Abstract
Pancreatic cancer (ductal adenocarcinoma) remains a deadly cancer with ~85% mortality, and a 5-year survival rate of ~6% or less for the past 30 years. The factors and events associated with the development of pancreatic cancer are poorly identified. As such, effective biomarkers for early detection of malignancy are lacking. Efficacious chemotherapy once the cancer is identified does not exist. Recent clinical studies have revealed that the zinc levels are consistently and markedly decreased in adenocarcinoma as compared with normal/benign pancreatic tissue. The decreased zinc is exhibited in well-differentiated malignancy and in progressing malignancy, and also exists throughout the development of PanIN. Concurrent with the decrease in zinc, RREB1 transcription factor and ZIP3 zinc uptake transporter are downregulated. Thus, a RREB1/ZIP3/Zinc transformation appears to be an early event in the development of pancreatic cancer. We propose that this transformation is necessary to prevent the accumulation of high cellular zinc levels, which result in cytotoxic effects on the developing malignant cells. This report now demonstrates that exposure of Panc1 cells to physiological concentrations of zinc that result in increased zinc uptake and accumulation also inhibits cell proliferation. The study further shows that ZIP3 is the important transporter required for the accumulation of zinc and its inhibition of proliferation. RREB1 is identified as the positive regulator of ZIP3 expression. Therefore, the pathway of RREB1/ZIP3/Zinc and its downregulation during oncogenesis exist to prevent the accumulation of cytotoxic levels of zinc during the development and progression of the malignant cells in pancreatic adenocarcinoma.
Introduction
Pancreatic cancer (ductal adenocarcinoma) is among the deadliest of cancers, and is expected to result in ~44 000 new cases/year with ~38 000 deaths in the USA.Citation1 Despite intense research in recent decades, the 5-y survival rate has remained at ~6% or less for the past 35 y. Contributing to this unfortunate absence of significant progress is the poor understanding and resolution of the factors and events involved in the development and progression of pancreatic malignancy. As a consequence, the discovery and availability of effective biomarkers for identification of premalignant and early stage cancer is impeded, as has been the development of effective chemotherapeutic agents for treatment—and perhaps prevention—of pancreatic cancer.
The status of zinc and zinc transporters have been implicated in several cancers, although the specific relationships are largely undefined in most cancers.Citation2-Citation5 For most of the cancers in which zinc levels have been determined (such as prostate cancer, hepatocellular cancer, ovarian cancer, among others), decreased zinc levels are associated with the cancer compared with the corresponding normal/benign zinc status. An exception to this relationship is breast cancer, in which malignant tissue zinc levels are increased compared with normal breast tissue levels.
In view of the widespread reported studies of the implications of zinc in various cancers, it is surprising that aside from our reported studies in 2011 and 2012,Citation6,Citation7 no other reports currently exist that provide the determination of the zinc levels in human pancreatic adenocarcinoma tissue compared with normal/benign pancreas. Our studies of in situ zinc levels in human pancreatic tissue sections have identified a consistent major decrease in zinc in the malignant cells compared with the normal/benign ductal and acini epithelium. The marked decrease in zinc is evident in highly-differentiated early malignancy and in the putative premalignant PanIN lesions, and it persists in advanced malignancy. The studies also identified ZIP3 as an important zinc uptake transporter in the normal ductal/acinar epithelium, which is consistently downregulated concurrently with the decrease in zinc. In addition, RREB1 (ras responsive element binding protein) downregulation was also identified concurrently with the silencing of ZIP3 in PanIN lesions and malignancy.
These observations lead to the new evolving concept that RREB1/ZIP3 downregulation results in the decrease in zinc as an early event in the development of pancreatic cancer. Since increased cellular accumulation of zinc exhibits cytotoxic effects in malignant cells,Citation2-Citation5 the decrease in zinc is essential during oncogenesis to permit the development and progression of malignancy. We propose that this relationship applies to pancreatic cancer. In support of this concept, we show in this report that treatment with zinc that results in its cellular accumulation inhibits malignant cell proliferation, and that the RREB1/ZIP3/zinc pathway is implicated in this effect.
