Abstract
miRNA-218 is a highlighted tumor suppressor and its underlying role in tumor progression is still unknown. Here, we restored the expression of miRNA-218 in pancreatic cancer to clarify the function and potent downstream pathway of miRNA-218. The expressions of both miRNA-218 and its potent target gene ROBO1 were revealed by RT-PCR and western blotting analysis. Transfection of miRNA-218 precursor mimics and luciferase assay were performed to elucidate the regulation mechanism between miRNA-218 and ROBO1. Cells, stably expressing miRNA-218 followed by forced expression of mutant ROBO1, were established through co-transfections of both lentivirus vector and plasmid vector. The cell migration and invasion abilities were evaluated by migration assay and invasion assay respectively. An increased expression of ROBO1 was revealed in cell BxPC-3-LN compared with cell BxPC-3. Elevated expression of miRNA-218 would suppress the expression of ROBO1 via complementary binding to a specific region within 3′UTR of ROBO1 mRNA (sites 971–978) in pancreatic cancer cells. Stably restoring the expression of miRNA-218 in pancreatic cancer significantly downregulated the expression of ROBO1 and effectively inhibited cell migration and invasion. Forced expression of mutant ROBO1 could reverse the repression effects of miRNA-218 on cell migration and invasion. Consequently, miRNA-218 acted as a tumor suppressor in pancreatic cancer by inhibiting cell invasion and migration. ROBO1 was a functional target of miRNA-218’s downstream pathway involving in cell invasion and migration of pancreatic cancer.
Introduction
Pancreatic cancer is one of the most malignant human solid tumors, characterized by its extremely aggressive nature including local invasion and early metastasis.Citation1,Citation2 Although great progress in diagnosis and treatment has been made, the 5-y survival rate remains dismal in pancreatic cancer.Citation3 Recently, researches on molecular mechanism of pancreatic cancer are highlightedCitation4 and the critical pathways involving in tumor invasion and metastasis may provide a better understanding of tumor progression, finally contributing to early diagnosis and novel targets of comprehensive therapies in pancreatic cancer.
miRNAs are endogenous, small and non-coding RNAs and primarily regulate genes via complementary binding to 3′UTR of their targets, leading to degradation of mRNAs or suppression of translation.Citation5 Cumulative evidence has found that miRNAs function in multiple processes of tumor development, including cell proliferation, apoptosis, differentiation, invasion, migration, and so on.Citation6 Previously we had found dysregulation of both miRNA-218 and its potent target gene ROBO1 in pancreatic cancerCitation7 and suggested that miRNA-218 and ROBO1 might participate in the progression of pancreatic cancer. In this study, we were to investigate the regulation mechanism between miRNA-218 and ROBO1 in pancreatic cancer. Through restoring the expression of miRNA-218 or mutant ROBO1, we found enhanced expression of miRNA-218 would inhibit migration and invasion of tumor cells via decreasing the expression of its target gene ROBO1 in pancreatic cancer.
Results
miRNA-218 inhibited the expression of ROBO1 in pancreatic cancer cells
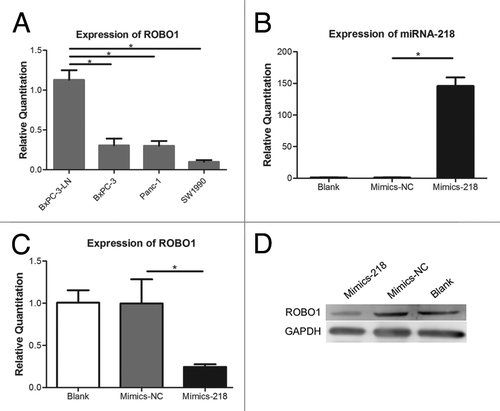
Previously we found a decreased expression of miRNA-218 in BxPC-3-LN compared with BxPC-3, Panc-1, or SW1990.Citation7 In this study, the expressions of ROBO1 in several pancreatic cancer cells were investigated () and it showed a significantly increased expression of ROBO1 in BxPC-3-LN compared with BxPC-3, Panc-1, or SW1990 (P = 0.0007, 0.0005, and 0.0001). Consequently, an inverse correlation between the expression of miRNA-218 and ROBO1 was showed in BxPC-3-LN compared with its parental cell line BxPC-3 or other 2 cell lines. Mimics-218 or Mimics-NC was transfected into cell BxPC-3-LN. The expression of miRNA-218 () increased significantly in cells transfected with Mimics-218 compared with Mimics-NC (P < 0.0001), while the expression of ROBO1 () decreased obviously in cells transfected with Mimics-218 compared with Mimics-NC (P = 0.0107).
