Abstract
The tumor suppressor p53 is extensively regulated by post-translational modification, including modification by the small ubiquitin-related modifier SUMO. We show here that MDM2, previously shown to promote ubiquitin, Nedd8 and SUMO-1 modification of p53, can also enhance conjugation of endogenous SUMO-2/3 to p53. Sumoylation activity requires p53-MDM2 binding but does not depend on an intact RING finger. Both ARF and L11 can promote SUMO-2/3 conjugation of p53. However, unlike the previously described SUMO-1 conjugation of p53 by an MDM2-ARF complex, this activity does not depend on the ability of MDM2 to relocalize to the nucleolus. Interestingly, the SUMO consensus is not conserved in mouse p53, which is therefore not modified by SUMO-2/3. Finally, we show that conjugation of SUMO-2/3 to p53 correlates with a reduction of both activation and repression of a subset of p53-target genes.
Introduction
The p53 protein plays an important role in the cell's response to stressors such as DNA damage or oncogene activation. Activation of p53 can both contribute to the repair and survival of stressed cells, or eliminate and arrest damaged cells through the induction of programs such as apoptosis and senescence.Citation1 Under normal conditions, p53 prevents the accumulation of malignant cells, demonstrated by the development of spontaneous sarcomas and lymphomas in p53-knockout miceCitation2 and so functions as a tumor suppressor. Tumor cells have therefore escaped the control by p53 in some way, either by disruption upstream and downstream of p53, or by mutating p53 itself.Citation3
p53 is a transcription factor that regulates the expression of many genes.Citation1 In a simplified model, transient or mild stress predominantly leads to activation of cell cycle arrest via activation of p53-target genes like the cyclin-dependent kinase inhibitor p21,Citation4 whereas sustained or severe stress leads to apoptosis through the induction of p53-target genes such as the BH3-domain proteins BaxCitation5 and PUMA.Citation6 p53 can also repress the expression of a number of genes, including anti-apoptotic and cell cycle genes that contribute to p53-mediated apoptosisCitation7 and cell cycle arrest.Citation8 There are several proposed mechanisms of transcriptional repression, ranging from interaction with other transcription factors and cofactors, recruitment of chromatin remodeling enzymes, such as histone deacetylases, and indirect repression via p53-target genes (reviewed in ref. Citation9). Among the p53-repressed cell cycle genes are G2/M phase-regulating genes, cyclin-dependent kinase 1 (Cdk1),Citation10 cyclin B2Citation11 and cyclin A2.Citation12
To keep p53 tightly in check in normal cells, p53-protein levels are kept low by the ubiquitin-E3-ligase MDM2, which constantly degrades newly synthesized p53 protein.Citation13,Citation14 The importance of this control is demonstrated by the MDM2-knockout mouse, which is embryonic lethal due to an overactive p53, leading to widespread apoptosis.Citation15,Citation16 Activation of p53 in response to stress is accompanied by a rapid stabilization of the p53 protein, reflecting an inhibition of MDM2. Various post-translational modifications of both p53 and MDM2 have been shown to regulate their interaction and the ability of MDM2 to target p53 for degradation.Citation17–Citation19 The activity of MDM2 is further controlled by a number of ribosomal proteins, such as L11,Citation20 which inhibit MDM2-mediated ubiquitination in response to nucleolar stress and the alternate reading frame (ARF) protein, which binds to a similar region of MDM2 in response to oncogene activation.Citation21
However, p53 regulation by MDM2 is more complex than simply polyubiquitination and degradation. Low levels of MDM2 can also catalyze monoubiquitination, which leads to nuclear export of p53.Citation22,Citation23 The interaction of MDM2 with p53 can mask the DNA-binding domain in p53, silencing its transcriptional activities.Citation24 Furthermore, MDM2 has been shown to promote modification of p53 with the ubiquitin-like protein NEDD8,Citation25 resulting in the modulation of transcriptional activity and nuclear export.Citation26 MDM2 has also been shown to promote the SUMO-1 modification of p53, an activity enhanced by ARF and related to the relocalization of p53 to the nucleolus.Citation27 Interestingly, ARF has also been shown to induce sumoylation of the p53 family member p63.Citation28
There are four SUMO family members; by far the best studied being SUMO-1, which is about 50% homologous to the almost identical SUMO-2 and 3. While SUMO-1, -2 and -3 have a broad tissue distribution, the expression of SUMO-4, which is also similar in structure to SUMO-2/3, is limited to certain organ typesCitation29 and contains a proline residue close to the diglycine motif, which may prevent protease cleavage into the mature protein.Citation30 Interestingly, although SUMO-1 and SUMO-2/3 are conjugated via the same set of enzymes, they can preferentially target specific proteins and show different dynamics and distribution in the cell.Citation31 SUMO-2/3 is more abundant in a large free pool than SUMO-1 and is also much more dynamically conjugated and removed,Citation32 consistent with a role for SUMO-2/3, in stress responses.Citation33 It is also becoming apparent that the modification of proteins with SUMO-2/3 may have different consequences than modification with SUMO-1.Citation34
Most studies on p53 sumoylation have so far focused on SUMO-1, which has been shown to modify a single lysine (K386) residue residing within a SUMO consensus motif in the p53's C terminus. Consequences of SUMO-1 modification of p53 activity are still a matter for debateCitation35 (the early p53-SUMO results are reviewed in ref. Citation36). Various publications have shown that SUMO-1 modification of p53 results in increased,Citation37,Citation38 decreasedCitation39 or unchanged transcriptional activity.Citation40 A more recent study has shown that SUMO-1 modification of p53 plays a role in the inhibition of C-terminal acetylation, thereby inhibiting DNA binding and transcriptional activity.Citation41 Further studies suggest that acetylation of SUMO-1 that is conjugated to p53 can also modulate the transcriptional activity of p53.Citation42 In addition to MDM2, a number of SUMO-E3-ligases that mediate the SUMO-1 conjugation of p53 have been identified, including Topors,Citation43 the PIAS proteins,Citation44 TRIM proteinsCitation45 an adenovirus E1B 55-Kilodalton protein.Citation46
By contrast, relatively few studies have addressed SUMO-2/3 modification of p53. SUMO-2/3 conjugation on lysine 386 was shown to be induced by H2O2 treatment of cells and was associated with an increase in transcriptional activity.Citation47 The viral protein K-bZIP from Kaposi's sarcoma-associated herpes virus that shows specificity toward SUMO-2/3 has also been reported to target p53,Citation48 resulting in the enhancement of p53-dependent transcriptional activity. We therefore set out to study the influence of MDM2 on the SUMO-2/3 conjugation on p53. We show that MDM2 can promote conjugation of SUMO-2/3 to p53, and that this modification of p53 modulates both the activation and repression of a number of p53-target genes.
Results
Mdm2 induces SUMO-3 conjugation of p53.
Since MDM2 can induce ubiquitination, neddylation and conjugation of SUMO-1, we tested its ability to promote conjugation of SUMO-2/3 to p53. Overexpression of p53, MDM2 and SUMO-3 induced sumoylation of p53 with SUMO-3 in U2OS cells (), HCT116 cells () and H1299 cells (). MDM2 can form a heterodimer with MDMX, a protein of the MDM2 family with a very similar p53-binding domain to MDM2.Citation49 Not only is this heterodimer the most predominant form of MDM2 in the cell,Citation50 but it is also a more efficient ubiquitin-E3-ligase than the MDM2 homodimer.Citation51 However, we were unable to show any effect of MDMX overexpression on p53 sumoylation, either alone ( and C) or together with MDM2 ().
Both p53 and MDM2 consist of clearly structured functional domains (). Interaction of MDM2 and p53 via their N-terminal domains is critical for efficient ubiquitination.Citation14 We therefore examined the role of p53-MDM2 binding on SUMO-3 modification of p53. An MDM2 protein (Δ58–89 MDM2), which does not contain the N-terminal p53-binding domain, cannot induce sumoylation of p53 (). Similarly wild-type MDM2 cannot enhance sumoylation of a p53 protein (ΔI) lacking the MDM2-binding domain (). These observations indicate that direct interaction of MDM2 and p53 via their N-terminal binding regions has to take place in order for MDM2 to efficiently promote SUMO-3 conjugation onto p53. This is, therefore, a similar requirement to other ubiquitin-like protein modifications of p53 by MDM2.Citation25,Citation2
The sumoylating and ubiquitinating activities of MDM2 are distinct features.
