Abstract
PTK6/Brk is a non-receptor tyrosine kinase overexpressed in cancer. Here we demonstrate that cytosolic PTK6 is rapidly and robustly induced in response to hypoxic conditions in a HIF-1-independent manner. Furthermore, a proportion of hypoxic PTK6 subsequently re-localized to the cell membrane. We observed that the rapid stabilization of PTK6 is associated with a decrease in PTK6 ubiquitylation and we have identified c-Cbl as a putative PTK6 E3 ligase in normoxia. The consequences of hypoxia-induced PTK6 stabilization and subcellular re-localization to the plasma membrane include increased cell motility and invasion, suggesting PTK6 targeting as a therapeutic approach to reduce hypoxia-regulated metastatic potential. This could have particular significance for breast cancer patients with triple negative disease.
Introduction
PTK6 (Protein Tyrosine Kinase 6) or Brk (Breast tumor Kinase) is a cytoplasmic non-receptor tyrosine kinase which has been shown to be highly expressed in various tumor types including breast carcinomas (85% of samples), as well as colorectal, prostate, lung, head and neck carcinomas, and B and T lymphomas.Citation1-Citation6 In normal tissues, PTK6 expression is restricted to the differentiated epithelium of the skin and gut, while in tumors the highest levels of PTK6 expression correlate with higher tumor grade, larger size, metastasis, and consequently a poorer prognosis.Citation7-Citation9
PTK6 has specific functions in different tissue types, including regulating differentiation in normal tissues and promoting proliferation and cell survival in tumors, brought about by variations in cellular localization.Citation1,Citation10 PTK6 is activated by a number of different ligands, as well as displaying a small amount of basal auto-phosphorylation in in vitro kinase assays.Citation11 EGF (epidermal growth factor) and IGF (insulin-like growth factor) induced signaling have been shown to activate PTK6,Citation9,Citation12,Citation13 as have HGF (hepatocyte growth factor) and osteopontin (OPN).Citation11,Citation14 Radiation treatment has also been reported to lead to the induction of PTK6 in both mouse intestine epithelial cells and human colorectal cancer cells, however, little is known about the mechanisms that regulate the de novo expression of PTK6 in tumors.Citation15,Citation16
The importance of the microenvironment, particularly hypoxia, for tumor establishment and metastasis is well characterized.Citation17 Tumor hypoxia arises as a consequence of high metabolic demand for oxygen caused by rapid tumor growth and the inefficiency of the tumor vasculature.Citation18 Many studies have shown that tumor hypoxia is significant as hypoxic tumors are associated with increased invasion, metastasis, poor patient survival and increased resistance to therapy.Citation19,Citation20 One of the key regulators of the hypoxic response is the hypoxia-inducible transcription factor 1 (HIF-1). Hypoxia-inducible genes regulate many biological processes including cell proliferation, angiogenesis, metabolism, apoptosis, immortalization, and migration.Citation21 Exposure to hypoxic conditions was recently shown to induce PTK6 in a HIF-dependent manner in breast cancer cell lines.Citation22 However, PTK6 has not previously been identified as a HIF target, since no HIF binding to PTK6 promoter was identified in larger genome-wide ChIP-seq studies.Citation23,Citation24 This indicated that there might be other parallel mechanisms for hypoxia-mediated PTK6 induction.
In this study, we showed that PTK6 was rapidly stabilized in hypoxic conditions in a posttranslational HIF-1-independent manner in both breast and colorectal cancer cell lines. Specifically, we demonstrated that, in normoxic conditions PTK6 was targeted to the proteasome and that this process was inhibited in hypoxia. Hypoxia-mediated PTK6 induction was associated with increased hypoxia-dependent migration and invasion. These findings are significant as they point to an additional mechanism of PTK6-induction by hypoxia in human cancers.
Results
Hypoxia induces a rapid and robust stabilization of PTK6
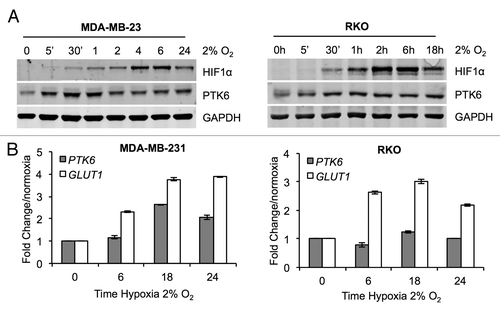
Hypoxia is a known driver of many key aspects of tumor development. Recently, PTK6 has been shown to be hypoxia-inducible in triple negative breast cancer cell lines.Citation22 In order to confirm whether PTK6 is hypoxia-inducible in different cancer cell types, the hypoxia-mediated induction of HIF-1α and PTK6 at both the mRNA and protein levels was examined in the MDA-MB-231 (breast) and RKO (colorectal) cell lines. When protein levels were examined, a rapid and robust induction of PTK6 at the protein level in both cell lines was observed, as early as 5 min after exposure to hypoxia (). PTK6 protein levels were induced in response to hypoxia prior to HIF-1α upregulation () in contrast to previous studies.Citation22 In MDA-MB-231 cells, PTK6 mRNA increased after 18 h exposure to hypoxia, but not at earlier timepoints (6 h), whereas no significant increase of PTK6 mRNA levels was observed for RKO cells () at any of the time points studied. Due to the observed rapid PTK6 protein level induction kinetics in hypoxia, preceding HIF-1α induction, it could be questioned whether PTK6 could in turn affect HIF-1α stabilization and/or activity. To address this, PTK6 was suppressed by RNA interference in RKO and MDA-MB-231 cells, which were then exposed to hypoxia (2% O2). HIF-1α induction in hypoxia was unaltered by the presence/absence of PTK6 (Fig. S1A and B). Similarly, no effect of PTK6 depletion was observed for the transcript levels of three well-characterized HIF-1 targets (Fig. S1C), indicating that hypoxic PTK6 does not affect HIF stability and function.