Figure 1. (A) Expression of ROBO1 in pancreatic cell lines. The relative quantitations of ROBO1 in BxPC-3-LN, BxPC-3, Panc-1, and SW1990 were 1.129 ± 0.1216, 0.3060 ± 0.8528, 0.3020 ± 0.06010, and 0.09967 ± 0.02255 respectively. (B) Expression of miRNA-218 in cells transfected with miRNA-218 precursor mimics. The relative quantitations of miRNA-218 in Blank Control group, Mimics-NC group, and Mimics-218 group were 1.311 ± 0.3077, 1.040 ± 0.3542, and 146.0 ± 13.48 respectively. (C) Expression of ROBO1 in cells transfected with miRNA-218 precursor mimics. The relative quantitation of ROBO1 in three groups were 1.007 ± 0.1475, 0.9970 ± 0.2872, and 0.2445 ± 0.03112 respectively. (D) The expression of ROBO1 protein in cells transfected with miRNA-218 precursor mimics. A significant decrease of ROBO1 protein was showed in Mimics-218 group. * represented the P value < 0.05.
miRNA-218 regulated ROBO1 via binding to 3′UTR of ROBO1 mRNA in pancreatic cancer cells
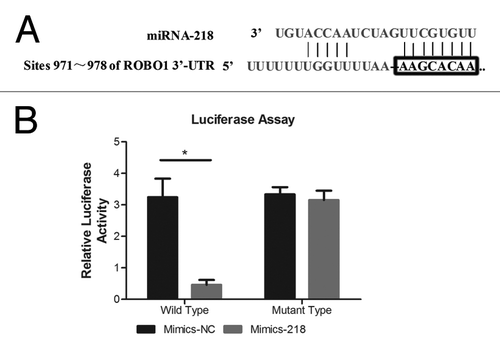
We established Luciferase assay to determine whether miRNA-218 inhibited the expression of ROBO1 through direct interaction with 3′UTR of ROBO1 mRNA (). The luciferase reporter plasmid included the wild type 3′UTR of ROBO1 (pLuc-ROBO1-wt) and the control reporter plasmid with an engineered mutant type 3′UTR of ROBO1 (pLuc-ROBO1-mu). Both plasmids were co-transfected with Mimics-218 or Mimics-NC into cell BxPC-3-LN respectively (). We found a significant decrease of luciferase activity (P < 0.0001) in the cells co-transfected with pLuc-ROBO1-wt and Mimics-218, compared with the cells co-transfected with pLuc-ROBO1-wt and Mimics-NC. Instead, no significant variation of luciferase activity (P = 0.4525) was observed between the cells co-transfected with pLuc-ROBO1-mu and Mimics-218, and the cells co-transfected with pLuc-ROBO1-mu and Mimics-NC.
Figure 2. (A) The predicted binding sites of miRNA-218 in the 3′UTR region of ROBO1. (B) miRNA-218 precursor mimics and pLuc-ROBO1-wt/mu were co-transfected into cells. The relative luciferase activities were 3.205 ± 0.2193 and 0.4857 ± 0.1466 in cells transfected with Mimics-NC+pLuc-ROBO1-wt, and Mimics-218+pLuc-ROBO1-wt respectively. The relative luciferase activities were 3.331 ± 0.2275 and 3.151 ± 0.2972 in cells transfected with Mimics-NC+pLuc-ROBO1-mu, and Mimics-218+pLuc-ROBO1-mu respectively. * represented the P value < 0.05.
Elevated expression of miRNA-218 inhibited the invasion and migration of pancreatic cancer cells
Lentivirus expressing vector containing miRNA-218 was transfected into cell BxPC-3-LN to generate cells stably overexpressing miRNA-218. The cells transfected with Lenti-218 or Lenti-NC, expressed green fluorescence protein (). It showed an increase expression of miRNA-218 (P < 0.0001) and a decrease expression of ROBO1 (P = 0.0014) in cells transfected with Lenti-218, relative to cells transfected with Lenti-NC (). In migration assay (), we found a significant decrease of migrated cell counts (P < 0.0001) on the inferior surface of the inserts, in Lenti-218 group compared with Lenti-NC group. Moreover, a notable decrease of invaded cell counts (P < 0.0001) was observed in Lenti-218 group compared with Lenti-NC group in invasion assay ().