Although the RING domain of MDM2 on its own can show ubiquitin ligase activity and can function in autoubiquitination assays,Citation52 both the N-terminal p53-binding domainCitation53 and the central acidic domain with adjacent Zinc fingerCitation54,Citation55 are also needed for MDM2's ability to ubiquitinate p53. As expected, MDM2 mutants deleted of the RING domain (ΔRING) or harboring mutation of a structurally critical cysteine residue within the RING (464A) both lost the ability to ubiquitinate p53 (). Similarly, deletion of the central part of MDM2 (ΔAD) strongly reduced the ability of MDM2 to drive ubiquitination of p53 (). However, in parallel assays, we found that deletion or mutation of the RING domain did not impede the SUMO-3 modification of p53 by MDM2, suggesting that the ability to promote sumoylation is distinct from the ubiquitin ligase activity (). Deletion of the acidic domain of MDM2 also did not prevent sumoylation of p53, consistent with previous work showing that this region is not necessary for SUMO-1 modification of p53 by MDM2.Citation27 Most previous studies of SUMO-1 modification of p53 by MDM2 have utilized ectopic expression of tagged SUMO as in our experiments so far. However, we were able to show that overexpression of MDM2 was also able to promote conjugation of endogenous SUMO-2/3 to p53 (). Using the MDM2 mutants, we observed even stronger endogenous SUMO-2/3 conjugation on p53 with ΔAD MDM2 and the RING domain point mutant of MDM2 mutants (464A), with a modest activity of the ΔRING MDM2. An MDM2 lacking the N-terminal p53-binding pocket (Δ58–89 MDM2) was once again unable to drive p53 sumoylation (). We also tested the effect of MDM2 on the SUMO-2/3 modification of endogenous p53 and once again detected an efficient activity with ΔAD MDM2 and 464A MDM2 (which was expressed at lower levels in this experiment) (). No clear activity of wild-type MDM2 was detected here, although this is likely to reflect the confounding effect of the ubiquitination and degradation of p53 by this protein. In order to further assess the activity of endogenous wild-type MDM2, we turned to small-molecule inhibitors of MDM2-mediated ubiquitination of p53. Treatment of cells with either Nutlin (which inhibits the N-terminal MDM2/p53 interactionCitation56) or HLI373 (which directly inhibits MDM2's ubiquitin ligase activityCitation57) stabilized MDM2 as previously shown (). While Nutlin treatment completely abolished p53 sumoylation, confirming the requirement of endogenous MDM2 to interact with p53 for efficient p53 sumoylation, HLI373 treatment did not affect the sumoylating activity of endogenous MDM2 (). These data show that endogenous MDM2 can drive the sumoylation of p53 when the ubiquitin ligase activity is inhibited.
Sumoylation of target proteins works via a similar three-step enzyme cascade to ubiquitination. In contrast to the plethora of ubiquitin-E2s described, the only SUMO-E2 reported so far is Ubc9.Citation58 We therefore tested the interaction of MDM2 with the Ubc9. Immunoprecipitation of Ubc9 in HCT116 cells showed a weak interaction between Ubc9 and MDM2 464A () and a much stronger interaction with ΔAD MDM2. This interaction with Ubc9 could explain how these MDM2 mutants promote sumoylation of p53. Since we reproducibly found that the ΔAD MDM2 mutant most efficiently promoted SUMO-2/3 conjugation of p53 without inducing ubiquitination, we used this mutant in in subsequent experiments to study p53 under maximal sumoylation conditions.
The SUMO consensus is important for SUMO-2/3 conjugation to p53.
Unlike ubiquitination, sumoylation is, in most cases, restricted to lysine residues in the environment of a SUMO motif ΨKxE (). The SUMO consensus motif consists of a hydrophobic amino acid (Ψ), a lysine residue (K), any amino acid (X) and a glutamic acid with a C-terminal glycine of SUMO forming an isopeptide bond with the ε-Amino group of the lysine residing in the consensus.Citation59 Previous studies showed that SUMO-2/3 is conjugated to p53 specifically on lysine 386 within this motif and we were able to confirm that a K386R mutant p53 cannot be sumoylated (). We find that mutation of the glutamic acid residue 388 in p53's SUMO consensus motif also abrogates sumoylation of p53 (), although this mutation would spare K386 for other modifications, such as ubiquitination or acetylation. Apart from the integrity of the SUMO consensus sequence, neither overall p53-conformation nor its DNA binding capability are required for p53 sumoylation, since cancer-derived p53 mutants that are impaired for DNA-binding (R273H) or overall conformation (R175H) retain the ability to be sumoylated ().
Surprisingly, alignment of the p53 C termini across different vertebrate species revealed that, although the SUMO-accepting lysine residue is conserved in mice, the required glutamic acid two residues downstream is not conserved (). Consistent with this observation, we show that human MDM2 does not modify mouse p53 by SUMO-2/3 () despite the fact that mouse p53 can interact with human MDM2 ().
In contrast to SUMO-1, SUMO-2/3 contain a SUMO consensus motif around lysine 11 () and can form chains.Citation60 Poly-sumoylation has functionally distinct consequences to mono-sumoylation,Citation61 for example, in the recruitment of SUMO-interacting proteins. When blotting for sumoylated p53, we consistently observed a ladder of three bands in about 20 kD intervals, which would be consistent with chains of up to three SUMO molecules. We therefore substituted the transfected HA-SUMO-3 in the SUMO assay for the K11R mutant HA-SUMO-3, in which the chain-accepting lysine 11 is substituted for an arginine residue (). Surprisingly, the band-pattern of immunoprecipitated p53 blotted with the HA-specific antibody did not change whether or not SUMO-3 contained K11, suggesting that the upper bands represent a combination of different modifications, possibly SUMO and ubiquitin on one p53 molecule, rather than a SUMO chain.
MDM2 sumoylates p53 in complex with ARF and L11 independent of relocalization to the nucleolus.
The interaction of MDM2 with ARF leads to the inhibition of ubiquitination of p53 but has been shown to promote the SUMO-1 modification.Citation27 We observed a similar result for endogenous SUMO-2/3 conjugation to p53, which was enhanced by addition of ARF both under endogenous and overexpressed MDM2 levels (). However, ARF has also been shown to inhibit desumoylation of proteins by increasing the turnover of the sentrin-specific protease SENP3Citation62 and to associate with the SUMO-E2 Ubc9, promoting sumoylation of Ubc9-targets.Citation63 These observations make it difficult to discriminate between specific p53-related and global sumoylation effects of ARF. We therefore examined the activity of the ribosomal protein L11, which functions like ARF in the ability to bind to the acidic domain of MDM2 and inhibit its ubiquitination activity,Citation20 but has not yet been linked to any sumoylating or desumoylating activity. Interestingly, the addition of L11 also strongly induced the sumoylation of p53 ().
Previous studies have suggested that the ability of MDM2 and ARF to drive SUMO-1 modification of p53 may be related to nucleolar relocalization of p53.Citation27 A cryptic nucleolar localization signal between amino acids 466 and 473 in MDM2, which is exposed upon ARF binding, has been previously characterized.Citation64 We therefore introduced a mutation of the nucleolar localization signal (NoLS), which has previously been shown to abolish nucleolar relocalization of MDM2,Citation64 into the strongly sumoylating ΔAD MDM2. However, mutation of the NoLS did not affect the ability of ΔAD MDM2 to promote SUMO-2/3 conjugation of p53. This is consistent with the observation that the ΔRING MDM2 mutant, which only consists of amino acids 1–440 and, therefore, lacks the nucleolar localization signal,Citation64 can efficiently sumoylate MDM2 ().
Previous studies of p53-SUMO fusion proteins have suggested a role for SUMO-1 in promoting p53 nuclear export.Citation65 We therefore analyzed the consequence of SUMO-3 conjugation to p53 by cellular fractionation, followed by immunoprecipitation of p53 (). Sumoylated p53 was exclusively found in the nuclear fraction, while overall p53 distribution was mainly nuclear and not altered when p53 mutants K386R and E388A, which cannot be sumoylated, were expressed, suggesting that SUMO-3 does not promote the nuclear export of p53.
Sumoylation modulates p53's transcriptional activation and repression.