Results
The normal range of plasma zinc is ~15 uM, from which the transportable zinc in the interstitial fluid derived from blood plasma is ~3–10 uM. We earlier reported,Citation6 as shown in , that exposure of Panc1 cells to physiological concentrations of zinc in the presence of pyrithione (a zinc ionophore) results in increased cellular zinc, which markedly inhibits cell proliferation. At concentrations of 2.5 and 5.0 uM zinc, the cellular accumulation of zinc resulted in ~65% and ~80% inhibition of cell proliferation. We now show () that exposure of Panc1 cells to zinc in the presence of clioquinol ionophore (5-chloro-7-iodo-8-quinolinol) results in ~55% and ~80% inhibition of cell proliferation. The nearly identical results with both zinc ionophores demonstrates that conditions that will increase cellular zinc uptake and accumulation at physiological zinc levels will inhibit cell proliferation. A reason for our election of clioquinol in this present study is its potential as a treatment approach for pancreatic cancer (discussed below). Clioquinol has been employed for the treatment of some conditions in humans, and exhibits therapeutic effects at concentrations that are of minimal or no toxicity,Citation8,Citation9 which makes it a potential candidate for a zinc-treatment approach in PanCa (discussed below). As shown in , our earlier studiesCitation6,Citation7 demonstrated that ZIP3 is the plasma membrane zinc uptake transporter in normal ductal/acinar epithelial cells, which is downregulated concurrently with the decrease in zinc in adenocarcinoma. Most importantly, the membrane localization occurs specifically at the basilar membrane, which is necessary for the capability of cellular uptake of zinc from plasma. This indicated the likelihood that ZIP3 is the important functional zinc uptake transporter in the normal ductal and acinar epithelium, and the loss of basilar membrane ZIP3 transporter is a major factor associated with the decreased cellular zinc in pancreatic adenocarcinoma. This prevents the inhibition of malignant cell proliferation during the development of adenocarcinoma. To test this concept, we determined the effects of zinc treatment on wild-type Panc1 cells that exhibit plasma membrane localized ZIP3 (Panc1/ZIP3 cells) compared with Panc1 cells with downregulated ZIP3 (Panc1/-ZIP3). shows that the zinc treatment (ZnTX) resulted in 53% inhibition of proliferation of the Panc1/ZIP3 cells, when compared with the proliferation of the Panc1/-ZIP3 cells in the presence of zinc. Thus, the downregulation of ZIP3, as occurs in the malignant cells in situ in PancCa, prevents the cytotoxic effects of physiological concentration of zinc.
Figure 1. The effect of zinc treatment on the cellular accumulation of zinc and proliferation of Panc1 cells. The cells were treated with zinc for 24 h. (A) Pyrithione (1 uM) was included in the medium. (B) Clioquinol (10 uM) was included in the medium.
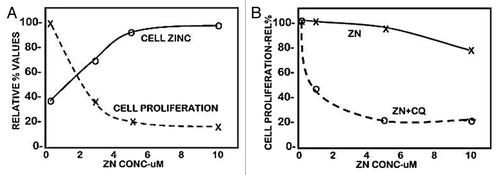
Figure 2. ZIP3 transporter and zinc treatment effects on cell proliferation. (A) Immunohistochemical identification of ZIP3 transporter in adenocarcinoma and normal human pancreatic tissue sections. Blue arrows show basal membrane localization of transporter in normal ductal and acinar epithelium. Black arrows show the absence of plasma membrane transporter in ductal adenocarcinoma and in PanIN epithelium. (B) Blue arrows show the presence of plasma membrane ZIP3 transporter in Panc1 cells. (C) Shows the effect of zinc treatment on proliferation of wildtype Panc1 cells that exhibit ZIP3 transporter and on Panc1 cells with downregulated ZIP3 (i.e., Panc1/ZIP3 cells vs. Panc1/-ZIP3 cells).
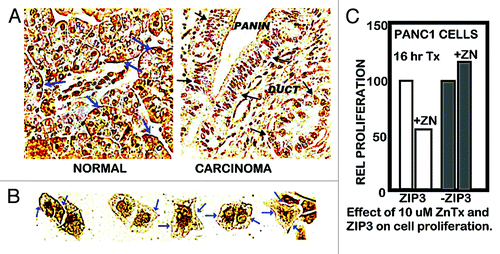
The Panc1 cell line was derived from ductal adenocarcinoma, in which the malignant cells in situ do not express ZIP3. The constitutive re-expression of ZIP3 in the Panc1 cells () reveals that the in situ downregulation of ZIP3 is not due to deletion or fatal mutation of the ZIP3 gene. More likely, it reveals the epigenetic silencing of ZIP3 expression under the in situ conditions of the pancreas, whereas those conditions or factors are not represented under in vitro culture conditions.
