Abstract
By orchestrating the sequential degradation of a large number of cell cycle regulators, the ubiquitin ligase anaphase-promoting complex (APC/C) is essential for proliferation in all eukaryotes. The correct timing of APC/C-dependent substrate degradation, a critical feature of progression through mitosis, was long known to be controlled by mechanisms targeting the core APC/C-machinery. Recent experiments, however, have revealed an important contribution of substrate-specific regulation of the APC/C to achieve accurate cell division. In this perspective, we describe different mechanisms of substrate-specific APC/C-regulation and discuss their importance for cell division.
Introduction
By determining the stability of cell cycle regulators, ubiquitination plays a crucial role in controlling cell division.Citation1–Citation3 The aberrant activation of ubiquitination enzymes can lead to unwanted cell division or survival, which often results in cancer. Dissecting regulatory mechanisms that control ubiquitination enzymes is, therefore of paramount importance to understanding tumorigenesis and to providing novel therapeutic strategies against this devastating disease.
The modification of proteins with ubiquitin requires a cascade of three distinct enzymes, referred to as E1 (ubiquitin-activating enzyme),Citation4 E2 (ubiquitin-conjugating enzyme)Citation5 and E3 (ubiquitin ligase).Citation6 Based on bioinformatic studies, human cells have between 600 and 1,000 different E3s.Citation6 Many of these E3s contain a characteristic RING-domain to recruit an E2 charged with ubiquitin, in addition to a wide variety of domains used to interact with specific substrates. In the cullin-family of RING-E3s (CRLs), a large cullin subunit provides a scaffold to bind both a RING-domain subunit and a substrate-recruitment factor. Several of these CRLs, including the Skp1-Cullin-F-box complex (SCF) and the anaphase-promoting complex (APC/C), have long been recognized as essential E3s required for cell cycle control.Citation1,Citation3
Although the SCF and APC/C both target cell cycle regulators for degradation, they differ in important aspects. For example, by recruiting the E2 Ube2R (yeast Cdc34), the SCF assembles K48-linked ubiquitin chains, the canonical proteasomal targeting signal. By contrast, the human APC/C utilizes an initiating E2, Ube2C (UbcH10) and an elongating E2, Ube2S, to catalyze the formation of K11-linked ubiquitin chains.Citation7–Citation10 The K11-linked ubiquitin chains also trigger the degradation of their substrates by the 26S proteasome.Citation7,Citation11 The reasons for why the APC/C assembles K11-linked ubiquitin chains to promote protein degradation are not known.
In addition to these differences in catalysis, the SCF and APC/C are controlled by distinct regulatory mechanisms. The SCF is active throughout all stages of the cell cycle, but usually recognizes its substrates only after these have been tagged for degradation through phosphorylation or related posttranslational modifications.Citation12 Thus, most of SCF-regulation is thought to occur on the level of substrates. By contrast, the activity of the APC/C is restricted to mitosis and G1 by phosphorylation of APC/C-subunits,Citation13 binding of several distinct APC/C-inhibitorsCitation14 or degradation of APC/C-specific E2s.Citation8,Citation15 The full activation of human APC/C during mitosis leads to a massive upregulation of K11-linked chain formation, resulting in the sequential degradation of its many substrates.Citation11,Citation16 Much of this substrate-ordering can be recapitulated in purified systems, indicating that substrates do not have to be modified for APC/C-recognition.Citation17 These observations have led to the suggestion that most of APC/C-regulation occurs on the level of the E3, and not the substrate.
Recent studies, however have revealed that in addition to the intrinsic regulation of the core APC/C machinery, substrate-specific control mechanisms are also critical for accurate cell cycle progression. Here, we describe how substrate-specific APC/C-regulation can be accomplished and discuss potential advantages of this type of APC/C-regulation for cell cycle control.
Substrate-Specific APC/C-Regulation through Post-Translational Modifications
The APC/C recruits its substrates through the adaptor proteins Cdc20 and Cdh1, which directly bind D-box and/or KEN-box sequences ().Citation14,Citation18–Citation20 Following substrate binding, the E2 Ube2C or a core subunit of the APC/C recognizes another substrate motif, referred to as TEK-box, to rapidly promote ubiquitin chain initiation ().Citation7 Additional APC/C-interaction motifs with less understood binding partners or functions have also been described ().Citation21–Citation23 The modification of residues within or in proximity to such substrate motifs has been found to allow for substrate-specific APC/C-regulation.