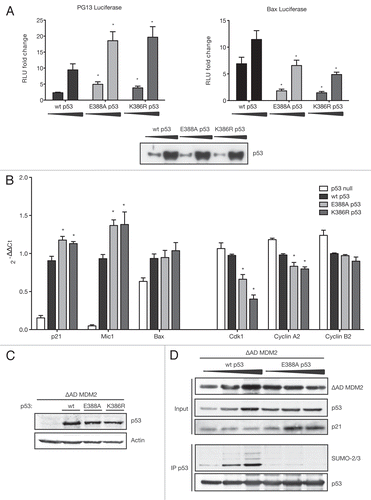
p53's major role as stress-induced transcription factor led us to investigate the effect of sumoylation on p53's transcriptional activity. First, we performed luciferase assays with two p53-responsive reporter gene contructs; one regulated by a series of optimised p53 binding sites-PG13Citation66 and one under the control of the naturally occurring p53 site in the Bax-promoter. Wild-type p53 and both mutants of the SUMO consensus, K386R and E388A p53, were titrated in the presence of ΔAD MDM2, and the luciferase assays [plotted as fold change of RLU: (relative luciferase units relative to TK Renilla)] revealed that both SUMO-site mutants of p53 were significantly more efficient in inducing the PG13 promoter than wild-type p53 (, left). Interestingly, we observed the opposite effect on the Bax-luciferase, which was more strongly induced by wild-type p53 than the SUMO-site mutants E388A and K386R (, right), suggesting that sumoylation can both positively and negatively modulate p53 transcriptional activity depending on the target promoter. Equal p53 protein level in the luciferase assays was confirmed by protein gel blotting (, bottom).
To investigate the impact of sumoylation on p53-target gene transcription under more physiological conditions, we extracted mRNA from p53-null H1299 cells retrovirally infected with empty vector, wild-type, E388A and K386R p53 in the presence of ΔAD MDM2. The difference between p21 mRNA levels depending on p53 SUMO status was small, yet significant and showed the same trend as observed on PG13 luciferase (). Furthermore, expression of the TGFβ family member macrophage inhibitory cytokine-1 [Mic-1, also known as growth/differentiation factor-15 (GDF15)], which is strongly induced by p53 expression, was also dampened by p53 sumoylation, since the SUMO-negative mutant E388A and K386R induced Mic-1 to significantly higher levels than wild-type p53. However, the decreased activation of Bax expression by the SUMO-dead mutants E388A and K386R observed using the Bax luciferase reporter could not be reproduced with endogenous mRNA levels. Bax was generally not induced strongly by p53 expression, and sumoylation status of p53 had no significant effect on its induction (). Equal p53 protein expression levels were confirmed by protein gel blotting (see ).
Since p53 also has an important role in repressing a range of genes, we studied the effect of p53 sumoylation on some established p53-repressed cell cycle genes. We observed that both non-sumoylatable mutants E388A and K386R p53 repressed the S- and G2-phase genes Cdk1 and cyclin A2 significantly more strongly than wild-type p53, suggesting that sumoylation can alleviate p53-mediated repression. Another similar p53-repressed gene, cyclin B2, did not show significant differences in repression levels depending on p53 sumoylation status. We conclude that sumoylation can dampen both activation and repression of p53 targets, and that modulation of p53-target genes by sumoylation is limited to a specific array of genes.
Finally, we analyzed the effect of p53 sumoylation on the expression of p21 at the protein level. Using ΔAD MDM2 to promote sumoylation of p53, we found that sumoylated wild-type p53 was impaired in driving the expression of p21 compared with E388A p53, which cannot be sumoylated (). This suggests that the modulation of p53's transcriptional activity by sumoylation is reflected on the protein level of its target genes.
Discussion
In this study, we show that MDM2 can promote conjugation of SUMO-2/3 to p53, and that this activity is independent of the RING domain () that is essential for the E3 ubiquitin ligase activity of MDM2. These results demonstrate a clear difference in requirements for MDM2 to function as a ubiquitin ligase or a SUMO-2/3 ligase and are consistent with other studies that have demonstrated that the PIASY-mediated sumoylation of Yin Yang1 can also occur independently of the PIAS RING finger.Citation67 MDM2 has previously been shown to drive the modification of p53 by SUMO-1 in cooperation with ARF. However, MDM2 could not promote endogenous SUMO-1 conjugation to p53,Citation27 while we observe conjugation of endogenous SUMO-2/3. It has previously been observed that overexpression of SUMO-1, which is normally restricted in availability in the cell, can compete with the conjugation of endogenous SUMO-2/3, which is naturally occurring in a large free pool.Citation68 We propose that under physiological expression levels of all SUMO isoforms, MDM2 predominantly promotes SUMO-2/3 conjugation to p53. Taken together, our data suggest that, in addition to promoting ubiquitin conjugation to p53, MDM2 can also enhance modification by SUMO-2/3, and that the requirements and consequences are different to those previously reported for modification by SUMO-1. Proteins that inhibit MDM2-mediated ubiquitination, like L11 and ARF, are able to stimulate sumoylation of p53. It would be of interest to assess the effect of nucleostemin, a more recently discovered modulator of MDM2-mediated p53 ubiquitination in response to nucleolar stress,Citation69 on MDM2-mediated p53 sumoylation.
We observed that MDM2 mutants that are unable to ubiquitinate are also better in promoting sumoylation of p53. This clearly shows distinct sumoylation and ubiquitination activities in the same protein and might also point toward a competition of both activities. There is no simple correlation between the degree of SUMO conjugation activity and the loss of ubiqutination activity, however. For example, the ΔAD MDM2 mutant retains some residual ubiquitination activity but is the strongest sumoylating mutant. This MDM2 mutant has previously been shown to promote SUMO-1 conjugation to p53 through relocalization to the nucleolus.Citation27 However, we show that MDM2 mutants that have lost the nucleolar relocalization signal (for example ΔRING and NoLS MDM2) or are not activated for nucleolar localization (for example 464A MDM2) retain SUMO-2/3-conjugating activity ( and ). Interestingly, only SUMO-1 but not SUMO-2/3 localizes to the nucleolus.Citation31 We propose that the strong SUMO-2/3 conjugating activity of ΔAD MDM2 depends on MDM2-interacting proteins, as we observed that this mutant interacted particularly well with the SUMO-E2 Ubc9. Binding to desumoylating enzymes might have an additional effect, since it has recently been published that the Sentrin-specific protease Senp3 interacts with MDM2 between amino acids 222–274,Citation70 which are missing in the ΔAD MDM2 protein. These results present the interesting possibility that the ability of wild-type MDM2 to ubiquitinate or sumoylate p53 may be subject to regulation, potentially following modification such as ATM-dependent phosphorylation, which specifically inhibits the ability of MDM2 to ubiquitinate p53.Citation71
Although the same SUMO-E2 enzyme (Ubc9) is responsible for conjugating all SUMO isoforms, some target genes are preferentially conjugated with SUMO-1, and others show much higher sumoylation with SUMO-2/3.Citation32,Citation72 Apart from different dynamics and subcellular distributions of the SUMO isoforms, functionally distinct consequences of SUMO-1 vs. SUMO-2/3 conjugation are emerging, including isoform-specific SUMO-interacting motifs for the recruitment of interacting proteinsCitation73 and polychain-specific (chain formation is limited to SUMO-2/3Citation60) functions.Citation74
Mutation of the SUMO consensus as in K386R and E388A p53 would prevent both SUMO-1 and SUMO-2/3 conjugation onto p53, but our experiments were performed under conditions where SUMO-2/3 conjugation of p53 was detectable, while SUMO-1 modification was not. The similar behavior of both negative control p53-SUMO-mutants (K386R and E388A) rule out effects of other modifications on K386.
Sumoylation is commonly associated with changes in sub-cellular localization. It has previously been reported that SUMO-1-fusion to p53 leads to nuclear export of p53.Citation65 Recently, PIASy-mediated induction of apoptosis in endothelial cells has been linked to nuclear export of p53 in PIASy-mediated conjugation of SUMO-3.Citation75 However, we did not observe any change in subcellular distribution of p53 whether or not it was modified with SUMO-2/3 ().
We suggest a model in which p53 sumoylation does not affect its localization, but dampens the transcriptional activity (both activation and repression) of p53 on a subset of target genes. An enhanced activation of p21 by the p53 K386R mutant has been observed previously (luciferase reporters,Citation37,Citation39 real-time-PCR,Citation41 microarrayCitation42) and is consistent with the report that the SUMO-dead mutant p53 K386R is more present at the p21 promoter in a chromatin immunoprecipitation experiment.Citation41
Although sumoylation contributes to the assembly of repressor complexes,Citation76,Citation77 the SUMO-dead p53 mutants showed enhanced repression of some targets (Cdk1 and cyclin A2) while having no effect on others (cyclin B2). This result is supported by a microarray performed with a p53-SUMO-1 fusion protein, which showed that only about a third of p53-repressed genes were still repressed by the p53-SUMO fusion.Citation42 It is possible that sumoylation can modulate the transcriptional activity of p53 by inhibiting p300-mediated acetylation of p53.Citation41 Although the effects of sumoylation on p53 activity are modest, there are numerous examples of how relatively small changes in p53 activity can have profound effects on physiological outcome. Interestingly, p53's ability to regulate transcription is reduced in senescent cells, and it will be of interest to determine whether this reflects changes in sumoylation.Citation78
The observation that mouse p53 cannot be sumoylated () limits the use of mouse models in which the C-terminal lysines in p53 have been mutated for the analysis of the consequences of this sumoylation.Citation79 Drosophila p53 is sumoylated, although the modified residues lie in different domains of the protein compared with human p53.Citation80 At present, the most promising animal model is in Zebrafish, which retain the conserved C-terminal SUMO motif.Citation81
Materials and Methods
Cells, transfections and infections.