Figure 1. Hypoxia induces the rapid stabilization of PTK6. (A) RKO colorectal and MDA-MB-231 breast cancer cells were exposed to hypoxia (2% O2) for the periods indicated. Cells were lysed and PTK6 and HIF-1α levels were determined by western blotting. (B) RKO and MDA-MB-231 cells were exposed to hypoxia (2% O2) for the periods indicated. PTK6 and GLUT-1 expression levels were determined by qRT-PCR.
PTK6 is ubiquitylated in an oxygen-dependent manner
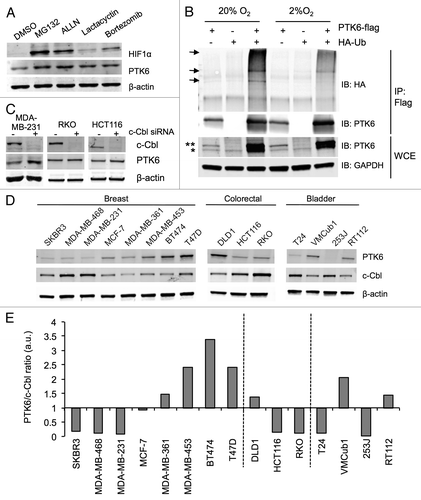
Due to its rapid kinetics, it was plausible that the transcription-independent PTK6 protein stabilization in hypoxia depicted in could occur via posttranslational turnover in the ubiquitin-proteasome system (UPS). In order to investigate this hypothesis, MDA-MB-231 cells were exposed to proteasome inhibitors MG132, ALLN, Lactacystin, and Bortezomib for 6 h in normoxia (20% O2). Increased PTK6 levels were observed after treatment with these inhibitors, suggesting that, in normoxic conditions, PTK6 is actively degraded via the UPS (). To investigate the role of direct protein ubiquitylation in PTK6 stabilization in hypoxia, constructs containing either tagged PTK6 (Flag-PTK6) and/or ubiquitin (HA-Ub) were transfected into HEK293T cells to ensure high levels of expression. The cells were then exposed to normoxia or hypoxia (2% O2) for 6 h and Flag-PTK6 was immunoprecipitated (). Higher molecular weight forms of PTK6 were detected in normoxic conditions when both constructs were present. This suggested that, under these conditions, PTK6 was ubiquitylated. The presence of these higher molecular weight forms of PTK6 was decreased in hypoxic conditions, indicating that the level of PTK6 ubiquitylation was lower in hypoxia than in normoxic conditions (). These data imply that an E3 ligase could control the level of PTK6 protein in an oxygen-dependent manner. The role of the known tyrosine kinase E3 ligase c-Cbl was investigated in this context. MDA-MB-231 (breast) and RKO and HCT116 (colorectal) cell lines were transfected with c-Cbl siRNA. In MDA-MB-231 cells, suppression of c-Cbl led to a statistically significant increase in PTK6 levels (; Fig. S2A). This effect was also evident (albeit not significant) to a lesser extent in the colorectal cell lines, including RKO (; Fig. S2A). To support this finding the levels of c-Cbl and PTK6 were determined in a range of cancer cell lines by western blotting (). This analysis demonstrated a reciprocal relationship between the levels of PTK6 and c-Cbl; that is, when one was relatively highly expressed the other was relatively low (; Fig. S2B). Altogether, these data support the hypothesis that PTK6 is ubiquitylated and degraded via the UPS in normoxic conditions and that this degradation is decreased in response to hypoxia, thereby allowing the protein to accumulate.