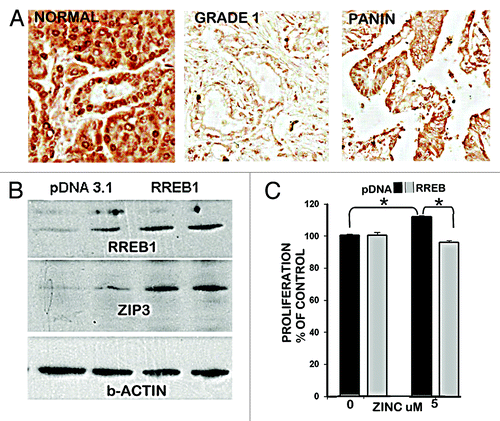
Based on this expectation, our previous reportsCitation6,Citation7 demonstrated that RREB1 transcription factor is expressed in the normal ductal/acinar epithelium and is concurrently downregulated with ZIP3 in PanIN epithelium and in early and progressing malignancy (), and the downregulation of RREB1 results in decreased expression of ZIP3 in Panc1 cells. This caused us to determine the effects of altered RREB1 expression on ZIP3 transporter abundance and proliferation of Panc1 cells. shows that the pcDNA Panc1 cells exhibit constituitive RREB1 and ZIP3, and that overexpression of RREB1 markedly increased the abundance of ZIP3. This confirms that RREB1 is a positive regulator of ZIP3 expression. When exposed to physiological concentration of zinc (), the Panc1/+RREB1 cells, which exhibited increased abundance of ZIP3 exhibited inhibition of proliferation, compared with the proliferation of the Panc1/pcDNA control cells that exhibit lower abundance of ZIP3 transporter. Collectively, these results are consistent with an important role of RREB1 regulation of ZIP3 gene expression and cellular zinc levels during the development of pancreatic cancer.
Figure 3. RREB1 in normal vs. adenocarcinoma pancreas and its regulation of ZIP3 and zinc effects on proliferation of Panc1 cells. (A) Immunohistochemistry showing abundant RREB1 in normal ductal and acinar epithelium; and marked decrease of RREB1 (especially nuclear RREB1) in well differentiated malignancy and in the PanIN epithelium. (B) Western blot of effects of overexpression of RREB1 on ZIP3 expression. Lanes are duplicates for pcDNA and for RREB1. (C) The effects of RREB1 expression of the Panc1 cells on cell proliferation in the presence of zinc.
Discussion
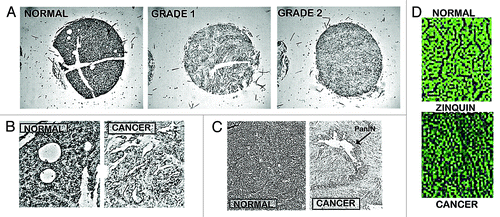
Presently, our studiesCitation6,Citation7 are the only reports that provide direct measurements of the relative zinc levels in human pancreatic tissue (). The combined studies involved ~45 cases of adenocarcinoma and ~15 normal/benign tissues. The relative zinc levels were determined by in situ histochemical staining with dithizone and confirmed with Zinquin. This is preferable over zinc measurements of tissue extracts since the pancreas is comprised of exocrine and endocrine components, and the latter includes islets, which contain extremely high zinc levels. The in situ determination permits the identification of the specific cells and their relative zinc levels, which cannot be identified by measurements with pancreatic tissue extracts. As represented in , our studiesCitation6,Citation7 demonstrated a consistent marked decrease in zinc in adenocarcinoma cells vs. normal/benign ductal/acinar epithelium. The decrease in zinc is evident in the premalignant PanIN lesions, in early Grade1 malignancy, and in progressing malignancy. This compelling clinical evidence leads to the conclusion that pancreatic adenocarcinoma is characterized by a consistent marked decrease in zinc, which occurs early in the development of malignancy. This calls attention to the question, “Why and how is zinc decreased in situ in the malignant cells in pancreatic cancer?”
