Abstract
Retinoblastoma gene (Rb1) is required for proper cell cycle exit in the developing mouse inner ear and its deletion in the embryo leads to proliferation of sensory progenitor cells that differentiate into hair cells and supporting cells. In a conditional hair cell Rb1 knockout mouse, Pou4f3-Cre-pRb-/-, pRb-/- utricular hair cells differentiate and survive into adulthood whereas differentiation and survival of pRb-/- cochlear hair cells are impaired. To comprehensively survey the pRb pathway in the mammalian inner ear, we performed microarray analysis of pRb-/- cochlea and utricle. The comparative analysis shows that the core pathway shared between pRb-/- cochlea and utricle is centered on E2F, the key pathway that mediates pRb function. A majority of differentially expressed genes and enriched pathways are not shared but uniquely associated with pRb-/-cochlea or utricle. In pRb-/- cochlea, pathways involved in early inner ear development such as Wnt/β-catenin and Notch were enriched, whereas pathways involving in proliferation and survival are enriched in pRb-/-utricle. Clustering analysis showed that the pRb-/- inner ear has characteristics of a younger control inner ear, an indication of delayed differentiation. We created a transgenic mouse model (ER-Cre-pRbflox/flox) in which Rb1 can be acutely deleted postnatally. Acute Rb1 deletion in the adult mouse fails to induce proliferation or cell death in inner ear, strongly indicating that Rb1 loss in these postmitotic tissues can be effectively compensated for, or that pRb-mediated changes in the postmitotic compartment result in events that are functionally irreversible once enacted. This study thus supports the concept that pRb-regulated pathways relevant to hair cell development, encompassing proliferation, differentiation and survival, act predominantly during early development.
Introduction
Cell cycle control is governed by a balanced act between cell cycle activators and cell cycle suppressors. During early inner ear development, genes involved in promoting cell cycle, including many cell division cycle genes (Cdc), cyclins and cyclin-dependent kinases (Cdk), are highly expressed. Their expression correlates with the proliferation status of the inner ear. In the postnatal inner ear, the sensory epithelial cells are in permanent quiescent status, due to the activation of negative cell growth genes and downregulation of cell cycle genes.Citation1–Citation3
In lower vertebrates including birds and fish, the inner ear maintains the capacity to regenerate hair cells throughout life.Citation4–Citation6 After hair cell loss, lower vertebrate inner ear cells either re-enter the cell cycle and differentiate into hair cells, or directly transdifferentiate into hair cells without cell division. Significantly, hair cell regeneration leads to functional recovery of auditory function.Citation7 In contrast, the mammalian inner ear has lost the capacity to regenerate hair cells due to the failure to re-enter the cell cycle or to transdifferentiate into hair cells spontaneously. Limited hair cell regeneration was observed in the mammalian utricle, a vestibular organ, after hair cell damage, suggesting that under certain conditions the mammalian inner ear may be induced for hair cell regeneration.Citation8,Citation9
One potential route to achieve mammalian hair cell regeneration is through renewed proliferation following suppression of the function of negative cell growth genes. Recent studies on the regulation of the cell cycle have uncovered a number of genes, including pRb, p27Kip1, p21Cip1 and p19Ink4d, with important functions in cell cycle exit, as well as maintenance of quiescence, in the mammalian inner ear. Deletion of p27Kip1 resulted in the partial failure of cell cycle exit in the cochlear prosensory region, with the production of supernumerary supporting cells and hair cells.Citation10,Citation11 Deletion of p19Ink4d or p19/p21 together resulted in attempted cell cycle re-entry and subsequent cell death in post-natal hair cells.Citation12,Citation13
pRb has been shown to play a prominent role in inner ear cell cycle exit and the maintenance of quiescence. Rb1 deletion in mouse embryos resulted in the escape of cell cycle exit from otherwise postmitotic sensory progenitor cells, which continued proliferation in the postnatal stage with concomitant differentiation of hair cells and supporting cells.Citation14–Citation18 Further, Rb1 deletion in postmitotic hair cells or supporting cells prompted cell cycle re-entry, demonstrating that pRb function is important in the maintenance of quiescent inner ear sensory epithelial cells.Citation16–Citation18 Deletion of Rb1 had different effects on inner ear cell survival. In the cochlea, the auditory organ, Rb1 deletion led to hair cell death in postnatal mice, whereas in the utricle, the vestibular organ, pRb™/™ hair cells survived to adulthood, strongly suggesting that pRb may play different roles in the auditory and vestibular organs.Citation16