Figure 3. (A–D) Cells transfected with Lenti-218 (A andC) or Lenti-NC (B andD) in regular optical vision and GFP vision (original magnification 100×). (E) Expression of miRNA-218 in cells transfected with lenti-218 or control. The relative quantitations of miRNA-218 in BxPC-3-LN group (blank control), Lenti-NC group, and Lenti-218 group were 1.007 ± 0.1466, 0.7627 ± 0.1704, and 143.5 ± 9.142 respectively. (F) The expression of ROBO1 protein in cells transfected with lenti-218 or control. A lower expressing level of ROBO1 protein was showed in Lenti-218 group compared with Lenti-NC group or Blank control. (G) Expression of ROBO1 in cells transfected with Lenti-218 or control. The relative quantitations of ROBO1 in three groups were 1.024 ± 0.2723, 0.9920 ± 0.1863, and 0.1327 ± 0.04005 respectively. P value < 0.05 was denoted as *.
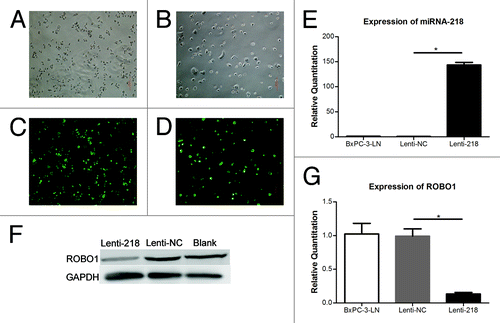
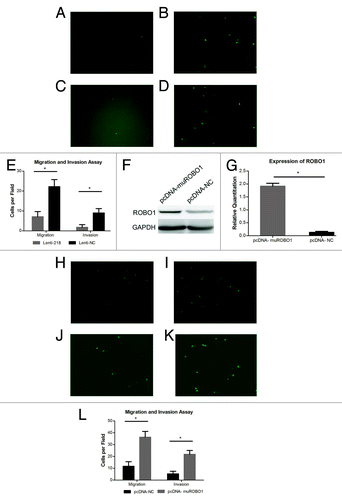
Figure 4. (A–D) Cells transfected with Lenti-218 (A) or Lenti-NC (B), migrated to the inferior surface of the transwell inserts in GFP vision (original magnification 100×). Cells transfected with Lenti-218 (C) or Lenti-NC (D), invaded to the inferior surface of the transwell inserts in GFP vision (original magnification 100×). (E) The mean migrated or invaded cell counts per field in each group. The mean migrated cell counts in Lenti-218 and Lenti-NC group were 7.100 ± 2.601 and 22.20 ± 3.584 respectively. The mean invaded cell counts in Lenti-218 and Lenti-NC group were 1.800 ± 1.317 and 9.000 ± 2.211 respectively. (F) The expression of ROBO1 protein in cells transfected with pcDNA-muROBO1 or control. An increased expressing level of ROBO1 protein was showed in pcDNA-muROBO1 group compared with pcDNA-NC group. (G) Expression of ROBO1 in cells transfected with pcDNA-muROBO1 or control. The relative quantitations of ROBO1 in pcDNA-muROBO1 group and pcDNA-NC group were 1.913 ± 0.1149 and 0.1307 ± 0.03800 respectively. (H–K) Cells transfected with pcDNA-NC (H) or pcDNA-muROBO1 (I), migrated to the inferior surface of the transwell inserts in GFP vision (original magnification 100×). Cells transfected with pcDNA-NC (J) or pcDNA-muROBO1 (K), invaded to the inferior surface of the transwell inserts in GFP vision (original magnification 100×). (L) The mean migrated or invaded cell counts per field in each group. The mean migrated cell counts in pcDNA-NC and pcDNA-muROBO1 group were 11.70 ± 3.917 and 36.30 ± 4.832 respectively. The mean invaded cell counts in pcDNA-NC and pcDNA-muROBO1 group were 5.300 ± 2.214 and 21.80 ± 3.259 respectively. P value < 0.05 was denoted as *.