As the APC/C needs to recognize D-boxes for substrate-binding, phosphorylation of residues near these motifs provides an intuitive means of stabilization. Indeed, such a mechanism was reported to prevent the degradation of the APC/C-substrate Cdc6 in cells that have entered the cell cycle after quiescence ().Citation24 Cdc6 is required for the assembly of pre-replication complexes during G1, although the APC/C is still active at this time of the cell cycle.Citation24–Citation27 To counteract the APC/C, the cyclin E-Cdk2 kinase phosphorylates serine residues in the vicinity of the D-box of Cdc6, which interferes with binding of Cdc6 to the substrate recruitment factor of the APC/C, Cdh1, and disrupts the APC/C-dependent degradation of Cdc6.Citation28 A similar mechanism was shown to ensure the proper timing of the APC/CCdc20-dependent degradation of the yeast anaphase inhibitor securin.Citation29 Moreover, recognition of the ribonucleotide reductase subunit RRM2 by APC/CCdh1 is inhibited through phosphorylation near a KEN-box motif, showing that such regulation is not restricted to D-boxes (Michele Pagano, pers. communication). The phosphorylation of residues in proximity to APC/C-recognition motifs can, therefore, selectively inhibit the degradation of specific APC/C-substrates by blocking their recognition through Cdc20 or Cdh1.
A slightly different mechanism regulates the levels of Aurora A kinase, a critical regulator of mitosis. The APC/C-dependent degradation of Aurora A was found to be inhibited by phosphorylation of a specific serine residue within an N-terminal sequence motif referred to as the “A-box”.Citation21 This serine residue is phosphorylated during early stages of mitosis, most likely by cyclin B1/Cdk1, in both Xenopus laevis and human cells.Citation30 Consistent with these observations, Aurora A needs to be dephosphorylated by the protein phosphatase PP2A in order to be efficiently degraded during mitotic exit.Citation31 The function of the A-box in APC/C-dependent reactions is not fully understood, and it has remained unclear how exactly serine phosphorylation leads to stabilization of Aurora A. As epitope-tagging of Aurora A at its N-terminus interferes with its proper degradation,Citation30 the A-box might serve to correctly orient Aurora A on the APC/C, and phosphorylation might interfere with this event. Nevertheless, these results suggest that phosphorylation of substrate residues might also interfere with APC/C-dependent ubiquitination at steps other than substrate binding.
Although most is known about phosphorylation-dependent substrate stabilization, it is possible that other posttranslational modifications serve similar roles in regulating the proteolysis of specific APC/C-substrates. Indeed, it has been suggested that acetylation of cyclin A or BubR1 is able to modulate their stability during mitosis, potentially by interfering with APC/C-dependent ubiquitination.Citation32,Citation33 In addition, farnesylation has recently been reported to regulate the APC/C-dependent degradation of CENP-F and CENP-E.Citation34 Farnesylation also promotes the targeting of these proteins to the kinetochore, suggesting that their APC/C-dependent ubiquitination and intracellular localization are tightly connected to each other.Citation35 Together, these observations indicate that posttranslational modifications provide powerful and versatile means of regulating the ubiquitination of specific substrates by the APC/C.
Substrate-Specific APC/C-Inhibition through Reversible Protein Interactions
Similar to phosphorylation, reversible protein interactions are able to inhibit the APC/C-dependent degradation of specific substrates, as recently observed for the turnover of the spindle assembly factors (SAFs) HURP and NuSAP.Citation36 HURP and NuSAP promote spindle formation by bundling microtubules, thereby stabilizing kinetochore fibers that connect the mitotic chromosomes to the spindle.Citation37,Citation38 Both SAFs are inhibited by binding to nuclear transport receptors of the importin-family, with the precise mechanism of this inhibition still being poorly understood. It is known, however, that the interaction between SAFs and importins can be disrupted by the small protein Ran, when it is bound to GTP (RanGTP).Citation39,Citation40 As the guanine-nucleotide exchange factor of Ran, Rcc1, associates with mitotic chromosomes,Citation41,Citation42 RanGTP is enriched in the proximity of chromatin, leading to the localized activation of HURP and NuSAP at these sites. The reversible binding of importins to HURP and NuSAP can, therefore, regulate their activity in chromatin-driven spindle formation.