The capacity of inner ear sensory epithelial cells to divide and differentiate in the absence of pRb suggests a possibility of manipulating pRb function for cell cycle re-entry and hair cell regeneration in mammals. However the differential response to Rb1 deletion in the cochlea and utricle, as well as in the inner ear at different ages, suggests more complex functions of pRb that are context dependent. To identify the shared and unique pRb pathways in cochlea and utricle, we studied expression profiles of pRb™/™ cochlea and utricle with microarray. We show that the pRb-E2F pathway is a core pathway shared between pRb™/™ cochlea and utricle. We identified distinct pRb pathways associated with the cochlea or utricle, which provides a basis for the study of their specific functions. We further created an inducible Rb1 knockout mouse model in which Rb1 was deleted in adult inner ear. We found that in contrast to young mice, Rb1 deletion in adult inner ear failed to induce either proliferation or cell death, strongly suggesting that additional mechanisms are involved in the maintenance of quiescence in the adult inner ear.
Results
Identification of the shared pRb pathway in the inner ear.
The current study was based on the use of conditional Rb1 knockout mice (Pou4f3-Cre-pRbflox/flox; referred to as pRb™/™) in which Rb1 was deleted consequent to Cre activity driven by the promoter of Pou4f3, a hair cell transcription factor.Citation16 In the pRb™/™ mouse, cochlear and vestibular hair cells showed different phenotypes in response to Rb1 deletion. In the vestibular system, pRb™/™ hair cells continued proliferation as late as 3 months of age and were viable in 6 month-old mice. At 6 months old, the vestibular pRb™/™ hair cells formed synapse with ganglion neurons and took up the dye FM1-43, demonstrating that the pRb™/™ hair cells function at a cellular level. Furthermore, the pRb™/™ mice maintained partial vestibular function, strongly indicating that the Rb1-null vestibular hair cells also function at a system level. In contrast, the cochlear pRb™/™ hair cells died in early postnatal weeks, and by 2 months the pRb™/™ mice exhibited complete hearing loss due to total cochlear hair cell loss.Citation16
To identify pRb pathways influencing inner ear cell proliferation, differentiation and survival, including those differing between pRb™/™ vestibular and cochlear hair cells, we performed microarray analysis of pRb™/™ cochlea and utricle at P6, and pRb™/™ utricle at 2 months post partum. The heterozygous Pou4f3-Cre-pRbflox/+ mice were used as controls. We first identified the differentially expressed genes and associated pathways within each tissue, and then compared those genes and pathways between different tissues, to identify the common and unique pathways. With this analysis, we identified not only the genes and pathways differentially regulated in the pRb™/™ cochlea and utricle, but also extracted information regarding the shared and unique pathways between pRb™/™ cochlea and utricle, or between young and adult pRb™/™ utricles. Individually, 866 and 905 genes were differentially expressed in the P6 pRb™/™ cochlea and utricle, whereas 268 were differentially expressed in 2-month-old pRb™/™ utricle (Fig. 1 and Sup. Table 1).
Of the differentially expressed genes, 232 were common to P6 pRb™/™ cochlea and utricle, representing 27% and 26% of differentially expressed genes in each tissue, respectively. Remarkably, 95% (220/232) of them showed expression changes (i.e., up or downregulated) in the same direction between pRb™/™ cochlea and utricle (), strongly indicating that they belong to a shared, core pRb pathway. Between 2-month and P6 pRb™/™ utricles, 68 differentially expressed genes were shared, representing 25% and 7.5% of differentially expressed genes in each tissue. In contrast to a high concordance of gene expression shared between P6 pRb™/™ cochlea and utricle, only 50% of the genes showed expression changes in the same direction between 2 month and P6 utricles (), whereas the rest showed expression level changes in the opposite direction, indicating the mechanisms that regulate gene expression differ from P6 to 2 months. Finally, only 32 differentially expressed genes were shared among P6 pRb™/™ cochlea and utricle and 2-month pRb™/™ utricle (), again suggesting a drastic change of the pRb pathway from P6 to adult inner ear.