ROBO1 participate in the process of miRNA-218’s regulation on tumor cell invasion and migration
Whether ROBO1 was involved in miRNA-218’s regulation pathway related to cell invasion and migration of pancreatic cancer was underdetermined. Accordingly, the plasmid pcDNA-muROBO1 lacking the 3′UTR of ROBO1, was transfected into cell BxPC-3-LN-Lenti-218 which had already been transfected with Lenti-218 and stably overexpressed miRNA-218. We found the transfection of pcDNA-muROBO1 reversed the inhibiting effects of miRNA-218 in cell BxPC-3-LN-Lenti-218, accompanied by obviously increased expression of ROBO1 (P < 0.0001) compared with the cell BxPC-3-LN-Lenti-218 transfected with pcDNA-NC (). Moreover, in migration assay and invasion assay (), we confirmed that the cell BxPC-3-LN-Lenti-218 transfected with pcDNA-muROBO1 showed increased migrated cells (P < 0.0001) and increased invaded cells (P < 0.0001), compared with the cell BxPC-3-LN-Lenti-218 transfected with pcDNA-NC.
Discussion
In several human solid tumors, miRNA-218 was reported to downregulate and function as a tumor suppressor by targeting different genes.Citation8-Citation11 Previously we found miRNA-218’s expression decreasing in pancreatic cancer and it was associated with lymph nodes metastasis of pancreatic cancer. Furthermore, we verified that ROBO1, a predicted target gene of miRNA-218, showed an increased expression in pancreatic cancer in previous study.Citation7 Therefore, it indicated that the decreased expression of miRNA-218 might mediate the elevated expression of ROBO1 in pancreatic cancer.
In this study we focused on whether and how miRNA-218 or ROBO1 would affect the biological processes of pancreatic cancer cells. BxPC-3-LN that a subclone of pancreatic cell line BxPC-3 was established from the metastatic lymph nodes. We found a decreased expression of miRNA-218 and correspondingly increased expression of ROBO1 in cell BxPC-3-LN, compared with its parental cell BxPC-3. The inverse expressing tendency between miRNA-218 and ROBO1 in metastatic cell subclone, might provide an insight for pivotal pathways driving tumor progression including invasion or migration of pancreatic cancer cell.
Whether the variation of ROBO1’s expression was induced by aberrant expression of miRNA-218 in pancreatic cancer was still underdetermined. Forced expression of miRNA-218 in cell BxPC-3-LN was performed and it showed a significantly elevated expression of miRNA-218, followed by decreased expression of ROBO1, suggesting that miRNA-218 might inhibit ROBO1’s expression in pancreatic cancer cells. This result was consistent to what had been found about miRNA-218 and ROBO1 in other tumors.Citation12,Citation13 Additionally, in order to elucidate the detailed mechanism how miRNA-218 regulated ROBO1, luciferase assay was performed and verified that miRNA-218 could bind to a complementary region within 3′UTR of ROBO1’s mRNA. As a specific and direct interaction between miRNA-218 and ROBO1 was found, it provided a reasonable explanation for the inverse correlation of miRNA-218 and ROBO1’s expressions in pancreatic cancer. At least, the decreased expression of miRNA-218 contributed in great part to the increased expression of ROBO1 in pancreatic cancer.
Although miRNA-218 was commonly considered to be a tumor suppressor in various human solid tumors involving in tumor progression such as cell invasion, migration, proliferation, and so on,Citation14-Citation16 the role of miRNA-218 was unexplored in pancreatic cancer. In this study, we established cells stably overexpressing miRNA-218 via the transfection of a designed lentivirus vector into cell BxPC-3-LN, to investigate whether the variation of miRNA-218’s expression truly take an effect on biological processes of pancreatic cancer cells. It was of note that novel increased expression of miRNA-218 was found in cells transfected with Lenti-218 vs. control and it inhibited the migration and invasion of pancreatic cancer cells. These results indicated that miRNA-218 took an effect on inhibiting cell migration and invasion. The decreased expression of miRNA-218 might promote tumor dissemination in pancreatic cancer.