In a beautiful example of co-regulation, Ran and importins also regulate the APC/C-dependent degradation of HURP and NuSAP.Citation36 The ubiquitination of these SAFs by the APC/C depends on specific D-, KEN- and TEK-boxes. By binding to overlapping motifs, importin-β blocks the association of HURP and NuSAP with the substrate-adaptors of the APC/C, Cdc20 and Cdh1 ().Citation36 RanGTP in turn disrupts the interaction between HURP and NuSAP with importin-β, thereby exposing the SAFs to recognition by Cdc20/Cdh1 and ubiquitination by the APC/C. The regulation of APC/C-dependent degradation by Ran and importin-β is thus conceptually similar to phosphorylation, in that importin-β reversibly interferes with substrate recognition by Cdc20 or Cdh1.
What is the relevance of this mechanism for cell cycle control? Importin-β-binding deficient mutants of HURP are prematurely degraded during spindle assembly, at the time when the SAF is normally active.Citation36 Conversely, aberrant spindles form with high frequency in cells that express higher levels of HURP or NuSAP,Citation36,Citation43,Citation44 suggesting that the levels of active SAFs have to be tightly controlled to achieve proper spindle assembly or spindle dynamics. By using importin-β as a common regulator of activity and degradation, the cell ensures that APC/C specifically targets the active pool of SAFs. This might provide spatial (i.e., degrade SAFs that were released from importin-β at an inappropriate location in the mitotic cell) or temporal (i.e., degrade SAFs that had been active for a sufficient amount of time) control of spindle formation. These two mechanisms of regulation are not mutually exclusive, as motor-proteins could remove active SAFs from spindle microtubules, thereby leading to SAF-degradation. Consistent with this hypothesis, the yeast APC/C-substrate Cik1 appears to require an interaction with the kinesin Kar3 for efficient degradation.Citation45
Substrate-Specific APC/C-Activation through Protein Interactions
While the masking of APC/C-recognition motifs through modifications or binding partners provides means of APC/C-inhibition, offering additional routes of APC/C-binding should accelerate the degradation of specific substrates. An example for this type of regulation is centered around the homologous proteins Cks1 and Cks2, which bind to complexes between cyclin-dependent kinase Cdk1 and cyclin A or cyclin B, two long known substrates of the APC/C.Citation46 Cks1/2 facilitate the degradation of the cyclins,Citation47,Citation48 but not other APC/C-substrates, demonstrating that they act as substrate-specific activators of the APC/C ().
How do Cks1/2 promote cyclin degradation on a mechanistic level? The Cks proteins were known to contain a phospho-peptide binding domain, the function of which is important for mitotic progression.Citation49 The APC/C is heavily phosphorylated during mitosis,Citation13,Citation50 and consistent with this, it was shown that Cks1/2 are able to directly bind phosphorylated APC3 during prometaphase.Citation48,Citation51,Citation52 The interaction between Cks1/2 and the APC/C occurs in parallel to the interaction between the substrate, cyclin, and the APC/C-substrate recruitment factors, Cdc20 or Cdh1.Citation48,Citation53 Such multivalent binding is predicted to increase the affinity of the substrates for the APC/C, thereby positively affecting the processivity of ubiquitin chain formation. It had been shown before that the processivity of ubiquitin chain formation is a major determinant of the timing of APC/C-substrate degradation.Citation17 Thus, binding partners that provide additional ways to APC/C-recruitment are an effective means of increasing the processivity of chain formation, thereby accelerating substrate degradation during the cell cycle.
Regulated Ubiquitination of the APC/C-Specific E2s Ube2C and Ube2S
Several activators of the APC/C, including Cdc20, Cdh1 or the E2s Ube2C and Ube2S, are also substrates of the APC/C.Citation8,Citation14,Citation15 To ensure the correctly timed degradation of canonical APC/C-substrates, it is critical to prevent the premature turnover of APC/C-activators. Especially for the E2s Ube2C and Ube2S, the mechanisms achieving this regulation are different from those restricting the binding of APC/C-substrates, thereby representing another layer of substrate-specific APC/C-control.