We used two methods to identify the pathways that respond to Rb1 deletion in pRb™/™ and control inner ear. First, we used Database for Annotation, Visualization and Integrated Discovery (DAVID),Citation19,Citation20 to identify overall enrichment of biological process as well as molecular and cellular function-based Gene Ontology (GO) classification. We then performed Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, www.ingenuity.com) to identify highly connected networks formed by the differentially expressed genes, possible interactions among them, and to identify enriched canonical pathways.
Of the 232 differentially expressed genes shared between P6 pRb™/™ cochlea and utricle, GO classification by DAVID revealed that the most highly enriched GO biological process groups are involved in different aspects of proliferation including cell cycle, DNA replication, response to DNA damage stimulus and regulation of cell cycle, as well as microtubule cytoskeleton organization (). GO classification thus showed that the cell cycle related activities were the most prominent events mediated by the differentially expressed genes shared between the P6 pRb™/™ cochlea and utricle, correlating with proliferation status of the pRb™/™ inner ear at this stage.
To study interactive pathways formed between 232 shared genes, we used IPA and identified highly connected networks and canonical pathways. Six highly connected networks were identified, with enrichment in cell cycle and DNA replication, recombination and repair, correlating with GO classification analysis (Sup. Table 2). We further merged the top two networks to illustrate the extensive interactions among the genes. The merged network was anchored around nodes that include Rb1, E2F, cyclin A and E. In total, 97 out of 100 genes of the merged network showed expression level changes in P6 pRb™/™ cochlea and utricle, with significantly enriched canonical pathways including G2/M DNA damage checkpoint regulation, role of Brca1 in DNA damage response, mitotic roles of polo-like kinase, and role of Chk proteins in cell cycle checkpoint control (), strongly indicating that the network represented the core pRb pathway in P6 mouse inner ear. Cell cycle control by pRb is mediated largely through interaction with E2F, which was reflected by the known pRb-E2F target genes, including cyclins (Ccna2, Ccnb1, Ccnb2 and Ccne1), cell division cycle (Cdc2 and 6), minichromosome maintenance complex components (Mcm3, 5, 6 and 7) and ribonucleotide reductase M (Rrm1, 2).Citation21 Deletion of Rb1 also directly leads to activation of its target genes including Ccna2, topoisomerase II DNA binding protein 1 (Topbp1) and Chk1 checkpoint homolog (Chek1), which are normally suppressed by pRb function.Citation22–Citation24 Among 100 shared genes, 10 were downregulated in the pRb™/™ inner ear, including Rb1, Lum (lumican), Dtna (alpha dystrobrevin), Dmd (dystrophin), Scin (scinderin), Sdc4 (syndecan 4), Cald1 (caldesmon 1), Pldn (pallidin homolog), Ncoa1 (nuclear receptor coactivator 1) and Rbm39 (RNA binding motif protein 39). Interestingly, 6 out of 10 downregulated genes are involved in skeletal and muscular system development (Rb1, Rbm39, Ldb2, Cald1, Dtna and Dmd), with 3 (Sdc4, Dtna and Cald1) involved in muscle contraction.
Other genes involved in the cell cycle were also commonly upregulated, including Kif22 and Prc1 in centromere separation and cytokinesis,Citation25–Citation27 and Rfc2 in DNA damage checkpoint control.Citation28,Citation29 It has been shown that, in the absence of pRb, negative cell growth genes, including other Rb family members, may be upregulated to compensate for pRb function.Citation30–Citation32 Correlating with this, microarray analysis showed upregulation of Rbl1 (p107), Cdkn1a (p21Cip1) and Cdkn2d (p19Ink4d) in the pRb™/™ inner ear.