Figure 2. PTK6 is ubiquitylated in an oxygen-dependent manner. (A) MDA-MB-231 cells were treated for 6 h with either vehicle (DMSO), 10 μM MG132, 50 μM ALLN, 5 μM lactacystin, or 50 nM bortezomib. PTK6 levels were determined by western blotting. HIF-1α was used as a positive control for proteasomal inhibition in normoxic conditions and GAPDH as a loading control. (B) HEK293T cells were transfected with constructs expressing either Flag-PTK6, HA-Ub, or both and exposed to normoxia (Norm) or hypoxia 2% O2 (Hyp) for 6h in the presence of 10 μM MG132. Flag-PTK6 was immunoprecipitated (IP) and analyzed by western blotting for the presence of ubiquitinated PTK6 (indicated by arrows). Whole cell extracts (WCE) were analyzed for the presence of PTK6 and GAPDH (loading control). Endogenous and ectopically expressed PTK6 in WCE are indicated as * and **, respectively. (C) MDA-MB-231 (breast), RKO, and HCT116 (colorectal) cells were transfected with either Scr (scramble) or c-Cbl siRNA for 72h. Western blotting was performed to detect the endogenous levels of c-Cbl and PTK6. (D) Whole cell extracts were prepared from the breast, colorectal, and bladder cancer cell lines indicated and western blotting was performed for PTK6 and c-Cbl. β-actin was used as a loading control. (E) Histogram represents PTK6/c-Cbl ratios from panel in (D). Quantification values are depicted in Figure S2B. (a.u., arbitrary units of fold increase relative to β-actin).
Hypoxia-induced PTK6 promotes cell motility and invasion
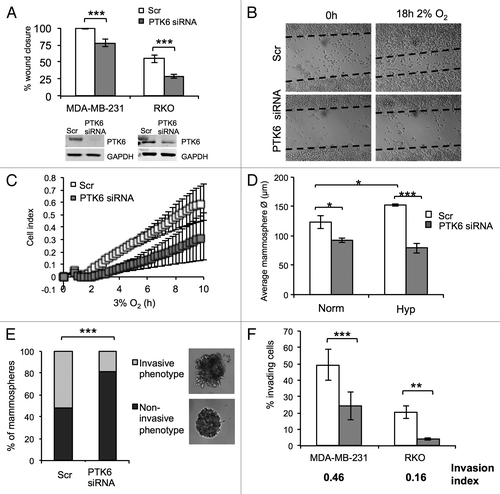
The role of hypoxia in regulating the ability of cancer cells to disseminate and proliferate to secondary sites clearly contributes for the metastatic process.Citation25 Furthermore, PTK6 has been reported to regulate a number of processes that are central for cellular proliferation and metastatic spread, when associated with different membrane subcellular fractions.Citation26,Citation27 The subcellular localization of normoxic and hypoxic PTK6 was investigated by biochemical and immunofluorescence approaches, where a clear increase in cytoplasmic hypoxic PTK6 was observed (Fig. S3). Although most PTK6 protein in hypoxic MDA-MB-231 cells remained cytoplasmic, a fraction of it translocated to the cell membrane and co-localized with F-actin (Fig. S3), indicating a potential role in cell motility under hypoxic conditions. In order to investigate this, scratch wound assays were performed in both MDA-MB-231 and RKO cells transfected with Scr (scramble) or PTK6 siRNA and exposed to hypoxic conditions (2% O2) (). Wound closure was significantly delayed in the absence of PTK6 in hypoxic conditions for both cell lines (). The xCELLigence real-time cell analyzer (RTCA) system was used to allow real-time kinetic analysis of early motility events. This system allows the differentiation between early motility and proliferation events.Citation28 Real-time hypoxic cell motility of MDA-MB-231 cells was decreased in the absence of PTK6 (). Although PTK6 knockdown did not affect cell proliferation and clonogenic survival in hypoxia using 2D models (Fig. S4), its potential role in 3D growth in hypoxic conditions was investigated using the mammosphere system. This allows the evaluation of the ability of breast cancer cells to survive and proliferate in an ECM-like substrate. Control Scr transfected MDA-MB-231 cells formed larger mammospheres in hypoxic conditions (2% O2) when compared with normoxia (; Fig. S5). However, mammospheres were significantly smaller in the absence of PTK6 (), indicating a role for PTK6 in anchorage-independent 3D cell growth. Interestingly, mammospheres formed from the cells lacking PTK6 were predominantly smooth, whereas PTK6-expressing mammospheres presented a spiky/invasive appearance (). This phenotype has been previously associated with increased invasive and tumorigenic ability.Citation29 The decreased invasive phenotype in the absence of PTK6 was further tested using conventional transwell assays, where both MDA-MB-231 and RKO cells had a lower invasive ability in the absence of PTK6 (; Fig. S6). This was reflected by an invasion index below 1 (0.46 for MDA-MB-231 and 0.16 for RKO). These results indicate that PTK6 is important for hypoxia-mediated cellular motility and invasion, which are key factors in metastasis.