H1299 cells (p53-null human non-small cell lung adenocarcinoma cells), HCT116 cells with and without wild-type p53 (human colorectal carcinoma cells), A2780 cells (wild-type, p53-expressing ovarian cancer cells), U2OS cells (wild-type, p53-expressing human osteosarcoma cells) and Phoenix cells expressing the ecotropic receptor were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 2 mM glutamine and antibiotics. Cells were transfected with Effectene (QIAgen) or GeneJuice (MERCK Biosciences) according to the manufacturer's instructions.
For retroviral infections, 10-cm plates of Phoenix cells were transfected with GeneJuice. The supernatant was collected 36, 48 and 60 h later; 4 µg/ml Polybrene (Sigma) was added and used for retroviral infections of H1299 cells.
Cells were treated with 20 µM Nutlin-3a (Roche) and 7.5 µM HLI373 (kind gift of A. Weissman) overnight.
Plasmids.
Plasmids expressing human wild-type p53 and Box I deletion (ΔI p53) mutants, hotspot mutations R175H and R273H,Citation82 SUMO-site mutants K386R and E388A p53,Citation83 wild-type MDM2, MDM2 Δ58–89,Citation84 MDM2 ΔAD (Δ222–437) and ΔRING (1–440), MDM2 464A mutant,Citation20 MDM2 NoLSCitation64 and flag-L11 Citation20 have been described previously. pcDNA3.1 mouse p53 and the retroviral plasmids pWZL blast MDM2 and pWZL neo p53 were supplied by K. Ryan. The ΔAD MDM2 mutation was introduced into pWZL blast MDM2 and MDM2 NoLS with the primers Δ222–437 fw: CTA CAG GGA CGC CAT CGA ATT GTG TGA TTT GTC AAG GTC G Δ222–437 rev: CGA CCT TGA CAA ATC ACA CAA TTC GAT GGC GTC CCT GTA G using the KOD Hot Start Mastermix (MERCK Biosciences) according to the manufacturer's protocol. pWZL neo p53 was subject to site-directed mutagenesis using the KOD Hot Start Mastermix (MERCK Biosciences) to create pWZL neo p53 K386R and E388A with the following primer pairs: K386R fw: AAA CTC ATG TTC AGG ACA GAA GGG CCT GAC, K386R rev: GTC AGG CCC TTC TGT CCT GAA CAT GAG TTT, E388A fw: CTC ATG TTC AAG ACA GCA GGG CCT GAC TCA GAC, E388A rev: GTC TGA GTC AGG CCC TGC TGT CTT GAA CAT GAG, HA-tagged ubiquitin, HA-tagged SUMO-3 and SV5-tagged Ubc9 were kindly provided by R. Hay. HA-SUMO-3 was mutated to K11R SUMO-3 using the KOD Hot Start Mastermix (MERCK Biosciences) with the following primers: K11R fw: CAA GGA GGG TGT GAG GAC AGA GAA TGA C and K11R rev: GTC ATT CTC TGT CCT CAC ACC CTC CTT G.
Luciferase assays were performed on Bax LuciferaseCitation85 and PG13 Luciferase,Citation66 using TK RenillaCitation86 as an internal control.
Protein analysis.
Protein gel blot analysis was performed out as previously described in reference Citation87. The following antibodies were used for blotting and immunoprecipitation: human p53, DO-1 antibody;Citation88 mouse p53, 1C12 (Cell Signaling Technology); MDM2, Ab-1 antibody (Calbiochem); p21, C19 goat antibody (Santa Cruz Biotechnology); p14ARF, 4037 raised in rabbit;Citation89 flag, Flag-M2-HRP (Sigma) HA, 16B12 antibody (Covance); SV5-tag, SV5-PK1 (AbD Serotec); SUMO-2/3, rabbit antibody PW9465 (ENZO Life Sciences) and MDMX, MDM4-antibody (Bethyl Laboratories); Histone-acetyl-transferase GCN5, H-75 antibody from (Santa Cruz); Lactate Dehydrogenase LDHB, 2H6 antibody (Sigma).
Sumoylation of p53 in cells.
Cells were seeded in 10-cm plates to reach 70% confluency 24 h prior to transfection with Effectene (QIAgen) or GeneJuice (MERCK Biosciences) reagent according to the manufacturer's instructions. Cells were washed 20 h later and lysed in 200 µl 1% SDS in Tris-buffered saline. The lysate was boiled for 10 min with intermediate vortexing and diluted with 400 µl 1.5% Triton-X in Tris-buffered saline. Lysates were precleared with 25 µl Protein G Sepharose FastFlow (Sigma) for 1 h and p53 immunoprecipitated with 5 µl DO1 antibody and 30 µl Protein G beads overnight. Beads were washed three times with HUNT buffer (20 mM Tris pH 8, 120 mM NaCl, 1 mM EDTA, 0.5% NP-40) and taken up in 3x sample buffer.
Immunoprecipitation under native conditions.
Cells were transfected at 70% confluency in 10-cm plates with 2 µg SV5-Ubc9 and 2 µg MDM2 using GeneJuice (MERCK Biosciences). Twenty-four hours later, cells were lysed in NP-40 buffer (150 mM NaCl, 50 mM Tris pH 8.0 and 1% NP-40) with proteasome inhibitor cocktail (Complete, Roche), frozen and thawed three times and debris spun down. Immunoprecipitation was performed with 5 µl SV5-antibody SV5-PK1 (AbD Serotec) and 30 µl Protein G Dynabeads (Invitrogen) overnight, rotating at 4°C. Beads were washed three times with NP-40 buffer and resuspended in 3x sample buffer.
Cellular fractionation.
Cells were seeded to 70% confluency in 10-cm dishes the day before transfection. Transfection was performed with GeneJuice following the manufacturer's guidelines. Twenty-four hours later, cells were trypsinated off the plate and spun down (5 min, 1,000 rpm). Fractionation into cytoplasmic and nuclear fraction was performed with Epigentek's Nuclear Extraction Kit (two extra washes with NE1 buffer were included after collection of the cytoplasmic fraction to prevent contamination of the nuclear fraction). Both cytoplasmic and nuclear fractions were then boiled in 1% SDS and subject to an in vivo SUMO assay. Successful fractionation was confirmed using LDHB as cytoplasmic and GCN5 as nuclear marker.
Luciferase assays.
Cells were seeded to 70% confluency into 24-well plates the day before transfection with 10 or 100 ng p53, 30 ng ΔAD MDM2, 20 ng TK Renilla Luciferase and 100 ng PG13 or Bax luciferase with GeneJuice (MERCK Biosciences). Twenty hours after transfection, cells were lysed in 100 µl lysis buffer provided by the Promega Renilla Luciferase Kit for 30 min at 4°C. Twenty µl lysate were transferred to luminometer plates, and readings with both Renilla and Luciferase substrate were performed at the Veritas Microplate lumi'nometer (Turner Biosystems) using the Glomax Software. Relative luciferase units were determined by dividing the Luciferase readings by the values obtained for Renilla luciferase to correct for cell number and transfection efficiency. Data are plotted as fold change to p53-null control readings. Error bars represent the standard error of the mean for three independent experiments.
RNA extraction and real-time-PCR.
H1299 cells were retrovirally infected with pWZL blast ΔAD MDM2, selected for 5 d in 5 µg/ml Blasticidin. RNA was extracted using the RNeasy kit from QIAgen, following the manufacturer's instructions. cDNA was synthesized from 1 µg RNA using Oligo d(T) primers and the DyNAmo SYBR Green two-step kit (Finnzymes) according to the manufacturer's instructions. The real-time-PCR reaction was performed on 5 µl cDNA, diluted 1:20 using the DyNAmo SYBR Green two-step kit (Finnzymes). The amount of fluorescent PCR product accumulating during the PCR program (15 min 95°C hot start, 2 min 94°C annealing, 40 cycles of 30 sec 94°C, 30 sec 60°C and 1 min 72°C; 10 min 72°C incubation) was detected by the Chromo4 Reader (Bio-Rad) and analyzed using the Opticon Monitor 3 software. Gene expression was quantified relative to the housekeeping genes β2-microglobulin (β2M) and ribosomal protein, large P0 (RPLPO) according to the comparative ΔΔCt-method. Results are presented as fold change relative to wild-type p53 induction. Error bars represent the standard error of the mean of three independent experiments.