To confirm these general conclusions from microarray analysis, we performed in situ hybridization using Anxa4 (annexin A4), Mlf1 (myeloid leukemia factor 1) and Cdkn2d (p19Ink4d) ribo-probes, respectively. We observed upregulation of these three genes to varying degrees in hair cells of P6 pRb™/™ cochlea and utricle in comparison with control pRb+/− inner ear, in agreement with microarray results (). Combined, the data suggested a compensatory mechanism mediated by Rbl1, Cdkn1a and Cdkn2d, in which Rb1 deletion led to upregulation of Rbl1 and activation of Cdkn2d, as well as upregulation of Cdkn1a, and illustrated the sensitivity of the microarray analysis in detection of gene expression changes.
In the adult pRb™/™ utricle, no proliferation was observed, suggesting that either genes involved in proliferation were no longer active or other compensatory mechanisms could have replaced pRb function.Citation16 50% of the genes shared between P6 and 2 month pRb™/™ utricles showed changes in the same direction (), in contrast to 95% of shared genes with the expression change in the same direction between P6 pRb™/™ cochlea and utricle. DAVID analysis showed that only two GO categories were enriched that included cell cycle (9 genes, with 6 upregulated in both) and positive regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolic process (10 genes) (Sup. Table 3). In contrast, between P6 pRb™/™ cochlea and utricle, the GO category of cell cycle was enriched for 57 genes. Further, the degree to which cell cycle genes were upregulated was less in adult compared to P6 pRb™/™ utricle (an increase of an average of 1.4-fold in adult vs. 2.7-fold in P6 utricles). Combined, these results support the idea that the diminished proliferation activity in adult pRb™/™ utricle is likely a result of a reduced number of active cell cycle genes as well as a reduction in their transcription. The higher expression level of a few cell cycle genes in adult pRb™/™ utricle may indicate a sustained activity related to proliferation, which is nevertheless not sufficient to drive proliferation.
IPA analysis showed that the top enriched canonical pathways in 2-month pRb™/™ utricles were growth hormone signaling, April mediated signaling, G2/M DNA damage checkpoint regulation and IGF-1 signaling. With the exception of checkpoint regulation, these enriched pathways were drastically different from those shared between P6 pRb™/™ cochlea and utricle, in which the enriched pathways are involved in different aspects of proliferation ().
Distinct pRb pathways in the cochlea and utricle.
Surprisingly, a majority of the differentially expressed pRb cochlear and utricular genes are different (∼73% are unique to each tissue), suggesting that pRb is likely to control distinct pathways in each tissue. To identify the unique pathways associated with the pRb™/™ cochlea or utricle, we performed comparative analysis of differentially expressed genes unique to each tissue, by identifying associated GO categories with DAVID and highly connected network with IPA.
DAVID analysis of differentially expressed genes unique to the pRb™/™ cochlea and utricle showed a major divergence of enriched GO categories. The top enriched GO categories in the pRb™/™ cochlea included cell adhesion, neuron differentiation, mechano-receptor differentiation, and actin cytoskeleton organization. In contrast, the top GO categories for the pRb™/™ utricle included cell cycle, DNA replication, microtubule cytoskeleton organization, DNA packaging and DNA metabolic processes and chromosome segregation (Sup. Table 4). Thus cellular events such as development and differentiation were prominently affected in the pRb™/™ cochlea, whereas expanded cell cycle control was primarily affected in the pRb™/™ utricle.
Using IPA, we identified and compared canonical pathways that showed drastic divergence between pRb™/™ cochlea and utricle. A large number of canonical pathways were enriched uniquely in pRb™/™ cochlea and utricle, with the exception of aryl hydrocarbon receptor signaling that was downregulated in pRb™/™ cochlea and upregulated in pRb™/™ utricle (). In the pRb™/™ cochlea, the most significantly enriched canonical pathways included human embryonic stem cell pluripotency (p < 3.46 × 10−4), Notch (p < 7.94 × 10−4), Wnt/β-catenin (p < 1.17 × 10−3) and hepatic fibrosis (p < 4.68 × 10−3), whereas the enriched canonical pathways in the pRb™/™ utricle included the role of CHK in cell cycle checkpoint control (p < 6.42 × 10−5), breast cancer signaling, glioma signaling (p < 8.46 × 10−5), RAR activation (p < 2.45 × 10−4), IGF1 signaling (p < 6.56 × 10−4) and G2/M DNA damage checkpoint regulation (p < 1.03 × 10−3). Similar to DAVID analysis results, pRb™/™ cochlea showed differential expression patterns with enriched signaling pathways in the development of the mouse inner ear, whereas pRb™/™ utricle contained pathways enriched in aspects of the cell cycle such as tumor development (breast cancer and glioma), DNA checkpoint regulation (CHK and G2/M) and possibly cell survival (IGF1) (Sup. Table 5).