Figure 3. Hypoxia-induced PTK6 promotes cell motility and invasion. (A) MDA-MB-231 and RKO cells were transfected with Scr (scramble) or PTK6 siRNA. Graph represents the percentage of wound closure after 18 h in 2% O2. Graphs represent the mean of n = 3 independent experiments. (B) Representative images of scratch wound assays for (A). (C) MDA-MB-231 cells were transfected with Scr or PTK6 siRNA. Kinetic real-time migration assays were performed at 3% O2 using the xCelligence Real Time Cell Analyzer (RTCA) DP instrument. The graph depicts changes in the cell index (CI). Graph represents n = 2 experiments. (D) MDA-MB-231 cells were transfected with Scr or PTK6 siRNA. Mammospheres were established and exposed to normoxia (Norm) or 2% O2 (Hyp) for 24 h. The graph represents a quantification of the average size of at least 150 mammospheres per condition. Data represent n = 6 individual experiments. (E) MDA-MB-231 mammospheres were generated from cells transfected with Scr or PTK6 siRNA and treated as described in (D). Mammospheres were scored according to their morphological phenotype as non-invasive or invasive. Representative images of both phenotypes depicted in inset. Graph represents the percentage of different morphologies under each condition. Data represent n = 6 individual experiments. (F) MDA-MB-231 and RKO cells were transfected with Scr or PTK6 siRNA as before. Cells were seeded in control (uncoated) or matrigel coated Transwell inserts with 8 μm pore size and allowed to invade for 18 h at 2% O2. Invasion index = % invasion PTK6 siRNA/% invasion Scr. Results are representative of n = 3 individual experiments. *P < 0.05; **P < 0.005; ***P < 0.0001
PTK6 expression is linked to distant metastasis-free survival
To determine whether our in vitro data suggesting that PTK6 expression is linked to metastatic potential correlated with findings in human tumors we examined the effects of PTK6 expression on distant metastasis-free survival (DMFS) in 1609 breast cancer patients using data from Györffy and colleagues (2010).Citation30 High Brk expression was correlated with a reduced metastasis-free survival (P = 0.0017) ().
Figure 4. High PTK6 expression is associated with decreased distant metastasis-free survival in breast cancer patients. (A and B) Kaplan–Meier curves depicting the effect of PTK6 expression in distant metastasis-free survival (DMFS) in 1609 breast cancer patients (A) and a subset of 220 triple negative (basal-like) patients (B). Kaplan–Meier curves were generated using the KMplot online tool. Median expression was used as a cut-off for grouping into low (black) or high (gray) PTK6 expression. HR, Hazard ratio
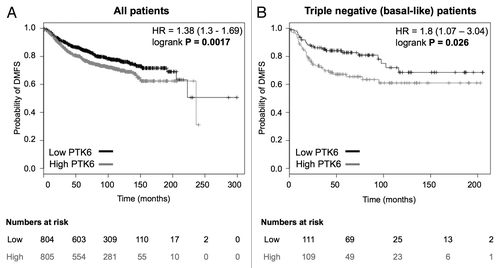
Given our findings in the triple-negative breast cancer cell line, MDA-MB-231, we determined whether PTK6 expression was particularly linked with DMFS in the 220 patients from the Györffy data set with triple-negative (basal-like) breast tumors. The Kaplan–Meier plot in shows that, as with the overall cohort, PTK6 expression is a poor prognostic indicator for DMFS in patients with basal-like breast cancer. Notably, there was a greater difference between the probabilities for basal-like breast cancers than was observed for the overall patient group.
Discussion
The role of hypoxia in tumor development and spread is well characterized.Citation17 However, although many key players in this process have been identified, many others remain uncharacterized. Lange and coworkers identified hypoxia to be an inducer of the non-receptor tyrosine kinase PTK6 in normal and neoplastic cells and that this induction was dependent on HIF-1α.Citation22 In our study we describe the rapid, posttranslational induction of PTK6 protein levels in response to hypoxia in both breast and colorectal cancer cell lines () in a much shorter time frame than previously reported.Citation22 PTK6 induction occurred prior to HIF-1α stabilization, implying that there is an additional HIF-independent mechanism mediating PTK6 protein level increase in hypoxic conditions. We did observe an increase of PTK6 mRNA after prolonged exposures to hypoxia for the breast cancer cell line MDA-MB-231, similarly to the published findings by Lange and coworkers.Citation22 However, as we observed no PTK6 transcript upregulation on shorter exposures to hypoxia in this cell line model, our data suggest that PTK6 induction could initially be independent of mRNA expression in breast cancer cells. Importantly, there was no hypoxia-dependent transcriptional upregulation of PTK6 in the RKO colorectal cancer cell line model, indicating the hypoxia-mediated transcriptional upregulation of PTK6 could be cancer type-dependent. To confirm that PTK6 induction in hypoxia could be independent of HIF and transcriptional mechanisms we showed that, in response to hypoxia, proteasomal-mediated degradation of PTK6 is reduced and that this correlated with decreased ubiquitylation of PTK6 (). A candidate-based approach was used to investigate the possible role of specific E3 ligases in PTK6 stabilization. c-Cbl was prioritized as it is known to have substrates including both receptor and non-receptor tyrosine kinases.Citation31,Citation32 