Ube2C acts as the physiological initiating E2 for the APC/C,Citation7,Citation11,Citation54 which adds the first ubiquitin to most, if not all APC/C-substrates. In addition to modifying substrates, Ube2C is also subject to APC/C-dependent autoubiquitination and degradation.Citation8,Citation15 The latter reaction is inhibited, when substrates are bound to the APC/C, as Ube2C then ubiquitinates the substrate, but not itself (). Given the recent finding that APC/C-activation leads to a dramatic upregulation of bulk K11-linked chain formation,Citation11 the APC/C is likely saturated with substrates throughout mitosis, which efficiently delays Ube2C-degradation and APC/C-inactivation until G1. The correct timing of Ube2C-degradation was not observed with amino-terminally tagged Ube2C,Citation55 most likely because the epitope tag interferes with known functions of the N-terminus of Ube2C in controlling charging by E1,Citation56 binding to the APC/C,Citation57 and catalyzing ubiquitin transfer to itself.Citation15 These observations, however, implied that the peculiar timing of Ube2C-degradation is linked to sequences in its N-terminus, which requires further investigation. Results from these studies should also provide insight into mechanisms underlying the competition between canonical APC/C-substrates during mitosis.Citation17
The chain elongating E2 of the APC/C, Ube2S, is also subject to APC/C-dependent degradation during G1, which requires a functional active site in Ube2S itself.Citation8 In addition to providing E2 activity towards substrates, Ube2C and Ube2S collaborate with p31comet to silence the spindle checkpoint, thus driving full APC/C-activation at metaphase.Citation8,Citation9,Citation57–Citation59
The role of the APC/C in silencing the spindle checkpoint was recently also observed upon chemical inhibition of the APC/C.Citation60 Accordingly, the tightly regulated degradation of Ube2C and Ube2S facilitates APC/C-inactivation during G1, but it may also function to protect cells from premature checkpoint silencing and inaccurate sister chromatid separation. In agreement with this hypothesis, the overexpression of Ube2C and Ube2S has been linked to the generation of aneuploidy and tumorigenesis,Citation61–Citation63 underscoring the importance of substrate-specific APC/C-regulation.
Conclusions
As an E3 required for the accurate execution of the mitotic program, the APC/C has to be tightly controlled. This is in part achieved by mechanisms that modulate the activity of the core APC/C-machinery, including the spindle checkpoint or inhibitors, such as Emi1. The APC/C is also able to discriminate between its many substrates by catalyzing their ubiquitination with distinct degrees of processivity, thereby providing a blueprint for the timing of substrate degradation. As discussed here, these intrinsic features of APC/C-regulation are overlain by substrate-specific control mechanisms, including phosphorylation or sequestration in specific protein complexes. In this manner, the APC/C can fine-tune the degradation of especially important mitotic regulators, such as cyclins or spindle assembly factors, to faithfully orchestrate cell cycle progression.
Figures and Tables
Figure 1 Inhibition of APC/C-dependent ubiquitination by posttranslational modifications. The ubiquitination of Cdc6 by the APC/C can be inhibited by phosphorylation of serine residues in proximity to an important D-box in the substrate. Cdc6 is phosphorylated by the cyclin E/Cdk2 kinase during late stages of G1. It can be assumed that a phosphatase is required for Cdc6 degradation during the next G1 phase.

Figure 2 Inhibition of APC/C-dependent ubiquitination by reversible protein interactions. The ubiquitination of the spindle assembly factor HURP by the APC/C can be inhibited by importin-β, which binds to sites in HURP that overlap with the D-, KEN- and TEK-boxes required for HURP-degradation. GTP-charged Ran can dissociate importin-β from HURP, thereby exposing it to recognition by Cdc20/Cdh1 and ubiquitination by the APC/C.
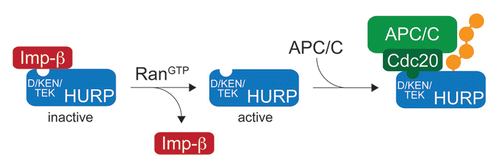
Figure 3 Stimulation of APC/C-dependent ubiquitination by activator-independent APC/C-binding motifs. The ubiquitination of cyclin B1 is stimulated by Cks1, a component of the cyclin B1/Cdk1 complex. Cks1 contains a phospho-peptide binding motif, which interacts with phosphorylated APC3 during mitosis. The multivalency resulting from the simultaneous recognition of the D-box in cyclin B1 and Cks1 by phosphorylated APC/C accelerates cyclin B1 ubiquitination and degradation during mitosis.

Figure 4 Competitive inhibition of APC/C-dependent ubiquitination. Ube2C is the physiological chain-initiating E2 for the APC/C. When substrates are bound to the APC/C, Ube2C transfers ubiquitin to substrates lysine residues. By contrast, after substrates have been degradation, Ube2C modifies lysine residues within itself, leading to Ube2C-degradation.