Figure 4. In situ staining of zinc levels in human pancreatic normal and adenocarcinoma tissues. (A) Low power tissue arrays showing marked decreased dithizone zinc staining in adenocarcinoma. (B) Enlargement to show the high zinc in the normal ductal and acini epithelium vs. zinc depletion in adenocarcinoma. (C) Shows the marked loss of zinc in PanIN and in the surrounding ductal adenocarcinoma. (D) Zinquin staining showing decreased zinc (loss of fluorescence) in adenocarcinoma.
The data presented in this report demonstrate that, at physiological concentrations of zinc, conditions that result in increased cellular uptake and accumulation of zinc by Panc1 cells consistently result in the inhibition of cell proliferation. OthersCitation10,Citation11 have similarly reported cytotoxic effects of increased zinc accumulation in malignant pancreatic cells. Donadelli et al.Citation12 additionally showed that non-malignant cells were less sensitive to zinc exposure than the malignant pancreatic cells. Consequently, the decreased zinc that occurs early in the development of pancreatic cancer is an essential malignant event to prevent the cytotoxic effects that would result from the higher zinc levels that exist in the normal cells. Some other cancers (such as prostate cancer or hepatocellular cancer) also exhibit decreased tissue zinc levels compared with their normal/benign tissue, and numerous reports identified similar cytotoxic effects of increased zinc with the corresponding malignant cells.Citation2-Citation4
Despite the published availability of this clinical evidence, Li et al. have maintained a conclusion that zinc is increased in pancreatic adenocarcinoma.Citation13-Citation17 However, they have not provided any zinc measurements in human pancreatic tissues in support of their conclusion. Nor have they acknowledged in any of their reports the existence of the clinical evidence that presently contradicts their clinically unfounded conclusion. Instead, they draw their conclusion from experimental studies with engineered cell lines, which purport to show that increased zinc facilitates pancreatic malignant cell proliferation and tumor growth. In the absence of clinical evidence of increased zinc levels in human adenocarcinoma tissue, the substitution of their experimental evidence to support their conclusion is inappropriate.
Moreover, the experimental studies of Li et al. (such as refs. Citation13, Citation14, Citation16, and Citation17) largely employed malignant pancreatic cell lines in which the cells were transfected with ZIP4, and were depleted of zinc by pretreatment with TPEN (N,N,N′,N-tetrakis[2-pyridylmethyl ethylenediamine]). TPEN is a cell permeable zinc chelator with a formation constant logKf~15. Mammalian cells contain ~100–500 uM zinc, of which ~95% is strongly bound (mainly as metalloprotein) as immobile zinc, and ~5% exists as mobile exchangeable zinc (ZnLigands with logKf ~10 or lower).Citation18 The latter comprises the essential reactive pool of zinc. Consequently, treatment with TPEN strongly binds and eliminates virtually all of the cellular mobile reactive zinc.Citation2,Citation4,Citation5 Such abnormal zinc-depleted cells do not exist in natural living systems, and such cells do not exhibit their normal growth, proliferation, and functional activities. Consequently, the survival effect of zinc treatment under such conditions involves the restoration of the cellular zinc that is essential for the recovery of the dying zinc-depleted cell. It is notable that Donadelli et al.Citation12 reported that pancreatic cells depleted of zinc by TPEN treatment exhibit cytotoxicity including increased apoptosis, decreased proliferation, and decreased viability, and that zinc treatment restored the zinc-depleted cells to their “normal” condition. Therefore, such conditions do not represent the effects of exposure to zinc on cells that exist with their normal physiological composition and status of zinc, and in which exposure to physiological concentration of zinc results in inhibition and cytotoxicity of the malignant cells.
Moreover, Jansen et al.,Citation19 in a recent Mayo Clinic/NIH collaborative study, reported that the incidence of pancreatic cancer is inversely associated with the level of dietary zinc consumption. The study revealed that the increased daily consumption of zinc from 5.19 to 9.13 (mg/1000 kcal) was associated with a 52% decrease in the incidence of pancreatic cancer, with a statistical significance of P = 0.0001. Also, plasma zinc levels are reportedly decreased in pancreatic cancer,Citation20,Citation21 which could contribute to decreased zinc uptake and accumulation in the developing ZIP3-deficient malignant cells. These relationships are consistent with our evidence of decreased zinc in pancreatic cancer and the effect of zinc as a cytotoxic/tumor suppressor agent, and that a zinc supplement regimen that restores increased zinc levels in malignancy, might provide a treatment approach for pancreatic cancer. Conversely, the results are inconsistent with the supposition of Li et al.Citation17-Citation21 that increased zinc will promote the development of pancreatic cancer, and with their proposal that decreased availability and depletion of zinc will prevent pancreatic cancer.