It appears that c-Cbl could, in part, be responsible for regulating PTK6 levels during early hypoxia, independently of HIF, although how c-Cbl is itself regulated in an oxygen-dependent manner to effect PTK6 levels is still unclear. Recently, PTK6 has been reported to promote the ubiquitylation and degradation of c-Cbl through targeted phosphorylation, which raises the intriguing possibility that a reciprocal feedback loop exists between c-Cbl and PTK6.Citation33 Furthermore, other E3 ligases, namely CHIP (C terminus of Hsc70-interacting protein) and SOCS3 (suppressor of cytokine signaling 3) were recently reported to enhance the proteasomal degradation of PTK6.Citation34,Citation35 These data add further support to our finding that PTK6 levels are regulated by the proteasome and the regulation of E3 ligases in hypoxia warrants further investigation. This study also demonstrates that hypoxic PTK6 has a role in regulating cellular invasion and migration (). Importantly, this is associated with a relocalization to the cell membrane, a process that is reported to be essential for PTK6’s role in oncogenesis.Citation36
Finally we show in a large patient cohort (1609 samples) that high PTK6 expression is correlated with reduced metastasis-free survival () across all tumor subtypes. This supports our previous findings that elevated PTK6 expression is associated with breast tumors that are either invasive, more likely to metastasize, as well as data from other studies using much smaller sample sizes (less than 300).Citation8,Citation9,Citation22,Citation37 Aubele and colleagues reported that high PLA signals, indicating a physical interaction between PTK6 and HER2, correlated with reduced metastasis-free survival, although their earlier findings in 193 invasive breast cancers suggest that PTK6 may be a positive prognostic indicator.Citation38 This discrepancy has been discussed elsewhere and does highlight the difference between expression at the mRNA level compared with protein-based studies.Citation8
Additionally, given that the reduction in metastasis free survival with high PTK6 expression appeared to be more marked in the 220 patients with triple-negative or basal-like breast cancer (), it is possible that, in the absence of other prognostic factors such as HER2, ER, and PR, PTK6 levels become more important in predicting prognosis.
It has been suggested that inhibition of PTK6 would be an effective therapeutic approach.Citation5,Citation27 However, the lack of commercially available specific inhibitors has not allowed for further investigations. Our in vitro results in both breast and colorectal cell line models show that PTK6 induction in hypoxia can be regulated by HIF-independent mechanisms, such as posttranslational modifications. Combined with the findings in breast cancers, our data add to the wealth of information describing the role of hypoxia in driving cell motility and invasion, indicating that targeting of PTK6 through the development of pharmacological inhibitors could potentially be used to decrease tumor metastatic potential and that this may be of particular benefit to patients with basal-like/triple negative breast cancers.
Materials and Methods
Cell lines, hypoxia, drug treatment, and siRNA transfections
MDA-MB-231, MDA-MB-453, MDA-MB-468, MDA-MB-361, BT474, T47D, MCF-7 and SKBR3 (breast), RKO, HCT116 and DLD1 (colorectal), and RT112, VmCuB1, T24 and 253J (bladder) cancer cell lines were grown in DMEM or RPMI-1640 (Sigma) with 10% FBS. HEK293T (kidney) cells were grown in DMEM/10% FBS. All cell lines were purchased from ATCC or ECCAC and routinely tested as negative for mycoplasma. Hypoxia treatments were performed in an in vivo2 400 (Ruskinn) or Heracell incubator (ThermoFisher). For experiments at < 0.1% O2, cells were plated in glass dishes and placed in a Bactron II anaerobic chamber (Shell labs). MG132 (Sigma), ALLN (Ac-LLnL-CHO, Sigma), lactacystin (Merck Millipore), and bortezomib (Selleck Chemicals) stocks were prepared in dimethyl sulfoxide (DMSO, Sigma). Cells were transfected with PTK6 siRNA (GGUGAUUUCU CGAGACAAC dTdT)Citation8 or scramble siRNA (Life Technologies) using DharmaFECT1 (Thermo Scientific). Knockdown was obtained after double transfection over 48 h. Transfection with Flag-PTK6 and HA-Ub (gift from Jason Parsons) was done using PEI (Polyethylenimine, Polysciences).
Cell lysis and western blotting
For whole cell extract (WCE) preparation, cells were lysed in UTB (9 M urea, 75 mM TRIS-HCl pH 7.5, and 0.15 M β-mercaptoethanol) and immunoblotted as previously described.Citation39 Antibodies used were PTK6 (ICR-100),Citation12 HIF-1α and GAPDH (BD Biosciences), c-Cbl, EGFR, and α-tubulin (Cell Signaling Technology), HA-tag (Abcam) and β-actin (Santa Cruz). The Odyssey infrared imaging technology was used for protein detection (LI-COR Biosciences). Densitometry was done using ImageJ software (NIH).
Immunoprecipitation (IP)
Cells were lysed in IP lysis buffer: 150 mM NaCl, 20 mM Hepes pH 7.5, 0.5 mM EDTA, 0.5% NP40, 1× Complete protease inhibitor cocktail, and 1× PhosStop phosphatase inhibitor cocktail (Roche). Flag-PTK6 was immunoprecipitated using Flag-M2 agarose (Sigma).
