55; http://dx.doi.org/10.1158/1078-0432.CCR-06-0260; PMID: 17062680
- Dingemans AM, de Langen AJ, van den Boogaart V, Marcus JT, Backes WH, Scholtens HT, van Tinteren H, Hoekstra OS, Pruim J, Brans B, et al. First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging. Ann Oncol 2011; 22:559 - 66; http://dx.doi.org/10.1093/annonc/mdq391; PMID: 20702788
- Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W, Melezínek I, et al, SATURN investigators. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11:521 - 9; http://dx.doi.org/10.1016/S1470-2045(10)70112-1; PMID: 20493771
- Juhász E, Kim JH, Klingelschmitt G, Walzer S. Effects of erlotinib first-line maintenance therapy versus placebo on the health-related quality of life of patients with metastatic non-small-cell lung cancer. Eur J Cancer 2013; 49:1205 - 15; http://dx.doi.org/10.1016/j.ejca.2012.11.006; PMID: 23477998
- Wu YL, Kim JH, Park K, Zaatar A, Klingelschmitt G, Ng C. Efficacy and safety of maintenance erlotinib in Asian patients with advanced non-small-cell lung cancer: a subanalysis of the phase III, randomized SATURN study. Lung Cancer 2012; 77:339 - 45; http://dx.doi.org/10.1016/j.lungcan.2012.03.012; PMID: 22494567
- Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R, Ward C, Mayne K, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol 2011; 29:4113 - 20; http://dx.doi.org/10.1200/JCO.2010.31.8162; PMID: 21969500
- Coudert B, Ciuleanu T, Park K, Wu YL, Giaccone G, Brugger W, Gopalakrishna P, Cappuzzo F, SATURN Investigators. Survival benefit with erlotinib maintenance therapy in patients with advanced non-small-cell lung cancer (NSCLC) according to response to first-line chemotherapy. Ann Oncol 2012; 23:388 - 94; http://dx.doi.org/10.1093/annonc/mdr125; PMID: 21610154
- Zhang L, Ma S, Song X, Han B, Cheng Y, Huang C, Yang S, Liu X, Liu Y, Lu S, et al, INFORM investigators. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012; 13:466 - 75; http://dx.doi.org/10.1016/S1470-2045(12)70117-1; PMID: 22512843
- Reckamp KL. Is benefit of maintenance therapy for NSCLC best defined by progression-free survival?. Lancet Oncol 2012; 13:435 - 6; http://dx.doi.org/10.1016/S1470-2045(12)70157-2; PMID: 22512846
- Piperdi B, Ling YH, Kroog G, Perez-Soler R. Schedule-dependent interaction between epidermal growth factor inhibitors (EGFRI) and G2/M blocking chemotherapeutic agents (G2/Mb) on human NSCLC cell lines in vitro. J Clin Oncol 2004; 22:14S 7028
- Mahaffey CM, Davies AM, Lara PN Jr., Pryde B, Holland W, Mack PC, Gumerlock PH, Gandara DR. Schedule-dependent apoptosis in K-ras mutant non-small-cell lung cancer cell lines treated with docetaxel and erlotinib: rationale for pharmacodynamic separation. Clin Lung Cancer 2007; 8:548 - 53; http://dx.doi.org/10.3816/CLC.2007.n.041; PMID: 18186959
- Pérol M, Chouaid C, Pérol D, Barlési F, Gervais R, Westeel V, Crequit J, Léna H, Vergnenègre A, Zalcman G, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30:3516 - 24; http://dx.doi.org/10.1200/JCO.2011.39.9782; PMID: 22949150
- Ahn MJ, Yang JC, Liang J, Kang JH, Xiu Q, Chen YM, Blair JM, Peng G, Linn C, Orlando M. Randomized phase II trial of first-line treatment with pemetrexed-cisplatin, followed sequentially by gefitinib or pemetrexed, in East Asian, never-smoker patients with advanced non-small cell lung cancer. Lung Cancer 2012; 77:346 - 52; http://dx.doi.org/10.1016/j.lungcan.2012.03.011; PMID: 22534669
- Scagliotti GV, Park K, Patil S, Rolski J, Goksel T, Martins R, Gans SJ, Visseren-Grul C, Peterson P. Survival without toxicity for cisplatin plus pemetrexed versus cisplatin plus gemcitabine in chemonaïve patients with advanced non-small cell lung cancer: a risk-benefit analysis of a large phase III study. Eur J Cancer 2009; 45:2298 - 303; http://dx.doi.org/10.1016/j.ejca.2009.04.033; PMID: 19473833
- Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, Dickson R, Tudur Smith C, Davis H, Green J, et al. Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer. Health Technol Assess 2010; 14:Suppl 1 47 - 53; PMID: 20507803
- Genova C, Rijavec E, Truini A, Coco S, Sini C, Barletta G, Dal Bello MG, Alama A, Savarino G, Pronzato P, et al. Pemetrexed for the treatment of non-small cell lung cancer. Expert Opin Pharmacother 2013; 14:1545 - 58; http://dx.doi.org/10.1517/14656566.2013.802774; PMID: 23683110
- Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013; 24:2371 - 6; http://dx.doi.org/10.1093/annonc/mdt205; PMID: 23723294