The clinical evidence now shows that RREB1 is expressed in normal ductal and acinar epithelial cells, and its downregulation in malignancy occurs concurrently with the silencing of ZIP3 expression. Computer analysis shows the presence of potential RREB1 binding sites in the promoter region of the ZIP3 gene. We cloned a 5′ fragment and identified potential RREB1 binding sites around the transcription start site (data not shown). The experimental evidence with Panc1 cells demonstrated that alteration of REBB1 expression correspondingly alters the expression of ZIP3. Initial evidence shows the presence of potential RREB1 binding sites in the promoter region of the ZIP3 gene. Thus, it is very likely that the downregulation of RREB1, which occurs in PanIN and in early stage malignancy, initiates the silencing of ZIP3 leading to decreased zinc in the malignant cells. RREB1 is a transcription factor that has been reported to be either an activator,Citation22-Citation24 or a repressorCitation25-Citation28 of gene expression, the action of which is dependent upon the gene and cell type.Citation29 Our results reveal that REEB-1 is a positive regulator of ZIP3 gene expression. Consequently, the existence of downregulated RREB1 in PanIN and early malignancy must be accompanied by preceding upstream events that promote the downregulation of RREB1. One would expect that an activated (mutated) KRAS pathway might be involved in this regulation of RREB1 in pancreatic malignancy. However, very little information regarding RREB1 regulation exists, and much of the information is speculative. KRAS mutations are reportedly minimal in early PanIN development (e.g., PanIN-1A), and are progressively increased in PanIN-2 and PanIN-3, and their oncogenetic effects are manifested in ductal adenocarcinoma.Citation30-Citation32 This pattern of activated KRAS oncogenesis is not represented by the RREB1 downregulation, which is fully manifested in PanIN-1A and precedes the presence of activating KRAS mutations. It will be important to elucidate the oncogenic regulation of RREB1 by KRAS or possibly other oncogenetic factors.
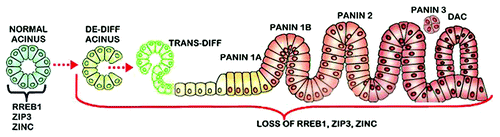
Another relevant issue regards the origin of the PanIN lesions. A prevailing view proposes that pancreatic conditions associated with the development of ductal adenocarcinoma causes de-differentiation and re-programming of acinar epithelium (reviewed in refs. Citation31–Citation36). These cells then transdifferentiate into PanIN epithelium leading to ductal adenocarcinoma. It is notable from our studies that the acinar epithelium exhibit downregulated RREB1/ZIP3/zinc in well-differentiated ductal adenocarcinoma, which is also evident in PanIN 1 epithelium. Therefore, as represented in , dedifferentiated acinar epithelium as the origin of PanIN in the development of ductal adenocarcinoma is supported by the identification of downregulated RREB1/ZIP3/zinc during pancreatic oncogenesis. Moreover, these changes were consistently evident in the pancreatic adenocarcinoma cases that were included in our studies, which indicates that downregulated RREB1/ZIP3/decreased zinc is required to permit the development of pancreatic adenocarcinoma, presumably to prevent the cytotoxic effects of increased zinc on the premalignant and malignant cells.
Figure 5. The concept of the “RREB1/ZIP3/Decreased Zinc” transformation and acini de-differentiation as the origin of PanlN leading to the development of ductal adenocarcinoma. De-diff, de-differentiation; Trans-diff, trans-differentiation; DAC, ductal adenocarcinoma. (The figure is a modified version from Morris et al.Citation31)
Conclusions
Clinical and experimental evidence reveals that zinc is consistently and markedly decreased in human pancreatic adenocarcinoma as compare with normal/benign pancreas. The decrease in zinc results from the downregulation of RREB1 resulting in silencing of expression of ZIP3 zinc uptake transporter. These coupled changes are evident in early PanIN, in well-differentiated malignancy, and in progressing malignancy, which leads to the conclusion that this transformation is an essential event initiated early in the pancreatic oncogenetic process, and which is consistent for all, or most, adenocarcinoma cases. This presents a new understanding of the development and progression of pancreatic adenocarcinoma, providing potential biomarkers for early detection of pancreatic cancer. Moreover, it reveals that human pancreatic malignancy can be characterized as being “ZIP3-deficient”. As such the potential of zinc treatment for cytotoxic/tumor suppressor effects against pancreatic cancer must recognize the necessity for a mechanism and/or agent that will facilitate the uptake and accumulation of zinc in the ZIP3-deficient malignant cells.Citation4,Citation5 An effective approach might employ a zinc ionophore such as Clioquinol, which, as shown in , effectively results in inhibition of cell proliferation. At this time, more clinical and experimental research is required to take advantage of the implications of zinc in dealing with this deadly cancer.