The following primers were used (all primers only amplified one product):
RPLPO fw: GCA ATG TTG CCA GTG TCT G, rev: GCC TTG ACC TTT TCA GCA A
B2M fw: GTG CTC GCG CTA CTC TCT C, rev: GTC AAC TTC AAT GTC GGA T
p21 fw: CTG GAG ACT CTC AGG GTC GAA A, rev: GAT TAG GGC TTC CTC TTG GAG AA
Mic-1 fw: GTT GCA CTC CGA AGA CTC CA, rev: GAG AGA TAC GCA GGT GCA GG
Bax fw: GGG TTG TCG CCC TTT TCT ACT T, rev: CAGC CCA TGA TGG TTC TGA TCA G
Cdk1 fw: CTT GCC AGA GCT TTT GGA ATA C, rev: TTC TGA ATC CCC ATG GAA AA
cyclin A2 fw: CCT GCA AAC TGC AAA GTT GA, rev: TGC TGT GGT GCT TTG AGG TA
cyclin B2 fw: TTG CAG TCC ATA AAC CCA CA, rev: GAA GCC AAG AGC AGA GCA GT
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed.
Figures and Tables
Figure 1 MDM2 induces SUMO-3 conjugation of p53. (A) MDM2 sumoylates p53 in U2OS cells. U2OS cells were transfected with 3 µg of p53, 1 µg of HA-SUMO-3 and 1 µg of either empty vector or MDM2. Cells were lysed under denaturing conditions according to the SUMO assay protocol and p53 immunoprecipitated (IP p53) with the p53-specific DO-1 antibody. (B) MDM2, but not MDMX sumoylates p53 in HCT116 cells. HCT116 cells were transfected with 3 µg of p53, 1 µg of HA-SUMO-3 and 1 µg of either empty vector, MDM2 or MDMX. Cells were lysed under denaturing conditions according to the SUMO assay protocol and p53 immunoprecipitated (IP p53) with the p53-specific DO-1 antibody. (C) MDM2, but not MDMX sumoylates p53 in H1299 cells. H1299 cells were transfected with 3 µg of p53, 1 µg of HA-SUMO-3 and 1 µg of either empty vector, MDM2, MDMX or 0.5 µg of MDM2 and MDMX. Cells were lysed under denaturing conditions according to the SUMO assay protocol and p53 immunoprecipitated (IP p53) with the p53-specific Do-1 antibody. (D) Model of p53 and MDM2 domains. p53 consists of an N-terminal transactivation domain, which is also the interaction-platform for MDM2, a central DNA binding domain and a C-terminal heavily post-translationally modified regulatory domain. MDM2 contains a hydrophobic p53-binding pocket in its C terminus, a central acidic domain, and adjacent zinc finger and a C-terminal RING finger domain. (E) MDM2 needs to bind p53 in order to sumoylate. U2OS-cells were transfected with 3 µg p53, 1 µg MDM2 or empty vector and 1 µg HA-SUMO-3 plasmids and lysed for the SUMO assay. p53 was immunoprecipitated (IP p53) using the DO-1 antibody.
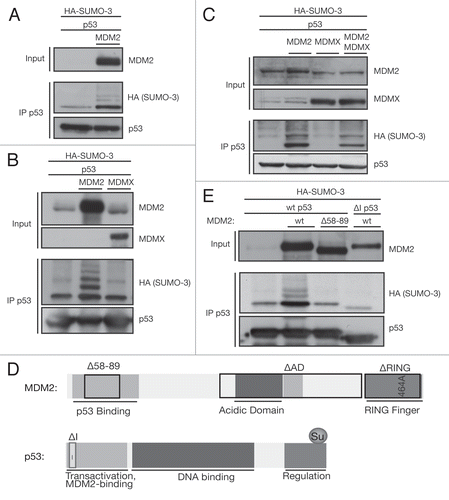
Figure 2 The sumoylating and ubiquitinating activities of MDM2 are distinct features. (A) Ubiquitination activity of different MDM2 mutants. A ubiquitination assay was performed on HCT116 cells, transfected with 3 µg of wild-type (wt) or mutant MDM2, 1 µg HA-ubiquitin and 1 µg of MDM2 constructs or empty vector. p53 was immunoprecipitated using the DO-1 antibody (IP p53). (B) Sumoylation activity of different MDM2 mutants. A SUMO assay was performed on HCT116 cells, transfected with 3 µg of MDM2, 1 µg HA-SUMO-3 and 1 µg of wild-type (WT) or mutant MDM2 constructs or empty vector. p53 was immunoprecipitated using the DO-1 antibody (IP p53). (C) Endogenous SUMO-2/3 is conjugated to p53 by MDM2. U2OS cells were transfected with 0.3 µg MDM2 and 0.6 µg p53 and subjected to the SUMO assay. p53 was immunoprecipitated using the DO-1 antibody (IP p53). (D) MDM2 mutants promote endogenous SUMO-2/3 conjugation to an even higher degree. U2OS cells were transfected with 0.3 µg MDM2 mutants and 0.6 µg p53 and subjected to the SUMO assay. p53 was immunoprecipitated using the DO-1 antibody (IP p53). (E) Endogenous p53 is sumoylated by MDM2. U2OS cells were transfected with 1.3 µg MDM2 wild-type and mutant plasmid. Cells were lysed under denaturing conditions, and the endogenous p53 was immunoprecipitated using the DO-1 antibody (IP p53). (F) Nutlin disrupts sumoylation, but HLI373 does not. A2780 cells were transfected with 1 µg p53 and, 8 h later, treated with 20 µM Nutlin and 7.5 µM HLI373 overnight. Cell lysis was performed according to the in vivo SUMO assay, and p53 was immunoprecipitated using the DO-1 antibody (IP p53). (G) The SUMO-E2 Ubc9 interacts strongly with ΔAD MDM2. HCT116 cells were transfected with 2.5 µg SV5-Ubc9 and 2.5 µg MDM2 mutant plasmids. Ubc9 was pulled down with the SV5-tag under mild lysis conditions according to the native immunoprecipitation protocol (IP SV5) to observe interacting proteins. (*IgG heavy chain).
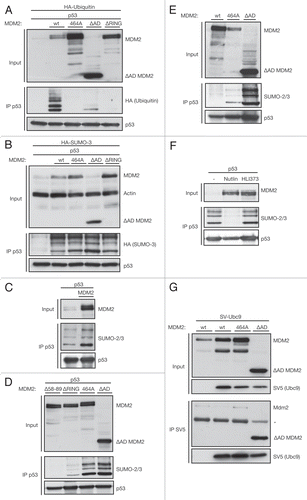
Figure 3 The SUMO-consensus is important for SUMO-2/3 conjugation to p53. (A) Alignment of p53's C-terminal amino acids of the human, mouse and zebrafish protein. Sumoylation is predominantly restricted to lysines in the SUMO consensus motif ΨKxE. While the lysine residue is conserved among all vertebrates, the glutamic acid residue, essential for the SUMO consensus, is lacking in mice. SwissProt protein accession numbers: Human p53, P04637; Mouse p53, P02340; Zebrafish p53, P79734. (B) Mutation of K386R and E388A stop sumoylation of p53. H1299 cells were transfected with 1 µg wild-type (wt) or mutant p53 and 0.4 µg ΔAD MDM2 and lysed according to the SUMO assay protocol. p53 was immunoprecipitated with the DO-1 antibody (IP p53). (C) p53 conformation does not affect its sumoylation. HCT116 p53-/- cells were transfected with 1.2 µg wild-type or mutant p53 and 0.3 µg ΔAD MDM2. Cells were lysed under denaturing condition and p53 was immunoprecipitated with the DO-1 antibody (IP p53). (D) Mouse p53 is not sumoylated. HCT116 p53-/- cells were transfected with 3 µg human or murine p53 and 1 µg ΔAD MDM2. Cells were lysed according to the SUMO assay protocol, and p53 was immunoprecipitated with the 1C12 antibody (IP p53). (E) Mouse p53 binds human MDM2. HCT116 p53-/- cells were transfected with 2 µg human or murine p53 and 2 µg ΔAD MDM2. Cells were lysed under native conditions to allow pull-down of interacting proteins and p53 was immunoprecipitated using the 1C12 antibody (co-IP p53). (F) Alignment of human SUMO-1, -2 and -3 around lysine 11 in SUMO-2/3. Sumoylation is predominantly restricted to lysines in the SUMO consensus motif ΨKxE. While both the lysine and glutamic acid residues are conserved among all SUMO isoforms, the hydrophobic amino acid (Ψ), essential for the SUMO consensus, is lacking in SUMO-1. Swissprot protein accession numbers: human SUMO-1, P63165; SUMO-2, P61956; SUMO-3, P55854. (G) The SUMO band pattern on p53 does not reflect a SUMO-2/3 chain. U2OS cells were transfected with 3 µg p53, 1 µg ΔAD MDM2 and 1 µg HA-SUMO-3 or HA-SUMO-3 K11R. Cells were lysed under denaturing conditions, and p53 was immunoprecipitated using the DO-1 antibody (IP p53).