Of particular interest were Notch and Wnt/β-catenin pathways enriched in the pRb™/™ cochlea identified by IPA, both of which are involved in inner ear development. Six members of Notch pathways including Hes5, Hey1, Jag1, Jag2, Lfng and Mfng showed upregulation, strongly indicating heightened Notch activity in the pRb™/™ cochlea. Notch signaling is essential in inner ear sensory epithelium development and patterning of hair cells.Citation33 Activation of Notch in supporting cells and suppression of Notch in hair cells generate the mosaic pattern in which hair cells are separated from each other by supporting cells. It has been shown that activation of Notch resulted in tumorigenesis in the Drosophila eye partly by downregulation of the Drosophila Rb1 geneCitation34 and Notch activation led to expression and nuclear localization of cyclin D1, which blocked pRb function by phosphorylation.Citation35 Further, pRb directly binds and inhibits the Notch target Hey1.Citation36 Our observation of an inverse relationship between Notch and pRb function is consistent with the studies. To confirm activation of the Notch pathway in the pRb™/™ cochlea, we performed in situ hybridization for a Notch ligand Jag2. Jag2 was upregulated in P6 pRb™/™ cochlear hair cells comparing to control, which were consistent with the microarray study ( and L).
Canonical Wnt/β-catenin pathway was highly enriched in the pRb™/™ cochlea by differential expression of 13 Wnt pathway genes (Sup. Table 5). Among them were many Wnt/β-catenin signaling inhibitors (Sox2, 6 and 11, and Dkk3) and Wnt/β-catenin activators (Tcf7l1, Tcf7l2). The upregulation of both inhibitor and activator may reflect cell type specificity. To study expression of Wnt/β-catenin pathway genes and to illustrate cellular distribution of members, we performed in situ hybridization and immunohistochemistry for Dkk3, Sox2 and β-catenin. We observed upregulation of Wnt inhibitor Dkk3 in the great epithelial ridge (GER), a non-sensory region of the cochlea ( and H), indicating a suppression of Wnt/β-catenin pathway. Within the sensory epithelium, Sox2, another gene with a role in antagonizing Wnt/β-catenin initiated transcription, showed expression in the pRb™/™ cochlear hair cells, whereas it was absent in control hair cells ( and J). Sox2 expression in pRb™/™ supporting cells was comparable to that in control. Using two anti-β-catenin antibodies, one against phosphorylated nuclear β-catenin and one against β-catenin that can be found in both membrane and nuclei, we detected a downregulation of both in the pRb™/™ hair cells (). The antibody against the phosphorylated nuclear form of β-catenin (p-Y489-β-catenin) has been shown to participate in the activation of transcription in combination with Tcf4/Lif.Citation37 Thus the elevated level of Sox2 and reduced level of β-catenin is a strong indication of reduced Wnt/β-catenin pathway activity in the pRb™/™ cochlear hair cells. The distribution of the Wnt members strongly suggested that, in the absence of the Rb1 gene, the Wnt/β-catenin pathway was actively inhibited in hair cells and the GER by upregulation of Sox2 and Dkk3 and downregulation of β-catenin.