miRNAs were a series of special regulators which could interact with multiple target genes simultaneously, and then influenced different downstream pathways. The regulatory mechanism of miRNA-218 was a complex framework and sometimes organ specific. Several predicted target genes of miRNA-218 had been reported. ROBO1 was targeted by miRNA-218 and acted as a promoter of tumor invasion and metastasis in both gastric cancer and nasopharyngeal cancer.Citation12,Citation13 The miRNA-218-LAMB3 pathway was reported to regulate cell invasion and migration in cervical cancer.Citation17 Caveolin-2, a target gene of miRNA-218 involving in tumor cell invasion and migration was verified in renal cell carcinoma.Citation14 Bmi1 and IKK-β were identified as functional targets of miRNA-218 correlated with tumor cell invasion and migration in glioma from two groups respectively.Citation15,Citation18 In pancreatic cancer, UDP-glycosyltransferase-8 (UGT-8) was reported to be regulated by miRNA-218 and promote tumor metastasis.Citation19 It implied that miRNA-218 was related to the progression of pancreatic cancer, However, UGT-8 was not the only functional target gene of miRNA-218 involved in metastasis of pancreatic cancer. Data are still scarce about miRNA-218 and its target genes in pancreatic cancer. We used plasmid vector containing a mutant ROBO1 (lacking of 3′UTR) to increase the expression of ROBO1 in cells overexpressing miRNA-218, and found that restoration of ROBO1’s expression would reverse the inhibiting effects of miRNA-218 on tumor cell migration and invasion, implying that ROBO1 might be a key target gene of miRNA-218-mediated pathway accounting for tumor cell migration and invasion in pancreatic cancer. Consequently, that decreased expression of miRNA-218 induced elevated expression of ROBO1 was probably an important mechanism responsible for tumor invasion or even metastasis in pancreatic cancer.
ROBO1 was a member of axon guidance receptor family (ROBO1–4) and its cognate ligand slit2 was from the large secreted guidance factor family (slit1–3).Citation20 Although it was recognized as pivotal regulator in the development of central nervous system,Citation21 slit/ROBO1 signal was also reported to play a role in modulating chemotaxis of T cells and tumor angiogenesis.Citation22,Citation23 More evidence showed that the secreted slit2 protein mediated chemorepulsive signal on ROBO1 expressing cells, and a chemorepulsive mechanism induced by the interaction between slit2 and ROBO1 might drive glioma and breast cancer cells migration.Citation24,Citation25 Additionally, intracellular PI-3K signaling cascades were reported to be activatied by slit2/ROBO1 interaction and then triggered cytoskeleton remodeling, finally promoting cell motility.Citation26 We suggested that interaction between slit2 and ROBO1 might be a potent downstream event of miRNA-218-ROBO1 signal pathway, driving the invasion and migration of pancreatic cancer cells. Further studies were still necessary in the future.
In this study we confirmed that the decreased expression of miRNA-218 was an important cause for the increased expression of ROBO1 in pancreatic cancer via binding to a complementary region within the 3′UTR of ROBO1’s mRNA. Elevated expression of miRNA-218 inhibited the migration and invasion of pancreatic cancer cells effectively. ROBO1 was a novel target gene of miRNA-218 signal pathway involved in the cell migration and invasion of pancreatic cancer. So miRNA-218 may be an attractive target for developing new therapeutic strategy of pancreatic cancer. An insight of the mechanism and role of miRNA-218-ROBO1 pathway during tumor invasion and metastasis is significant.
Materials and Methods
Cell culture
Human pancreatic cancer cell lines Panc-1, SW1990, and BxPC-3 were obtained from Cell Library of the Chinese Academy of Sciences. Panc-1 was cultured in DMEM medium (12491-015, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS, 10099-141, Gibco). SW1990 was cultured in Leibovitz’s L-15 medium (11415-064, Gibco) supplemented with 10% FBS. BxPC-3-LN, generated from the metastatic lymph nodes, was a subclone of cell line BxPC-3 as previously described,Citation7 and its parental cell lines BxPC-3 were both cultured in RPMI-1640 medium (11875-093, Thermo Fisher Scientific) supplemented with 10% FBS. All the cells were maintained at 37 °C in a humidified incubator containing 5% carbon dioxide.