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed using Thermo Scientific Verso™ QRT-PCR (Thermo Scientific) as previously described.Citation40,Citation41 The qPCR reaction was performed using the 7500 Fast Real Time PCR System (Life Technologies). PTK6 and GLUT-1 expression levels were normalized to 18S rRNA. Primer sequences are available in the Supplementary Material (Table S1).
Scratch wound assay and measurement of cellular invasion
Scratch wound assays were performed as described previously.Citation42 For cellular invasion assays, cells were plated in control or Matrigel invasion chambers with 8 μm pore size (BD Biosciences). Cells were allowed to invade for 18 h before fixing and staining with DAPI to visualize nuclei.
Measurement of cellular proliferation and motility using the xCelligence system
Experiments were performed using the xCelligence Real Time Cell Analyzer (RTCA) DP instrument (Cambridge Biosciences). Cell migration was assessed using 16-well CIM-plates 16 as described.Citation28,Citation43 DMEM/10% FBS was added to the lower chamber as chemotractant and cells were seeded into the upper chamber at 40 000/well in serum free medium.
Mammosphere formation assay
Matrigel (BD Biosciences, USA) diluted 1:1 in serum free medium was added to 24-well plates. Cells were seeded at 2500 cells/well and allowed to adhere for 6 h before exposure to hypoxia (2% O2). After 24 h cells were returned to normal culture conditions. Medium was changed every 2–3 d. After 10 d the mammospheres were imaged using an Eclipse SE2000-E microscope (Nikon). Images were analyzed using ImageJ software (NIH). At least 150 mammospheres were measured per condition.
Breast cancer patient distant metastasis-free survival analysis
Kaplan–Meier curves for distant metastasis-free survival (DMFS) were generated using the KM-plotter online tool, (http://kmplot.com/analysis), which used microarray data for over 20 000 genes for 1609 breast cancer patients.Citation30 Analysis of PTK6 expression (Affymetrix ID 206482_at) was performed for 1609 breast cancer patient samples and a subset of 220 triple negative (basal-like) breast cancer patients. Both analyses were performed regardless of lymph node status. Patients were grouped as having high or low PTK6 expression, and median expression was used as the cut-off.
Statistical analysis
Statistical significance was determined using the Student t test and error bars represent ± SEM.
| Abbreviations: | ||
| PTK6 | = | protein tyrosine kinase 6 |
| Brk | = | breast tumor kinase |
| HIF | = | hypoxia-inducible transcription factor |
| EGF | = | epidermal growth factor |
| IGF | = | insulin-like growth factor |
| HGF | = | hepatocyte growth factor |
| OPN | = | osteopontin |
| ALLN | = | Ac-LLnL-CHO |
| GLUT1 | = | glucose transporter-1 |
| ALDOA | = | aldolase A |
| BNIP3 | = | BCL2/adenovirus E1B 19kDa interacting protein 3 |
| RTCA | = | Real Time Cell Analyser |
| c-Cbl | = | Casitas B-lineage lymphoma proto-oncogene |
| CHIP | = | C terminus of Hsc70-interacting protein |
| SOCS3 | = | suppressor of cytokine signaling 3 |
Additional material
Download Zip (818.7 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Dr Anderson Ryan, Dr Jason Parsons, Dr Stephen Maher, and Professor John Greenman for useful discussions. A Breast Cancer Campaign pilot grant awarded to E.M.H. supported this study. I.M.P. is supported by University of Hull HEFCE funding, and Royal Society and Breast Cancer Campaign pilot grants. S.A.E. is supported by ICR HEFCE funding and Cancer Research UK program grant C309/A11566. A.H. is supported by Brunel University HEFCE funding. E.M.H. is supported by a Cancer Research UK grant.