Among the top five enriched P6 pRb™/™ utricle canonical pathways, the role of CHK in cell cycle checkpoint control and breast cancer signaling were also enriched in the shared pathways between P6 pRb™/™ cochlea and utricle, indicating that pRb™/™ utricle had more genes participating in the two pathways. A canonical pathway, retinoic acid nuclear receptor (RAR) activation that included 15 genes, was significantly suppressed in pRb™/™ utricle ( and Sup. Table 5). This was shown by the downregulation of aldehyde dehydrogenase family 1, subfamily A2 (Aldh1a2) and retinol binding protein 4 (Rbp4), both of which are involved in the production of retinoic acid.Citation38 Peroxisome proliferative activated receptor gamma coactivator 1alpha (Ppargc1a), a gene with a role in binding and activation of retinoic acid-RAR-RXR complex,Citation39 was also downregulated. In contrast, genes whose functions are inhibited by the retinoic acid-RAR-RXR complex, including Akt, Adcy7, Pkd1, Jun and Fos, were upregulated, suggesting that RAR signaling is likely to be suppressed in the pRb™/™ utricle. Retinoic acid signaling is required for inner ear and hair cell development and blockade of the pathway has been associated with fewer hair cells and disruption of cochlea development,Citation40 whereas its activation led to a production of supernumerary hair cells in culture.Citation41 Retinoic acid has been shown to induce hypophosphorylation of pRb, leading to G0 arrest,Citation42 suggesting a positive correlation between RAR and the pRb pathway. Suppression of retinoic acid signaling by Rb1 deletion is consistent with the correlation and also suggests a feed back mechanism between the two pathways.
One of the significant differences between pRb™/™ cochlea and utricle is their differential responses to Rb1 deletion-induced hair cell death. Neonatal pRb™/™ cochlear hair cells die, whereas the pRb™/™ utricular hair cells survive to adulthood.Citation16,Citation17 IPA analysis identified a number of genes involved in cell death and survival that were differentially expressed in the pRb™/™ cochlea and utricle. Among them, the canonical IGF1 signaling pathway was significantly upregulated in the pRb™/™ utricle but not in the cochlea (Sup. Table 5). Ten IGF1 signaling genes showed expression level changes with 7 upregulated and 3 downregulated. The upregulated genes included those involved in cell survival such as Pdk1, Akt3, Prkar2a and Prkcz, as well as genes involved in cell growth and proliferation such as Fos, Jun and Nras (). In contrast, in the pRb™/™ cochlea, only Igf1 and an IGF binding protein Cry61 were downregulated. Upregulation of IGF1 signaling in the pRb™/™ utricle and downregulation of IGF1 signaling components in the pRb™/™ cochlea could play a role in different outcomes of hair cell survival in the two structures.
Rb1 deletion delays differentiation in the inner ear.
Rb1 deletion led to differential expression of hundreds of genes in the inner ear, and a majority of them were uniquely associated with either the cochlea or the utricle. In addition to identifying enriched interactive pathways in each tissue, we also wish to establish on a global level how Rb1 deletion affects the inner ear, by examining the normal expression profiles of genes differentially expressed in the pRb™/™ inner ear.
We have previously performed microarray studies of gene expression in the developing mouse utricle from E12.5 to 17 months post-partum, and have identified most of the genes differentially expressed throughout development (Manuscript in preparation). This study allowed us to examine the alteration in expression patterns of these genes upon deletion of Rb1.
Focusing on genes differentially expressed in the pRb™/™ inner ear, we performed sample clustering analysis based on their expression profiles in the developing mouse inner ear. Differentially expressed genes from each pRb™/™ inner ear organ (P6 utricle and cochlea, and adult utricle) were used separately for the analysis. During normal development, based on expression profiles, all embryonic utricles were clustered under one node, separated from all postnatal utricles that were clustered under another node (). Further, utricles from adjacent developmental stages tended to be clustered next to each other, reflecting an overall similarity in their expression profiles (). Based on 905 differentially expressed genes in the pRb™/™ utricle, the sample clustering showed that P6 pRb™/™ utricles were closer to the embryonic mouse utricles than to postnatal utricles, whereas the control P6 utricles were grouped together with age-appropriate P7 postnatal utricles (). Similarly, when using 268 differentially expressed adult pRb™/™ utricle genes for cluster analysis, pRb™/™ utricles were closer to early postnatal normal utricles whereas control adult utricles were clustered properly with their age group (Sup. Fig. 1). These results suggest that the differentially expressed genes in pRb™/™ utricles collectively had expression profiles more similar to the developing utricles of younger age. We performed a similar analysis with 866 genes differentially expressed in the P6 pRb™/™ cochlea, and found that the pRb™/™ and control cochleas were clustered together, separated from utricles at all age groups (Sup. Fig. 1). This result was likely an indication that gene expression in cochlea is sufficiently different from that in utricle that the cochlear samples were not clustered with any utricle sample. However, it is still likely that P6 pRb™/™ cochlea have an expression profile more similar to that of embryonic cochlea than to P6 cochlea, reminiscent of the utricle comparison.