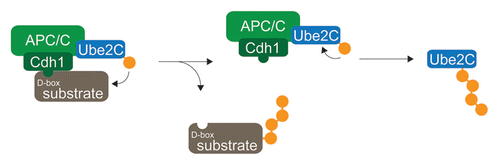
Table 1 Substrate motifs involved in APC/C-dependent ubiquitination
Acknowledgements
We thank Julia Schaletzky for discussions and for critically reading the manuscript. We are also grateful to all members of our lab for many discussions and ideas. Work in the lab is funded by March of Dimes, the NIGMS, an NIH Director's New Innovator Award, and the Pew Foundation.
References
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 2006; 7:644 - 656
- Wickliffe K, Williamson A, Jin L, Rape M. The multiple layers of ubiquitin-dependent cell cycle control. Chem Rev 2009; 109:1537 - 1548
- Skaar JR, Pagano M. Control of cell growth by the SCF and APC/C ubiquitin ligases. Curr Opin Cell Biol 2009; 21:816 - 824
- Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol 2009; 10:319 - 331
- Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol 2009; 10:755 - 764
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem 2009; 78:399 - 434
- Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell 2008; 133:653 - 665
- Williamson A, Wickliffe KE, Mellone BG, Song L, Karpen GH, et al. Identification of a physiological E2 module for the human anaphase-promoting complex. Proc Natl Acad Sci USA 2009; 106:18213 - 18218
- Garnett MJ, Mansfeld J, Godwin C, Matsusaka T, Wu J, Russell P, et al. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat Cell Biol 2009; 11:1363 - 1369
- Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A, Kirschner MW. UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci USA 107:1355 - 1360
- Matsumoto ML, Wickliffe KE, Dong KC, Yu C, Bosanac I, Bustos D, et al. K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol Cell 39:477 - 484
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 2005; 6:9 - 20
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, et al. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J 2003; 22:6598 - 6609
- Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol 2008; 24:475 - 499
- Rape M, Kirschner MW. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 2004; 432:588 - 595
- Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol 2007; 8:894 - 903
- Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 2006; 124:89 - 103
- da Fonseca PC, Kong EH, Zhang Z, Schreiber A, Williams MA, Morris EP, et al. Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature 1991; 349:132 - 138
- Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev 2000; 14:655 - 665
- Littlepage LE, Ruderman JV. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev 2002; 16:2274 - 2285
- Castro A, Vigneron S, Bernis C, Labbe JC, Lorca T. Xkid is degraded in a D-box, KEN-box and A-box-independent pathway. Mol Cell Biol 2003; 23:4126 - 4138
- Araki M, Yu H, Asano M. A novel motif governs APC-dependent degradation of Drosophila ORC1 in vivo. Genes Dev 2005; 19:2458 - 2465
- Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem 2002; 71:333 - 374
- Blow JJ, Dutta A. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 2005; 6:476 - 486
- Petersen BO, Wagener C, Marinoni F, Kramer ER, Melixetian M, Lazzerini Denchi E, et al. Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev 2000; 14:2330 - 2343
- McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 1998; 93:1043 - 1053
- Mailand N, Diffley JF. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell 2005; 122:915 - 926
- Holt LJ, Krutchinsky AN, Morgan DO. Positive feedback sharpens the anaphase switch. Nature 2008; 454:353 - 357
- Crane R, Kloepfer A, Ruderman JV. Requirements for the destruction of human Aurora-A. J Cell Sci 2004; 117:5975 - 5983
- Horn V, Thelu J, Garcia A, Albiges-Rizo C, Block MR, Viallet J. Functional interaction of Aurora-A and PP2A during mitosis. Mol Biol Cell 2007; 18:1233 - 1241
- Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J 2009; 28:2077 - 2089
- Mateo F, Vidal-Laliena M, Canela N, Busino L, Martinez-Balbas MA, Pagano M, et al. Degradation of cyclin A is regulated by acetylation. Oncogene 2009; 28:2654 - 2666
- Gurden MD, Holland AJ, van Zon W, Tighe A, Vergnolle MA, Andres DA, et al. Cdc20 is required for the post-anaphase, KEN-dependent degradation of centromere protein F. J Cell Sci 123:321 - 330
- Schafer-Hales K, Iaconelli J, Snyder JP, Prussia A, Nettles JH, El-Naggar A, et al. Farnesyl transferase inhibitors impair chromosomal maintenance in cell lines and human tumors by compromising CENP-E and CENP-F function. Mol Cancer Ther 2007; 6:1317 - 1328