Materials and Methods
Reagents
Culture media, antibiotic mixture, and phosphate buffered saline (PBS) were purchased from Invitrogen. Fetal bovine serum (FBS) was purchased from Atlantic Biologicals. All other chemicals, reagents and antibodies (unless otherwise indicated) were purchased from Sigma-Aldrich.
Zinc treatment and proliferation assay
Panc1 cells obtained from ATCC were cultured under standard conditions in DMEM medium supplemented with 10% FBS and 1% penicillin–streptomycin mixture at 37 °C in a humidified atmosphere of 5% CO2 and air with the medium changed twice a week. For studies on the effect of Zn treatment, cells were generally plated in multi-well plates (3 × 104 cells/well) and cultured overnight. The next day the medium was removed, cells washed, and the medium replaced with DMEM supplemented with 2% FBS and containing various concentrations of ZnCl2 and other supplements as described in the results. Following treatment for 24 h, the medium was removed and the wells washed with PBS and the plates placed at –80 °C overnight. Cell proliferation was measured using the CyQUANT Cell Proliferation Assay Kit (Invitrogen) according to the manufacturer’s protocol. Cellular zinc was measured using Zinquin fluorescent indicator as previously described.Citation6
Transfections
Panc1 cells were maintained in DMEM medium with l-glutamine and Hepes (Gibco) supplemented with 10% FBS and 1% penicillin–streptomycin mixture. Cells were plated in 12-well plates (500 000 cells/well) 24 h before the transfection. After attachment for 24 h, the cells were transfected with 0.5 µg/well of pcDNA3.1-Finb/RREB1,Citation37 or with empty pcDNA3.1 vector using Fugene HD transfection reagent from Roche Applied Science. Cells were cultured in the presence of pcDNA3.1 or pcDNA3.1-Finb/RREB1 for 72 h, washed and the medium replaced with medium for the appropriate experiments.
siRNA ZIP3 knockdown
Panc1 cells were plated in DMEM medium supplemented with 10% FBS and 1% Penicillin-Streptomycin mixture and allowed to attach for 24 h. After 24 h the cells were transfected with 50 nM Non-target siRNA or ZIP3 siRNA from Dharmacon RNAi Technologies using DharmaFECT 2 Transfection Reagent following the manufacturer’s instructions. Twenty-four hours after transfection the cells were treated with 10 µM ZnCl2 or an equal volume of PBS and cultured overnight. After ZnCl2 treatment, cells were washed and the cell number determined using the CyQUANT Cell Proliferation assay Kit.