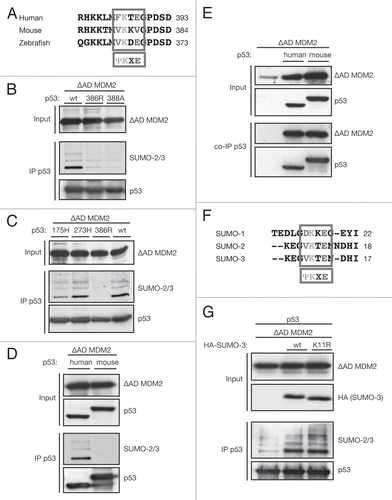
Figure 4 MDM2 strongly sumoylates p53 in complex with ARF and L11 independently of relocalization to the nucleolus. (A) ARF and L11 enhance p53 sumoylation. U2OS cells were transfected with 2.5 µg p53, 0.5 µg HA-SUMO-3, 1 µg MDM2 or empty vector and 2 µg ARF or flag-L11 and lysed according to the SUMO assay protocol. p53 was immunoprecipitated using the DO-1 antibody (IP p53). (B) sumoylation activity of ΔAD MDM2 does not depend on nucleolar relocalization. H1299 cells were transfected with 3 µg p53 and 1 µg ΔAD MDM2 or 1 µg ΔAD NoLS MDM2 (mutant nucleolar localization signal). Lysates were prepared for the in vivo SUMO assay, and p53 was immunoprecipitated using the DO-1 antibody (IP p53). (C) p53 subcellular distribution does not change upon sumoylation. HCT116 p53-/- cells were transfected with 1 µg ΔAD MDM2 and 3 µg wild-type (wt) p53, E388A p53 or K386R p53. Twenty-four hours later, cells were trypsinated and fractionated into cellular and nuclear fractions. Fractions were boiled with 1% SDS and p53 immunoprecipitated (IP p53). Efficient fractionation was confirmed using the histone acetyl transferase GCN5 as nuclear and lactate dehydrogenase LDHB as cytoplasmic marker.
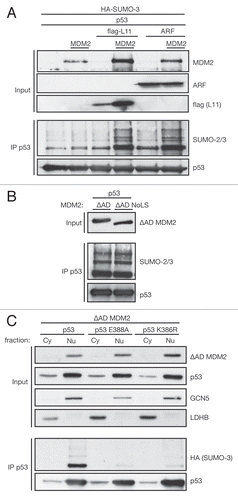
Figure 5 Sumoylation modulates p53's transcriptional activation and repression. (A) sumoylation modulates p53 transcriptional activation of luciferase reporters. HCT116 p53 cells were transfected with 10 or 100 ng wild-type (wt) or mutant p53, 30 ng ΔAD MDM2, 20 ng TK Renilla and 100 ng PG13 (left) or Bax (right) luciferase. Cells were lysed according to Promega's luciferase system instructions and luminescence measured in a microplate luminometer and analyzed using the Glomax Software. Relative Luciferase Units (RLU) were calculated dividing the readings obtained with Firefly divided by readings for Renilla luciferase and plotted as fold change relative to p53-null control. The diagram shows the mean of three independent triplicates of experiments with error bars as standard error of the mean. *indicates a p-value < 0.02. p-values were calculated using an unpaired, two-tailed Student t-test, comparing the RLUs of SUMO-mutant p53 to the RLU obtained after transfecting the same amount of wild-type (wt) p53. Triplicates were combined and equal p53-protein levels confirmed by protein gel blot. (B) sumoylation modulates mRNA levels of p53-activated and repressed target genes. H1299 cells were retrovirally infected with ΔAD MDM2 and empty vector, wild-type (wt) or SUMO-mutant p53. Half the cells were used for RNA extraction and reverse transcription PCR into cDNA. Quantitative Real-time-PCR was performed with a SYBR Green Master-mix for a number of p53-activated and -repressed genes. Gene expression was quantified relative to the housekeeping genes β2-microglobulin and ribosomal protein, large P0 (RPLPO) according to the comparative ΔΔCt-method. Results are displayed as mean of 2−ΔΔCt values obtained from three independent experiments. Error bars represent the standard error of the mean. * indicates a p-value ≤ 0.02 as calculated by an unpaired, two-tailed Student t-test comparing cells expressing SUMO-mutant p53 to those expressing wild-type p53. (C) p53 protein is expressed at comparable levels. The other half of infected cells generated for (B) was lysed in sample buffer, and equal p53-protein levels were confirmed by protein gel blotting with the p53-specific DO-1 antibody. (D) sumoylation of p53 represses activation of p21 at protein level. H1299 cells were transfected with 0.2, 0.5 and 1.0 µg of wild-type (wt) p53 or E388A p53 and 0.3 µg ΔAD MDM2. Cells were lysed under denaturing conditions and p53 immunoprecipitated using the DO-1 antibody (IP p53).
Acknowledgments
We thank Bob Ludwig for help with experiments and all members of the Tumour Suppression Laboratory for discussion. We are grateful to Ron Hay for HA-SUMO, HA-ubiquitin and SV5-Ubc9 constructs. The MDM2-inhibitor HLI373 was a kind gift of Allan Weissman. This work was funded by Cancer Research UK.
References
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell 2009; 137:413 - 431; PMID: 19410540; http://dx.doi.org/10.1016/j.cell.2009.04.037
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992; 356:215 - 221; PMID: 1552940; http://dx.doi.org/10.1038/356215a0
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991; 253:49 - 53; PMID: 1905840; http://dx.doi.org/10.1126/science.1905840
- el-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994; 54:1169 - 1174; PMID: 8118801
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995; 80:293 - 299; PMID: 7834749; http://dx.doi.org/10.1016/0092-8674(95)90412-3
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001; 7:683 - 694; PMID: 11463392; http://dx.doi.org/10.1016/S1097-2765(01)00214-3
- Ho J, Benchimol S. Transcriptional repression mediated by the p53 tumour suppressor. Cell Death Differ 2003; 10:404 - 408; PMID: 12719716; http://dx.doi.org/10.1038/sj.cdd.4401191
- Imbriano C, Gurtner A, Cocchiarella F, Di Agostino S, Basile V, Gostissa M, et al. Direct p53 Transcriptional Repression: In Vivo Analysis of CCAAT-Containing G2/M Promoters. Mol Cell Biol 2005; 25:3737 - 3751; PMID: 15831478; http://dx.doi.org/10.1128/MCB.25.9.373751.2005
- Böhlig L, Rother K. One Function-Multiple Mechanisms: The Manifold Activities of p53 as a Transcriptional Repressor. J Biomed Biotechnol 2011; 2011:464916; PMID: 21436991; http://dx.doi.org/10.1155/2011/464916
- Yun J, Chae HD, Choy HE, Chung J, Yoo HS, Han MH, et al. p53 negatively regulates cdc2 transcription via the CCAAT-binding NF-Y transcription factor. J Biol Chem 1999; 274:29677 - 29682; PMID: 10514438; http://dx.doi.org/10.1074/jbc.274.42.29677
- Krause K, Wasner M, Reinhard W, Haugwitz U, Dohna CL, Mossner J, et al. The tumour suppressor protein p53 can repress transcription of cyclin B. Nucleic Acids Res 2000; 28:4410 - 4418; PMID: 11071927; http://dx.doi.org/10.1093/nar/28.22.4410
- Badie C, Bourhis J, Sobczak-Thepot J, Haddada H, Chiron M, Janicot M, et al. p53-dependent G2 arrest associated with a decrease in cyclins A2 and B1 levels in a human carcinoma cell line. Br J Cancer 2000; 82:642 - 650; PMID: 10682678
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 1997; 387:296 - 299; PMID: 9153395; http://dx.doi.org/10.1038/387296a0
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature 1997; 387:299 - 303; PMID: 9153396; http://dx.doi.org/10.1038/387299a0
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995; 378:206 - 208; PMID: 7477327; http://dx.doi.org/10.1038/378206a0
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995; 378:203 - 206; PMID: 7477326; http://dx.doi.org/10.1038/378203a0
- Alarcon-Vargas D, Ronai Z. p53-Mdm2—the affair that never ends. Carcinogenesis 2002; 23:541 - 547; PMID: 11960904; http://dx.doi.org/10.1093/carcin/23.4.541
- Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 2004; 4:793 - 805; PMID: 15510160; http://dx.doi.org/10.1038/nrc1455
- Meek DW, Knippschild U. Posttranslational modification of MDM2. Mol Cancer Res 2003; 1:1017 - 1026; PMID: 14707285
- Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell 2003; 3:577 - 587; PMID: 12842086; http://dx.doi.org/10.1016/S15356108(03)00134-X
- Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J 1999; 18:22 - 27; PMID: 9878046; http://dx.doi.org/10.1093/emboj/18.1.22
- Gu J, Nie L, Wiederschain D, Yuan ZM. Identification of p53 sequence elements that are required for MDM2-mediated nuclear export. Mol Cell Biol 2001; 21:8533 - 8546; PMID: 11713288; http://dx.doi.org/10.1128/MCB.21.24.8533-46.2001
- Lohrum MA, Woods DB, Ludwig RL, Balint E, Vousden KH. C-terminal ubiquitination of p53 contributes to nuclear export. Mol Cell Biol 2001; 21:8521 - 8532; PMID: 11713287; http://dx.doi.org/10.1128/MCB.21.24.8521-32.2001
- Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 1993; 362:857 - 860; PMID: 8479525; http://dx.doi.org/10.1038/362857a0
- Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 2004; 118:83 - 97; PMID: 15242646; http://dx.doi.org/10.1016/j.cell.2004.06.016
- Liu G, Xirodimas DP. NUB1 promotes cytoplasmic localization of p53 through cooperation of the NEDD8 and ubiquitin pathways. Oncogene 2010; 29:2252 - 2261; PMID: 20101219; http://dx.doi.org/10.1038/onc.2009.494
- Chen L, Chen J. MDM2-ARF complex regulates p53 sumoylation. Oncogene 2003; 22:5348 - 5357; PMID: 12917636; http://dx.doi.org/10.1038/sj.onc.1206851
- Vivo M, Di Costanzo A, Fortugno P, Pollice A, Calabro V, La Mantia G. Downregulation of DeltaNp63alpha in keratinocytes by p14ARF-mediated SUMO-conjugation and degradation. Cell Cycle 2009; 8:3537 - 3543; PMID: 19829080; http://dx.doi.org/10.4161/cc.8.21.9954
- Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach DA. M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem 2004; 279:27233 - 27238; PMID: 15123604; http://dx.doi.org/10.1074/jbc.M402273200
- Owerbach D, McKay EM, Yeh ET, Gabbay KH, Bohren KM. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem Biophys Res Commun 2005; 337:517 - 520; PMID: 16198310; http://dx.doi.org/10.1016/j.bbrc.2005.09.090
- Ayaydin F, Dasso M. Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol Biol Cell 2004; 15:5208 - 5218; PMID: 15456902; http://dx.doi.org/10.1091/mbc.E04-07-0589
- Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem 2000; 275:6252 - 6258; PMID: 10692421; http://dx.doi.org/10.1074/jbc.275.9.6252
- Tempé D, Piechaczyk M, Bossis G. SUMO under stress. Biochem Soc Trans 2008; 36:874 - 878; PMID: 18793154; http://dx.doi.org/10.1042/BST0360874
- Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein sumoylation. Biochem J 2010; 428:133 - 145; PMID: 20462400; http://dx.doi.org/10.1042/BJ20100158
- Stehmeier P, Muller S. Regulation of p53 family members by the ubiquitin-like SUMO system. DNA Repair (Amst) 2009; 8:491 - 498; PMID: 19213614; http://dx.doi.org/10.1016/j.dnarep.2009.01.002
- Melchior F, Hengst L. SUMO-1 and p53. Cell Cycle 2002; 1:245 - 249; PMID: 12429940; http://dx.doi.org/10.4161/cc.1.4.131
- Rodriguez MS, Desterro JM, Lain S, Midgley CA, Lane DP, Hay RT. SUMO-1 modification activates the transcriptional response of p53. EMBO J 1999; 18:6455 - 6461; PMID: 10562557; http://dx.doi.org/10.1093/emboj/18.22.6455
- Gostissa M, Hengstermann A, Fogal V, Sandy P, Schwarz SE, Scheffner M, et al. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J 1999; 18:6462 - 6471; PMID: 10562558; http://dx.doi.org/10.1093/emboj/18.22.6462
- Schmidt D, Muller S. Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity. Proc Natl Acad Sci USA 2002; 99:2872 - 2877; PMID: 11867732; http://dx.doi.org/10.1073/pnas.052559499
- Kwek SS, Derry J, Tyner AL, Shen Z, Gudkov AV. Functional analysis and intracellular localization of p53 modified by SUMO-1. Oncogene 2001; 20:2587 - 2599; PMID: 11420669; http://dx.doi.org/10.1038/sj.onc.1204362
- Wu SY, Chiang CM. Crosstalk between sumoylation and acetylation regulates p53-dependent chromatin transcription and DNA binding. EMBO J 2009; 28:1246 - 1259; PMID: 19339993; http://dx.doi.org/10.1038/emboj.2009.83
- Cheema A, Knights CD, Rao M, Catania J, Perez R, Simons B, et al. Functional mimicry of the acetylated C-terminal tail of p53 by a SUMO-1 acetylated domain, SAD. J Cell Physiol 2010; 225:371 - 384; PMID: 20458745; http://dx.doi.org/10.1002/jcp.22224
- Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett 2005; 579:5007 - 5012; PMID: 16122737; http://dx.doi.org/10.1016/j.febslet.2005.07.088
- Bischof O, Schwamborn K, Martin N, Werner A, Sustmann C, Grosschedl R, et al. The E3 SUMO ligase PIASy is a regulator of cellular senescence and apoptosis. Mol Cell 2006; 22:783 - 794; PMID: 16793547; http://dx.doi.org/10.1016/j.molcel.2006.05.016
- Chu Y, Yang X. SUMO E3 ligase activity of TRIM proteins. Oncogene 2011; 30:1108 - 1116; PMID: 20972456; http://dx.doi.org/10.1038/onc.2010.462
- Pennella MA, Liu Y, Woo JL, Kim CA, Berk AJ. Adenovirus E1B 55-kilodalton protein is a p53-SUMO1 E3 ligase that represses p53 and stimulates its nuclear export through interactions with promyelocytic leukemia nuclear bodies. J Virol 2010; 84:12210 - 12225; PMID: 20861261; http://dx.doi.org/10.1128/JVI.01442-10
- Li T, Santockyte R, Shen RF, Tekle E, Wang G, Yang DC, et al. Expression of SUMO-2/3 induced senescence through p53- and pRB-mediated pathways. J Biol Chem 2006; 281:36221 - 36227; PMID: 17012228; http://dx.doi.org/10.1074/jbc.M608236200
- Chang PC, Izumiya Y, Wu CY, Fitzgerald LD, Campbell M, Ellison TJ, et al. Kaposi's sarcoma associated herpesvirus (KSHV) encodes a SUMO E3 ligase which is SIM-dependent and SUMO-2/3-specific. J Biol Chem 2010; 285:5266 - 5273; PMID: 20034935; http://dx.doi.org/10.1074/jbc.M109.088088
- Böttger V, Bottger A, Garcia-Echeverria C, Ramos YF, van der Eb AJ, Jochemsen AG, et al. Comparative study of the p53-mdm2 and p53-MDMX interfaces. Oncogene 1999; 18:189 - 199; PMID: 9926934; http://dx.doi.org/10.1038/sj.onc.1202281
- Tanimura S, Ohtsuka S, Mitsui K, Shirouzu K, Yoshimura A, Ohtsubo M. MDM2 interacts with MDMX through their RING finger domains. FEBS Lett 1999; 447:5 - 9; PMID: 10218570; http://dx.doi.org/10.1016/S0014-5793(99)00254-9
- Badciong JC, Haas AL. MdmX is a RING finger ubiquitin ligase capable of synergistically enhancing Mdm2 ubiquitination. J Biol Chem 2002; 277:49668 - 49675; PMID: 12393902; http://dx.doi.org/10.1074/jbc.M208593200
- Poyurovsky MV, Priest C, Kentsis A, Borden KL, Pan ZQ, Pavletich N, et al. The Mdm2 RING domain C terminus is required for supramolecular assembly and ubiquitin ligase activity. EMBO J 2007; 26:90 - 101; PMID: 17170710; http://dx.doi.org/10.1038/sj.emboj.7601465
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem 2000; 275:8945 - 8951; PMID: 10722742; http://dx.doi.org/10.1074/jbc.275.12.8945