- Song L, Rape M. Regulated degradation of spindle assembly factors by the anaphase-promoting complex. Mol Cell 38:369 - 382
- Ribbeck K, Raemaekers T, Carmeliet G, Mattaj IW. A role for NuSAP in linking microtubules to mitotic chromosomes. Curr Biol 2007; 17:230 - 236
- Sillje HH, Nagel S, Korner R, Nigg EA. HURP is a Ran-importin beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr Biol 2006; 16:731 - 742
- Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell 2004; 16:319 - 330
- Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol 2008; 9:464 - 477
- Hutchins JR, Moore WJ, Hood FE, Wilson JS, Andrews PD, Swedlow JR, et al. Phosphorylation regulates the dynamic interaction of RCC1 with chromosomes during mitosis. Curr Biol 2004; 14:1099 - 1104
- Li HY, Wirtz D, Zheng Y. A mechanism of coupling RCC1 mobility to RanGTP production on the chromatin in vivo. J Cell Biol 2003; 160:635 - 644
- Wong J, Lerrigo R, Jang CY, Fang G. Aurora A regulates the activity of HURP by controlling the accessibility of its microtubule-binding domain. Mol Biol Cell 2008; 19:2083 - 2091
- Raemaekers T, Ribbeck K, Beaudouin J, Annaert W, Van Camp M, Stockmans I, et al. NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization. J Cell Biol 2003; 162:1017 - 1029
- Benanti JA, Matyskiela ME, Morgan DO, Toczyski DP. Functionally distinct isoforms of Cik1 are differentially regulated by APC/C-mediated proteolysis. Mol Cell 2009; 33:581 - 590
- Pines J. Cell cycle: reaching for a role for the Cks proteins. Curr Biol 1996; 6:1399 - 1402
- Wolthuis R, Clay-Farrace L, van Zon W, Yekezare M, Koop L, Ogink J, et al. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol Cell 2008; 30:290 - 302
- van Zon W, Ogink J, ter Riet B, Medema RH, te Riele H, Wolthuis RM. The APC/C recruits cyclin B1-Cdk1-Cks in prometaphase before D box recognition to control mitotic exit. J Cell Biol 190:587 - 602
- Bourne Y, Watson MH, Hickey MJ, Holmes W, Rocque W, Reed SI, et al. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell 1996; 84:863 - 874
- Steen JA, Steen H, Georgi A, Parker K, Springer M, Kirchner M, et al. Different phosphorylation states of the anaphase promoting complex in response to antimitotic drugs: a quantitative proteomic analysis. Proc Natl Acad Sci USA 2008; 105:6069 - 6074
- Di Fiore B, Pines J. How cyclin A destruction escapes the spindle assembly checkpoint. J Cell Biol 190:501 - 509
- Sudakin V, Shteinberg M, Ganoth D, Hershko J, Hershko A. Binding of activated cyclosome to p13(suc1). Use for affinity purification. J Biol Chem 1997; 272:18051 - 18059
- Fry AM, Yamano H. APC/C-mediated degradation in early mitosis: how to avoid spindle assembly checkpoint inhibition. Cell Cycle 2006; 5:1487 - 1491
- Yu H, King RW, Peters JM, Kirschner MW. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr Biol 1996; 6:455 - 466
- Walker A, Acquaviva C, Matsusaka T, Koop L, Pines J. UbcH10 has a rate-limiting role in G1 phase but might not act in the spindle checkpoint or as part of an autonomous oscillator. J Cell Sci 2008; 121:2319 - 2326
- Huang DT, Zhuang M, Ayrault O, Schulman BA. Identification of conjugation specificity determinants unmasks vestigial preference for ubiquitin within the NEDD8 E2. Nat Struct Mol Biol 2008; 15:280 - 287
- Summers MK, Pan B, Mukhyala K, Jackson PK. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol Cell 2008; 31:544 - 556
- Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature 2007; 446:921 - 925
- Miniowitz-Shemtov S, Teichner A, Sitry-Shevah D, Hershko A. ATP is required for the release of the anaphase-promoting complex/cyclosome from inhibition by the mitotic checkpoint. Proc Natl Acad Sci USA 107:5351 - 5356
- Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh DC, et al. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell 18:382 - 395
- van Ree JH, Jeganathan KB, Malureanu L, van Deursen JM. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J Cell Biol 188:83 - 100
- Jung CR, Hwang KS, Yoo J, Cho WK, Kim JM, Kim WH, Im DS. E2-EPF UCP targets pVHL for degradation and associates with tumor growth and metastasis. Nat Med 2006; 12:809 - 816
- Wagner KW, Sapinoso LM, El-Rifai W, Frierson HF, Butz N, Mestan J, et al. Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene 2004; 23:6621 - 6629