References
- Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene 2003; 22:4212 - 20; http://dx.doi.org/10.1038/sj.onc.1206465; PMID: 12833144
- Kasprzycka M, Majewski M, Wang ZJ, Ptasznik A, Wysocka M, Zhang Q, Marzec M, Gimotty P, Crompton MR, Wasik MA. Expression and oncogenic role of Brk (PTK6/Sik) protein tyrosine kinase in lymphocytes. Am J Pathol 2006; 168:1631 - 41; http://dx.doi.org/10.2353/ajpath.2006.050521; PMID: 16651629
- Lin HS, Berry GJ, Fee WE Jr., Terris DJ, Sun Z. Identification of tyrosine kinases overexpressed in head and neck cancer. Arch Otolaryngol Head Neck Surg 2004; 130:311 - 6; http://dx.doi.org/10.1001/archotol.130.3.311; PMID: 15023838
- Schmandt RE, Bennett M, Clifford S, Thornton A, Jiang F, Broaddus RR, Sun CC, Lu KH, Sood AK, Gershenson DM. The BRK tyrosine kinase is expressed in high-grade serous carcinoma of the ovary. Cancer Biol Ther 2006; 5:1136 - 41; http://dx.doi.org/10.4161/cbt.5.9.2953; PMID: 16855388
- Harvey AJ, Crompton MR. The Brk protein tyrosine kinase as a therapeutic target in cancer: opportunities and challenges. Anticancer Drugs 2004; 15:107 - 11; http://dx.doi.org/10.1097/00001813-200402000-00002; PMID: 15075665
- Zhao C, Chen Y, Zhang W, Zhang J, Xu Y, Li W, Chen S, Deng A. Expression of protein tyrosine kinase 6 (PTK6) in nonsmall cell lung cancer and their clinical and prognostic significance. Onco Targets Ther 2013; 6:183 - 8; PMID: 23525678
- Aubele M, Auer G, Walch AK, Munro A, Atkinson MJ, Braselmann H, Fornander T, Bartlett JM. PTK (protein tyrosine kinase)-6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomas. Br J Cancer 2007; 96:801 - 7; http://dx.doi.org/10.1038/sj.bjc.6603613; PMID: 17299391
- Harvey AJ, Pennington CJ, Porter S, Burmi RS, Edwards DR, Court W, Eccles SA, Crompton MR. Brk protects breast cancer cells from autophagic cell death induced by loss of anchorage. Am J Pathol 2009; 175:1226 - 34; http://dx.doi.org/10.2353/ajpath.2009.080811; PMID: 19661439
- Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res 2007; 67:4199 - 209; http://dx.doi.org/10.1158/0008-5472.CAN-06-3409; PMID: 17483331
- Brauer PM, Zheng Y, Wang L, Tyner AL. Cytoplasmic retention of protein tyrosine kinase 6 promotes growth of prostate tumor cells. Cell Cycle 2010; 9:4190 - 9; http://dx.doi.org/10.4161/cc.9.20.13518; PMID: 20953141
- Castro NE, Lange CA. Breast tumor kinase and extracellular signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res 2010; 12:R60; http://dx.doi.org/10.1186/bcr2622; PMID: 20687930
- Kamalati T, Jolin HE, Mitchell PJ, Barker KT, Jackson LE, Dean CJ, Page MJ, Gusterson BA, Crompton MR. Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J Biol Chem 1996; 271:30956 - 63; http://dx.doi.org/10.1074/jbc.271.48.30956; PMID: 8940083
- Qiu H, Zappacosta F, Su W, Annan RS, Miller WT. Interaction between Brk kinase and insulin receptor substrate-4. Oncogene 2005; 24:5656 - 64; http://dx.doi.org/10.1038/sj.onc.1208721; PMID: 15870689
- Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res 2008; 68:152 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-07-2126; PMID: 18172307
- Haegebarth A, Perekatt AO, Bie W, Gierut JJ, Tyner AL. Induction of protein tyrosine kinase 6 in mouse intestinal crypt epithelial cells promotes DNA damage-induced apoptosis. Gastroenterology 2009; 137:945 - 54; http://dx.doi.org/10.1053/j.gastro.2009.05.054; PMID: 19501589
- Zheng Y, Gierut J, Wang Z, Miao J, Asara JM, Tyner AL. Protein tyrosine kinase 6 protects cells from anoikis by directly phosphorylating focal adhesion kinase and activating AKT. Oncogene 2013; 32:4304 - 12; PMID: 23027128
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009; 15:35 - 44; http://dx.doi.org/10.1016/j.ccr.2008.11.012; PMID: 19111879
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 1998; 58:1408 - 16; PMID: 9537241
- Vaupel P, Höckel M. Tumor oxygenation and its relevance to tumor physiology and treatment. Adv Exp Med Biol 2003; 510:45 - 9; http://dx.doi.org/10.1007/978-1-4615-0205-0_8; PMID: 12580403
- Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. Methods Enzymol 2004; 381:335 - 54; http://dx.doi.org/10.1016/S0076-6879(04)81023-1; PMID: 15063685
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 2012; 148:399 - 408; http://dx.doi.org/10.1016/j.cell.2012.01.021; PMID: 22304911
- Regan Anderson TM, Peacock DL, Daniel AR, Hubbard GK, Lofgren KA, Girard BJ, Schörg A, Hoogewijs D, Wenger RH, Seagroves TN, et al. Breast tumor kinase (Brk/PTK6) is a mediator of hypoxia-associated breast cancer progression. Cancer Res 2013; 73:5810 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-13-0523; PMID: 23928995
- Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 2009; 284:16767 - 75; http://dx.doi.org/10.1074/jbc.M901790200; PMID: 19386601
- Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 2011; 117:e207 - 17; http://dx.doi.org/10.1182/blood-2010-10-314427; PMID: 21447827
- Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 2010; 16:5928 - 35; http://dx.doi.org/10.1158/1078-0432.CCR-10-1360; PMID: 20962028
- Ostrander JH, Daniel AR, Lange CA. Brk/PTK6 signaling in normal and cancer cell models. Curr Opin Pharmacol 2010; 10:662 - 9; http://dx.doi.org/10.1016/j.coph.2010.08.007; PMID: 20832360
- Brauer PM, Tyner AL. Building a better understanding of the intracellular tyrosine kinase PTK6 - BRK by BRK. Biochim Biophys Acta 2010; 1806:66 - 73; PMID: 20193745
- Scrace S, O’Neill E, Hammond EM, Pires IM. Use of the xCELLigence system for real-time analysis of changes in cellular motility and adhesion in physiological conditions. Methods Mol Biol 2013; 1046:295 - 306; http://dx.doi.org/10.1007/978-1-62703-538-5_17; PMID: 23868595
- Singh AJ, Meyer RD, Navruzbekov G, Shelke R, Duan L, Band H, Leeman SE, Rahimi N. A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCgamma1 activation and angiogenesis. Proc Natl Acad Sci U S A 2007; 104:5413 - 8; http://dx.doi.org/10.1073/pnas.0700809104; PMID: 17372230
- Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 2010; 123:725 - 31; http://dx.doi.org/10.1007/s10549-009-0674-9; PMID: 20020197
- Thien CB, Langdon WY. Negative regulation of PTK signalling by Cbl proteins. Growth Factors 2005; 23:161 - 7; http://dx.doi.org/10.1080/08977190500153763; PMID: 16019438
- Zhou L, Yang H. The von Hippel-Lindau tumor suppressor protein promotes c-Cbl-independent poly-ubiquitylation and degradation of the activated EGFR. PLoS One 2011; 6:e23936; http://dx.doi.org/10.1371/journal.pone.0023936; PMID: 21949687
- Kang SA, Lee ST. PTK6 promotes degradation of c-Cbl through PTK6-mediated phosphorylation. Biochem Biophys Res Commun 2013; 431:734 - 9; http://dx.doi.org/10.1016/j.bbrc.2013.01.046; PMID: 23352614
- Gao Y, Cimica V, Reich NC. Suppressor of cytokine signaling 3 inhibits breast tumor kinase activation of STAT3. J Biol Chem 2012; 287:20904 - 12; http://dx.doi.org/10.1074/jbc.M111.334144; PMID: 22547065
- Kang SA, Cho HS, Yoon JB, Chung IK, Lee ST. Hsp90 rescues PTK6 from proteasomal degradation in breast cancer cells. Biochem J 2012; 447:313 - 20; http://dx.doi.org/10.1042/BJ20120803; PMID: 22849407
- Palka-Hamblin HL, Gierut JJ, Bie W, Brauer PM, Zheng Y, Asara JM, Tyner AL. Identification of beta-catenin as a target of the intracellular tyrosine kinase PTK6. J Cell Sci 2010; 123:236 - 45; http://dx.doi.org/10.1242/jcs.053264; PMID: 20026641
- Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, Zou L, Yao J, Lu Y, Epstein CB, et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One 2010; 5:e11729; http://dx.doi.org/10.1371/journal.pone.0011729; PMID: 20668531
- Aubele M, Spears M, Ludyga N, Braselmann H, Feuchtinger A, Taylor KJ, Lindner K, Auer G, Stering K, Höfler H, et al. In situ quantification of HER2-protein tyrosine kinase 6 (PTK6) protein-protein complexes in paraffin sections from breast cancer tissues. Br J Cancer 2010; 103:663 - 7; http://dx.doi.org/10.1038/sj.bjc.6605836; PMID: 20700126
- Pires IM, Bencokova Z, Milani M, Folkes LK, Li JL, Stratford MR, Harris AL, Hammond EM. Effects of acute versus chronic hypoxia on DNA damage responses and genomic instability. Cancer Res 2010; 70:925 - 35; http://dx.doi.org/10.1158/0008-5472.CAN-09-2715; PMID: 20103649
- Hammond EM, Mandell DJ, Salim A, Krieg AJ, Johnson TM, Shirazi HA, Attardi LD, Giaccia AJ. Genome-wide analysis of p53 under hypoxic conditions. Mol Cell Biol 2006; 26:3492 - 504; http://dx.doi.org/10.1128/MCB.26.9.3492-3504.2006; PMID: 16611991
- Haglund K, Schmidt MH, Wong ES, Guy GR, Dikic I. Sprouty2 acts at the Cbl/CIN85 interface to inhibit epidermal growth factor receptor downregulation. EMBO Rep 2005; 6:635 - 41; http://dx.doi.org/10.1038/sj.embor.7400453; PMID: 15962011
- Pires IM, Olcina MM, Anbalagan S, Pollard JR, Reaper PM, Charlton PA, McKenna WG, Hammond EM. Targeting radiation-resistant hypoxic tumour cells through ATR inhibition. Br J Cancer 2012; 107:291 - 9; http://dx.doi.org/10.1038/bjc.2012.265; PMID: 22713662
- Coutts AS, Pires IM, Weston L, Buffa FM, Milani M, Li JL, Harris AL, Hammond EM, La Thangue NB. Hypoxia-driven cell motility reflects the interplay between JMY and HIF-1α. Oncogene 2011; 30:4835 - 42; http://dx.doi.org/10.1038/onc.2011.188; PMID: 21625218