Immunoblot (western blot)
Whole cell lysates were prepared by collecting cells using RIPA buffer (Upstate Biotech). The concentration of the protein in the lysates was determined using the BioRad protein assay based on the Bradford procedure.Citation38 Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes and blocked with PBS containing 5% non-fat dry milk and 0.1% Tween 20 for 2 h at room temperature. The membranes were then incubated overnight with hZIP3 chicken polyclonal antibody previously described.Citation39 Protein-bound antibody was detected using goat anti-chicken secondary antibody and enhanced chemiluminescence detection reagents. The membranes were stripped of antibody and re-probed with antiRREB1 and anti β-actin.Citation28
Statistical analysis was performed by a paired t test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Some of the studies of the authors described in this report were supported by NIH grants CA79903, CA93443, and DK42839.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem 2009; 106:750 - 7; http://dx.doi.org/10.1002/jcb.22049; PMID: 19160419
- Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys 2007; 463:211 - 7; http://dx.doi.org/10.1016/j.abb.2007.02.033; PMID: 17400177
- Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther 2012; 12:121 - 8; http://dx.doi.org/10.1586/era.11.190; PMID: 22149438
- Costello LC, Franklin RB. A review of the current status and concept of the emerging implications of zinc and zinc transporters in the development of pancreatic cancer. Pancreat Disord Ther 2013; Suppl 4 PMID: 24195024
- Costello LC, Levy BA, Desouki MM, Zou J, Bagasra O, Johnson LA, Hanna N, Franklin RB. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol Ther 2011; 12:297 - 303; http://dx.doi.org/10.4161/cbt.12.4.16356; PMID: 21613827
- Costello LC, Zou J, Desouki MM, Franklin RB. Evidence for changes in RREB-1, ZIP3, and Zinc in the early development of pancreatic adenocarcinoma. J Gastrointest Cancer 2012; 43:570 - 8; http://dx.doi.org/10.1007/s12029-012-9378-1; PMID: 22427155
- Mao X, Schimmer AD. The toxicology of Clioquinol. Toxicol Lett 2008; 182:1 - 6; http://dx.doi.org/10.1016/j.toxlet.2008.08.015; PMID: 18812216
- Bareggi SR, Cornelli U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci Ther 2012; 18:41 - 6; http://dx.doi.org/10.1111/j.1755-5949.2010.00231.x; PMID: 21199452
- Donadelli M, Dalla Pozza E, Scupoli MT, Costanzo C, Scarpa A, Palmieri M. Intracellular zinc increase inhibits p53(-/-) pancreatic adenocarcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim Biophys Acta 2009; 1793:273 - 80; http://dx.doi.org/10.1016/j.bbamcr.2008.09.010; PMID: 18951928
- Jayaraman AK, Jayaraman S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the ZnT-1 zinc transporter gene in pancreatic cancer cells. J Nutr Biochem 2011; 22:79 - 88; http://dx.doi.org/10.1016/j.jnutbio.2009.12.001; PMID: 20392624
- Donadelli M, Dalla Pozza E, Costanzo C, Scupoli MT, Scarpa A, Palmieri M. Zinc depletion efficiently inhibits pancreatic cancer cell growth by increasing the ratio of antiproliferative/proliferative genes. J Cell Biochem 2008; 104:202 - 12; http://dx.doi.org/10.1002/jcb.21613; PMID: 17979179
- Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A 2007; 104:18636 - 41; http://dx.doi.org/10.1073/pnas.0709307104; PMID: 18003899
- Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res 2009; 15:5993 - 6001; http://dx.doi.org/10.1158/1078-0432.CCR-09-0557; PMID: 19755388
- Yang J, Zhang Y, Cui X, Yao W, Yu X, Cen P, Hodges SE, Fisher WE, Brunicardi FC, Chen C, et al. Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr Mol Med 2013; 13:401 - 9; PMID: 23331012
- Zhang Y, Yang J, Cui X, Chen Y, Zhu VF, Hagan JP, Wang H, Yu X, Hodges SE, Fang J, et al. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol Med 2013; 5:1322 - 34; http://dx.doi.org/10.1002/emmm.201302507; PMID: 23857777
- Cui X, Zhang Y, Yang J, Sun X, Hagan JP, Guha S, Li M. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle 2014; 13:1180 - 6; http://dx.doi.org/10.4161/cc.28111; PMID: 24553114
- Costello LC, Fenselau CC, Franklin RB. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J Inorg Biochem 2011; 105:589 - 99; http://dx.doi.org/10.1016/j.jinorgbio.2011.02.002; PMID: 21440525