Quantitative real-time RT-PCR (qRT-PCR)
The details were conducted as previously described. Briefly speaking, total RNA was extracted from cells using TRIzol Reagent (15596-026, Invitrogen, USA). miRNAs and mRNAs were converted to cDNAs using PrimeScript miRNA cDNA Synthesis Kit (D350A, Takara) and the PrimeScript RT Master Mix Kit (DRR036A, Takara) respectively. SYBR green real-time PCR was performed to compare the expressing levels of miRNAs or mRNAs using SYBR Premix Ex TaqII (DRR041A, Takara) by Applied Biosystems 7500 Real-Time PCR. U6 snRNA and GAPDH were selected for normalization to the expressing levels of miRNAs and mRNAs respectively. Relative quantitations (RQs) of miRNAs and mRNAs were calculated using the 2−ΔΔCt method. The sequences of RT-PCR primers are listed in .
Table 1. Primer sequences
Western-blotting
Protein from cells was extracted and quantified. Western blotting was performed according to the standard procedure. The primary antibody, anti-Robo-1 (rabbit polyclonal antibody, sc-25672) was purchased from Santa Cruz Biotechnology and GAPDH (rabbit monoclonal antibody, 2251-1) was purchased from Epitomics. Chemiluminescent substrate (34079; Thermo Fisher Scientific) was used for detection of immunoblots. The images were developed by Fujifilm Las-3000 (Fuji).
miRNA-218 precursor transfection
A total of 2.5 × 104 cells were plated in 24-well plates with medium free of antibiotics and the cells would grow to 70% confluency at the time of transfection. miRNA-218 precursor Mimics-218 and negative control Mimics-NC (AM17100 and AM17110, Ambion) were transfected into cell line BxPC-3 using Lipofectamine 2000 Reagent (11668-019, Invitrogen) according to the manufacturer’s instruction. The group without transfection of neither Mimics-218 nor Mimics-NC was designated as blank control. Total RNA and protein were extracted from the cells 48 h after the transfection to evaluate the expressions of both miRNA-218 and ROBO1.
Luciferase reporter assay
The wild type 3′UTR of ROBO1 mRNA (NM_133631.3) containing predicted binding sites of miRNA-218 (position 971–978 of ROBO1 3′UTR) was amplified from Human Genome DNA and cloned into a construct with XhoI and NotI. The product was then subcloned into XhoI and NotI sites of the psiCHECK-2 dual-luciferase reporter plasmid (C8021, Promega). The plasmid containing the mutant type 3′UTR of ROBO1 with 8 nucleotides (5′-AAGCACAA-3′) deleting in the seed region (provided by Shanghai SBO Medical Biotechnology) was synthesized according to manufacturer’s instruction (C8021, Promega).
Cell line BxPC-3 was seeded in 24-well plates and grew to 70% confluency at the time of transfection. miRNA-218 precursor mimics, pLuc-ROBO1-wt/mu, Firefly, Renilla, and Lipofectamine 2000 Reagent were transfected into cells using Lipofectamine 2000 Reagent according to the manufacturer’s instruction. Luciferase assay was conducted 48 h after transfection using Dual-Luciferase Reporter Assay System (E1910, Promega, USA) according to the manufacturer’s protocol.
miRNA-218 expressing vector design
The stem-loop sequence miRNA-218-2 (MI0000295) was synthesized from Human Genome DNA with primer pair hsa-mir-218-2-P1 and hsa-mir-218-2-P2 (). The generated sequence was then cloned into the NheI site of a lentivirus expressing vector Ubi-EGFP-MCS-IRES-Puromycin (provided by Genechem) to generate a miRNA-218 stable expressing vector (Lenti-218). The lentivirus expressing vector without interest gene was selected as negative control (Lenti-NC). Lenti-218 or lenti-NC was transfected into cells and the cells would express EGFP stably 72 h after transfection.
ROBO1 expressing vector design
ROBO1 mRNA (NM_133631.3) without 3′UTR (muROBO1) was generated from the template (HC139237, YRGene) with primer pair ROBO1-P1 and ROBO1-P2 (). The products were then cloned into the XhoI and BamHI sites of a expressing vector pcDNA3.1(-)-3FLAG-Myc-His (provided by Shanghai SBO Medical Biotechnology) to generate a ROBO1 transient expressing vector (pcDNA-muROBO1). The expressing vector without interest gene was used as negative control (pcDNA-NC). PcDNA-muROBO1 or pcDNA-NC was transfected into cells using Lipofectamine 2000 Reagent according to the manufacturer’s instruction.