- Kawai H, Wiederschain D, Yuan ZM. Critical contribution of the MDM2 acidic domain to p53 ubiquitination. Mol Cell Biol 2003; 23:4939 - 4947; PMID: 12832479; http://dx.doi.org/10.1128/MCB.23.14.4939-47.2003
- Meulmeester E, Frenk R, Stad R, de Graaf P, Marine JC, Vousden KH, et al. Critical role for a central part of Mdm2 in the ubiquitylation of p53. Mol Cell Biol 2003; 23:4929 - 4938; PMID: 12832478; http://dx.doi.org/10.1128/MCB.23.14.4929-38.2003
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303:844 - 848; PMID: 14704432; http://dx.doi.org/10.1126/science.1092472
- Kitagaki J, Agama KK, Pommier Y, Yang Y, Weissman AM. Targeting tumor cells expressing p53 with a water-soluble inhibitor of Hdm2. Mol Cancer Ther 2008; 7:2445 - 2454; PMID: 18723490; http://dx.doi.org/10.1158/1535-7163.MCT-08-0063
- Desterro JM, Thomson J, Hay RT. Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett 1997; 417:297 - 300; PMID: 9409737; http://dx.doi.org/10.1016/S0014-5793(97)01305-7
- Rodriguez MS, Dargemont C, Hay RT. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem 2001; 276:12654 - 12659; PMID: 11124955; http://dx.doi.org/10.1074/jbc.M009476200
- Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, et al. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J Biol Chem 2001; 276:35368 - 35374; PMID: 11451954; http://dx.doi.org/10.1074/jbc.M104214200
- Ulrich HD. The fast-growing business of SUMO chains. Mol Cell 2008; 32:301 - 305; PMID: 18995828; http://dx.doi.org/10.1016/j.molcel.2008.10.010
- Kuo ML, den Besten W, Thomas MC, Sherr CJ. Arf-induced turnover of the nucleolar nucleophosminassociated SUMO-2/3 protease Senp3. Cell Cycle 2008; 7:3378 - 3387; PMID: 18948745; http://dx.doi.org/10.4161/cc.7.21.6930
- Rizos H, Woodruff S, Kefford RF. p14ARF interacts with the SUMO-conjugating enzyme Ubc9 and promotes the sumoylation of its binding partners. Cell Cycle 2005; 4:597 - 603; PMID: 15876874; http://dx.doi.org/10.4161/cc.4.4.1588
- Lohrum MA, Ashcroft M, Kubbutat MH, Vousden KH. Identification of a cryptic nucleolar-localization signal in MDM2. Nat Cell Biol 2000; 2:179 - 181; PMID: 10707090; http://dx.doi.org/10.1038/35004057
- Carter S, Vousden KH. p53-Ubl fusions as models of ubiquitination, sumoylation and neddylation of p53. Cell Cycle 2008; 7:2519 - 2528; PMID: 18719371; http://dx.doi.org/10.4161/cc.7.16.6422
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75:817 - 825; PMID: 8242752; http://dx.doi.org/10.1016/00928674(93)90500-P
- Deng Z, Wan M, Sui G. PIASy-mediated sumoylation of Yin Yang 1 depends on their interaction but not the RING finger. Mol Cell Biol 2007; 27:3780 - 3792; PMID: 17353273; http://dx.doi.org/10.1128/MCB.01761-06
- Azuma Y, Arnaoutov A, Dasso M. SUMO-2/3 regulates topoisomerase II in mitosis. J Cell Biol 2003; 163:477 - 487; PMID: 14597774; http://dx.doi.org/10.1083/jcb.200304088
- Lo D, Lu H. Nucleostemin: Another nucleolar “Twister” of the p53-MDM2 loop. Cell Cycle 2010; 9:3227 - 3232; PMID: 20703089; http://dx.doi.org/10.4161/cc.9.16.12605
- Nishida T, Yamada Y. The nucleolar SUMO-specific protease SMT3IP1/SENP3 attenuates Mdm2-mediated p53 ubiquitination and degradation. Biochem Biophys Res Commun 2011; 406:285 - 291; PMID: 21316347; http://dx.doi.org/10.1016/j.bbrc.2011.02.034
- Cheng Q, Chen J. Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle 2010; 9:472 - 478; PMID: 20081365; http://dx.doi.org/10.4161/cc.9.3.10556
- Vertegaal AC, Andersen JS, Ogg SC, Hay RT, Mann M, Lamond AI. Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Mol Cell Proteomics 2006; 5:2298 - 2310; PMID: 17000644; http://dx.doi.org/10.1074/mcp.M600212-MCP200
- Hecker CM, Rabiller M, Haglund K, Bayer P, Dikic I. Specification of SUMO1- and SUMO2-interacting motifs. J Biol Chem 2006; 281:16117 - 16127; PMID: 16524884; http://dx.doi.org/10.1074/jbc.M512757200
- Vertegaal AC. SUMO chains: polymeric signals. Biochem Soc Trans 2010; 38:46 - 49; PMID: 20074033; http://dx.doi.org/10.1042/BST0380046
- Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, et al. PKC{zeta} mediates disturbed flow-induced endothelial apoptosis via p53 sumoylation. J Cell Biol 2011; 193:867 - 884; PMID: 21624955; http://dx.doi.org/10.1083/jcb.201010051
- Chupreta S, Holmstrom S, Subramanian L, Iniguez-Lluhi JA. A small conserved surface in SUMO is the critical structural determinant of its transcriptional inhibitory properties. Mol Cell Biol 2005; 25:4272 - 4282; PMID: 15870296; http://dx.doi.org/10.1128/MCB.25.10.4272-82.2005
- Garcia-Dominguez M, Reyes JC. SUMO association with repressor complexes, emerging routes for transcriptional control. Biochim Biophys Acta 2009; 1789:451 - 459; PMID: 19616654
- Huang B, Vassilev LT. Reduced transcriptional activity in the p53 pathway of senescent cells revealed by the MDM2 antagonist nutlin-3. Aging 2009; 1:845 - 854; PMID: 20157557
- Krummel KA, Lee CJ, Toledo F, Wahl GM. The C-terminal lysines fine-tune p53 stress responses in a mouse model but are not required for stability control or transactivation. Proc Natl Acad Sci USA 2005; 102:10188 - 10193; PMID: 16006521; http://dx.doi.org/10.1073/pnas.0503068102
- Mauri F, McNamee LM, Lunardi A, Chiacchiera F, Del Sal G, Brodsky MH, et al. Modification of Drosophila p53 by SUMO modulates its transactivation and proapoptotic functions. J Biol Chem 2008; 283:20848 - 20856; PMID: 18492669; http://dx.doi.org/10.1074/jbc.M710186200
- Yuan H, Zhou J, Deng M, Liu X, Le Bras M, de The H, et al. Small ubiquitin-related modifier paralogs are indispensable but functionally redundant during early development of zebrafish. Cell Res 2010; 20:185 - 196; PMID: 19704416; http://dx.doi.org/10.1038/cr.2009.101
- Lukashchuk N, Vousden KH. Ubiquitination and degradation of mutant p53. Mol Cell Biol 2007; 27:8284 - 8295; PMID: 17908790; http://dx.doi.org/10.1128/MCB.00050-07
- Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol 2007; 9:428 - 435; PMID: 17369817; http://dx.doi.org/10.1038/ncb1562
- Chen J, Lin J, Levine AJ. Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol Med 1995; 1:142 - 152; PMID: 8529093
- Bálint E, Bates S, Vousden KH. Mdm2 binds p73 alpha without targeting degradation. Oncogene 1999; 18:3923 - 3929; PMID: 10435614; http://dx.doi.org/10.1038/sj.onc.1202781
- Muller PAJ, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139:1327 - 1341; PMID: 20064378; http://dx.doi.org/10.1016/j.cell.2009.11.026
- Kubbutat MH, Ludwig RL, Levine AJ, Vousden KH. Analysis of the degradation function of Mdm2. Cell Growth Differ 1999; 10:87 - 92; PMID: 10074902
- Cesková P, Chichger H, Wallace M, Vojtesek B, Hupp TR. On the mechanism of sequence-specific DNA-dependent acetylation of p53: the acetylation motif is exposed upon DNA binding. J Mol Biol 2006; 357:442 - 456; PMID: 16438982; http://dx.doi.org/10.1016/j.jmb.2005.12.026
- Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J 1998; 17:5001 - 5014; PMID: 9724636; http://dx.doi.org/10.1093/emboj/17.17.5001