- Jansen RJ, Robinson DP, Frank RD, Anderson KE, Bamlet WR, Oberg AL, Rabe KG, Olson JE, Sinha R, Petersen GM, et al. Fatty acids found in dairy, protein and unsaturated fatty acids are associated with risk of pancreatic cancer in a case-control study. Int J Cancer 2014; 134:1935 - 46; http://dx.doi.org/10.1002/ijc.28525; PMID: 24590454
- Farzin L, Moassesi ME, Sajadi F, Faghih MAA. Evaluation of trace elements in pancreatic cancer patients in Iran. Middle East J Cancer 2013; 4:79 - 86
- Fabris C, Farini R, Del Favero G, Gurrieri G, Piccoli A, Sturniolo GC, Panucci A, Naccarato R. Copper, zinc and copper/zinc ratio in chronic pancreatitis and pancreatic cancer. Clin Biochem 1985; 18:373 - 5; http://dx.doi.org/10.1016/S0009-9120(85)80078-3; PMID: 4092355
- Thiagalingam A, De Bustros A, Borges M, Jasti R, Compton D, Diamond L, Mabry M, Ball DW, Baylin SB, Nelkin BD. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol Cell Biol 1996; 16:5335 - 45; PMID: 8816445
- Liu H, Hew HC, Lu ZG, Yamaguchi T, Miki Y, Yoshida K. DNA damage signalling recruits RREB-1 to the p53 tumour suppressor promoter. Biochem J 2009; 422:543 - 51; http://dx.doi.org/10.1042/BJ20090342; PMID: 19558368
- Jiang W, Sequeira JM, Nakayama Y, Lai SC, Quadros EV. Characterization of the promoter region of TCblR/CD320 gene, the receptor for cellular uptake of transcobalamin-bound cobalamin. Gene 2010; 466:49 - 55; http://dx.doi.org/10.1016/j.gene.2010.07.004; PMID: 20627121
- Mukhopadhyay NK, Cinar B, Mukhopadhyay L, Lutchman M, Ferdinand AS, Kim J, Chung LW, Adam RM, Ray SK, Leiter AB, et al. The zinc finger protein ras-responsive element binding protein-1 is a coregulator of the androgen receptor: implications for the role of the Ras pathway in enhancing androgenic signaling in prostate cancer. Mol Endocrinol 2007; 21:2056 - 70; http://dx.doi.org/10.1210/me.2006-0503; PMID: 17550981
- Flajollet S, Poras I, Carosella ED, Moreau P. RREB-1 is a transcriptional repressor of HLA-G. J Immunol 2009; 183:6948 - 59; http://dx.doi.org/10.4049/jimmunol.0902053; PMID: 19890057
- Kent OA, Fox-Talbot K, Halushka MK. RREB1 repressed miR-143/145 modulates KRAS signaling through downregulation of multiple targets. Oncogene 2013; 32:2576 - 85; http://dx.doi.org/10.1038/onc.2012.266; PMID: 22751122
- Milon BC, Agyapong A, Bautista R, Costello LC, Franklin RB. Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate 2010; 70:288 - 96; PMID: 19802870
- Oxford G, Smith SC, Hampton G, Theodorescu D. Expression profiling of Ral-depleted bladder cancer cells identifies RREB-1 as a novel transcriptional Ral effector. Oncogene 2007; 26:7143 - 52; http://dx.doi.org/10.1038/sj.onc.1210521; PMID: 17496927
- Scarlett CJ, Salisbury EL, Biankin AV, Kench J. Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathology 2011; 43:183 - 200; http://dx.doi.org/10.1097/PAT.0b013e3283445e3a; PMID: 21436628
- Morris JP 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 2010; 10:683 - 95; http://dx.doi.org/10.1038/nrc2899; PMID: 20814421
- Murtaugh LC. Pathogenesis of pancreatic cancer: lessons from animal models. Toxicol Pathol 2014; 42:217 - 28; http://dx.doi.org/10.1177/0192623313508250; PMID: 24178582
- De La O JP, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A 2008; 105:18907 - 12; http://dx.doi.org/10.1073/pnas.0810111105; PMID: 19028876
- Rooman I, Real FX. Pancreatic ductal adenocarcinoma and acinar cells: a matter of differentiation and development?. Gut 2012; 61:449 - 58; http://dx.doi.org/10.1136/gut.2010.235804; PMID: 21730103
- Shi C, Hong SM, Lim P, Kamiyama H, Khan M, Anders RA, Goggins M, Hruban RH, Eshleman JR. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol Cancer Res 2009; 7:230 - 6; http://dx.doi.org/10.1158/1541-7786.MCR-08-0206; PMID: 19208745
- Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol 2007; 171:263 - 73; http://dx.doi.org/10.2353/ajpath.2007.061176; PMID: 17591971
- Ray SK, Nishitani J, Petry MW, Fessing MY, Leiter AB. Novel transcriptional potentiation of BETA2/NeuroD on the secretin gene promoter by the DNA-binding protein Finb/RREB-1. Mol Cell Biol 2003; 23:259 - 71; http://dx.doi.org/10.1128/MCB.23.1.259-271.2003; PMID: 12482979
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248 - 54; http://dx.doi.org/10.1016/0003-2697(76)90527-3; PMID: 942051
- Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer 2007; 6:37; http://dx.doi.org/10.1186/1476-4598-6-37; PMID: 17550612