Migration and invasion assay
The migration and invasion abilities of cells were evaluated using 24-well plates with 8-μm pore size inserts (3422, Corning). Cells were starved for 24 h before plating and then suspended in serum-free medium. A total of 1 × 105 cells were seeded in the upper chamber for migration assay and 2 × 105 cells were seeded in the upper chamber with Basement Membrane Matrix (356234, BD Matrigel) coated inserts for invasion assay. Medium supplemented with 20% FBS was added to lower chamber. The cells were incubated at 37 °C for 24 h, followed by removal of the cells on the superior surface of the inserts with cotton swabs. Migrated or invaded cells on the inferior surface of the inserts were fixed with 4% paraformaldehyde and counted under microscope. Each assay was repeated for three times and the mean cells of 10 random fields per well (100× magnification) were compared between groups.
Statistical analysis
Data was presented as mean ± standard deviation (SD). Differences between groups were determined by the Student t test. All statistical analyses were performed by SPSS for Windows 16.0 software. P < 0.05 was considered statistically significant.
| Abbreviations: | ||
| miRNA-218 | = | Homo sapiens microRNA-218 |
| ROBO1 | = | roundabout axon guidance receptor homolog 1 |
| pLuc-ROBO1-wt | = | the luciferase reporter plasmid with wild type 3′UTR of ROBO1 |
| pLuc-ROBO1-mu | = | the luciferase reporter plasmid with mutant type 3′UTR of ROBO1 |
| Mimics-218 | = | miRNA-218 precursor mimics |
| Mimics-NC | = | negative control of precursor mimics |
| Lenti-218 | = | lentivirus vector expressing miRNA-218 |
| Lenti-NC | = | negative control of lentivirus vector |
| pcDNA-muROBO1 | = | plasmid vector expressing mutant ROBO1 |
| pcDNA-NC | = | negative control of plasmid vector |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Minrui Liang Ph.D, Department of Rheumatology, Huashan Hospital, for her assistance on Molecular Biology. The study was supported by a grant from the National Natural Science Foundation of China (81172274), a grant from the Shanghai Committee of Science and Technology, China (11JC1401600), and a grant from the Shanghai Municipal Health Bureau, China (20114v141).
References
- Kato K, Yamada S, Sugimoto H, Kanazumi N, Nomoto S, Takeda S, Kodera Y, Morita S, Nakao A. Prognostic factors for survival after extended pancreatectomy for pancreatic head cancer: influence of resection margin status on survival. Pancreas 2009; 38:605 - 12; http://dx.doi.org/10.1097/MPA.0b013e3181a4891d; PMID: 19629002
- Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg 2008; 247:490 - 500; http://dx.doi.org/10.1097/SLA.0b013e31815b9cae; PMID: 18376195
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9 - 29; http://dx.doi.org/10.3322/caac.21208; PMID: 24399786
- Morris JP 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 2010; 10:683 - 95; http://dx.doi.org/10.1038/nrc2899; PMID: 20814421
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005; 123:631 - 40; http://dx.doi.org/10.1016/j.cell.2005.10.022; PMID: 16271387
- Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis 2012; 33:1126 - 33; http://dx.doi.org/10.1093/carcin/bgs140; PMID: 22491715
- He H, Di Y, Liang M, Yang F, Yao L, Hao S, Li J, Jiang Y, Jin C, Fu D. The microRNA-218 and ROBO-1 signaling axis correlates with the lymphatic metastasis of pancreatic cancer. Oncol Rep 2013; 30:651 - 8; PMID: 23733161
- Shi TY, Chen XJ, Zhu ML, Wang MY, He J, Yu KD, Shao ZM, Sun MH, Zhou XY, Cheng X, et al. A pri-miR-218 variant and risk of cervical carcinoma in Chinese women. BMC Cancer 2013; 13:19; http://dx.doi.org/10.1186/1471-2407-13-19; PMID: 23320911
- Gao C, Zhang Z, Liu W, Xiao S, Gu W, Lu H. Reduced microRNA-218 expression is associated with high nuclear factor kappa B activation in gastric cancer. Cancer 2010; 116:41 - 9; PMID: 19890957
- Davidson MR, Larsen JE, Yang IA, Hayward NK, Clarke BE, Duhig EE, Passmore LH, Bowman RV, Fong KM. MicroRNA-218 is deleted and downregulated in lung squamous cell carcinoma. PLoS One 2010; 5:e12560; http://dx.doi.org/10.1371/journal.pone.0012560; PMID: 20838434
- Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene 2008; 27:2575 - 82; http://dx.doi.org/10.1038/sj.onc.1210919; PMID: 17998940
- Alajez NM, Lenarduzzi M, Ito E, Hui ABY, Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res 2011; 71:2381 - 91; http://dx.doi.org/10.1158/0008-5472.CAN-10-2754; PMID: 21385904
- Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet 2010; 6:e1000879; http://dx.doi.org/10.1371/journal.pgen.1000879; PMID: 20300657
- Yamasaki T, Seki N, Yoshino H, Itesako T, Hidaka H, Yamada Y, Tatarano S, Yonezawa T, Kinoshita T, Nakagawa M, et al. MicroRNA-218 inhibits cell migration and invasion in renal cell carcinoma through targeting caveolin-2 involved in focal adhesion pathway. J Urol 2013; 190:1059 - 68; http://dx.doi.org/10.1016/j.juro.2013.02.089; PMID: 23454155
- Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin W, Zhang Y. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res 2013; 73:6046 - 55; http://dx.doi.org/10.1158/0008-5472.CAN-13-0358; PMID: 23950210
- Uesugi A, Kozaki K, Tsuruta T, Furuta M, Morita K, Imoto I, Omura K, Inazawa J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res 2011; 71:5765 - 78; http://dx.doi.org/10.1158/0008-5472.CAN-11-0368; PMID: 21795477
- Yamamoto N, Kinoshita T, Nohata N, Itesako T, Yoshino H, Enokida H, Nakagawa M, Shozu M, Seki N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int J Oncol 2013; 42:1523 - 32; PMID: 23483249
- Song L, Huang Q, Chen K, Liu L, Lin C, Dai T, Yu C, Wu Z, Li J. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-β. Biochem Biophys Res Commun 2010; 402:135 - 40; http://dx.doi.org/10.1016/j.bbrc.2010.10.003; PMID: 20933503
- Li CH, To KF, Tong JH, Xiao Z, Xia T, Lai PB, Chow SC, Zhu YX, Chan SL, Marquez VE, et al. Enhancer of zeste homolog 2 silences microRNA-218 in human pancreatic ductal adenocarcinoma cells by inducing formation of heterochromatin. Gastroenterology 2013; 144:1086 - , e9; http://dx.doi.org/10.1053/j.gastro.2013.01.058; PMID: 23395645
- Legg JA, Herbert JM, Clissold P, Bicknell R. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis 2008; 11:13 - 21; http://dx.doi.org/10.1007/s10456-008-9100-x; PMID: 18264786
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998; 92:205 - 15; http://dx.doi.org/10.1016/S0092-8674(00)80915-0; PMID: 9458045
- Wang B, Xiao Y, Ding BB, Zhang N, Yuan Xb, Gui L, Qian KX, Duan S, Chen Z, Rao Y, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003; 4:19 - 29; http://dx.doi.org/10.1016/S1535-6108(03)00164-8; PMID: 12892710
- Prasad A, Qamri Z, Wu J, Ganju RK. Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol 2007; 82:465 - 76; http://dx.doi.org/10.1189/jlb.1106678; PMID: 17565045
- Mertsch S, Schmitz N, Jeibmann A, Geng JG, Paulus W, Senner V. Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J Neurooncol 2008; 87:1 - 7; http://dx.doi.org/10.1007/s11060-007-9484-2; PMID: 17968499
- Schmid BC, Rezniczek GA, Fabjani G, Yoneda T, Leodolter S, Zeillinger R. The neuronal guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. Breast Cancer Res Treat 2007; 106:333 - 42; http://dx.doi.org/10.1007/s10549-007-9504-0; PMID: 17268810
- Wang Y, Huang ZH. Morphological phenotypes of olfactory ensheathing cells display different migratory responses upon Slit-2. Exp Cell Res 2012; 318:1889 - 900; http://dx.doi.org/10.1016/j.yexcr.2012.05.024; PMID: 22677040