The clustering of pRb™/™ inner ear genes with those of younger, control inner ears was driven by upregulation of early developmental genes and downregulation of differentiation genes in the pRb™/™ inner ear. In the P6 pRb™/™ utricle, 279 out of 572 upregulated genes were expressed in early inner ear development, which is significantly more compared to 17 out of 334 downregulated genes with the same expression pattern (Fisher's exact test: p-value =7.45 × 10−49). Thus, compared to control, Rb1 deletion led to a sustained expression of genes in the younger inner ear and delayed expression of later differentiation genes.
To confirm the conclusion of the developmental expression of the genes, we studied expression of Atoh1 and Isl1, two early prosensory genes, by in situ or immunohistochemistry. In P6 pRb™/™ mouse cochlea, Atoh1 was expressed in hair cells and Isl1 was expressed in both hair cells and supporting cells, whereas in control cochlea, Atoh1 expression was no longer detectable and Isl1 expression was restricted to the greater epithelial ridge (GER) ().Citation43–Citation46 The persistent expression of early genes Atoh1 and Isl1 in P6 pRb™/™ hair cells was consistent with the array results.
Acute deletion of Rb1 in the adult mouse inner ear is not sufficient for cell cycle re-entry.
We have previously shown that pRb was abundantly detected in adult mouse cochlea and utricle, suggesting that pRb may be required for the maintenance of postmitotic status of adult inner ear.Citation15 While it is known that acute deletion of the Rb1 in early postnatal inner ear led to cell cycle re-entry,Citation15,Citation17,Citation18 it is critical to establish the effect of acute deletion of the Rb1 in adult mouse inner ear.
To study the effect of Rb1 deletion in adult mouse inner ear, we created an inducible Rb1 deletion mouse line (ER-Cre-pRb) by breeding a transgenic mouse line with a mutant form of the estrogen receptor (ER) fused with Cre (ER-Cre),Citation47 with the pRbflox/flox mice, in which 4-hydroxy-tamoxifen (4OH-TM) induced Cre activity which led to Rb1 deletion. To demonstrate that 4OH-TM induced Cre activity in the adult inner ear, we bred ER-Cre with the Rosa26 reporter mice to produce the ER-Cre-Rosa26 mice. Upon injection of 4OH-TM, Cre activities were observed in the inner ear sensory epithelia including hair cells and supporting cells, as demonstrated by 5-bromo-4-chloro-3-indolyl-D-galactosidase (X-gal) histochemistry ( and B).
Three-month-old ER-Cre-pRb mice were injected with 4OH-TM daily for three days together with BrdU with the inner ear harvested three days later. The analysis of whole mount cochlea in the injected and un-injected control mice showed normal distribution of three rows of outer and one row of inner hair cells, demonstrating that Rb1 deletion did not lead to either an increase or decrease in the number of hair cells ( and D). This was further confirmed by the lack of any BrdU-positive hair cells or supporting cells in either cochlea or utricle (data not shown), illustrating that the adult inner ear lost the response to Rb1 deletion seen in young mice. We verified by genotyping that Rb1 was deleted by 4OH-TM exposure (data not shown). Further, in contrast to Rb1 deletion in neonatal cochlea in which hair cells undergo rapid cell death, immunohistochemistry with an anti-Casp3 antibody did not detect Casp3-positive cells (data not shown). Thus, acute deletion of Rb1 in the adult inner ear does not lead to cell cycle re-entry or cell death, suggesting other mechanism(s) are likely involved in the maintenance of the quiescent status of the adult inner ear.
Discussion
A hallmark of pRb loss in the developing mouse inner ear is continuous cell division of the sensory epithelium with concomitant differentiation, which raised the possibility of using the approach for hair cell regeneration. Differential responses in cell survival in the utricle and cochlea following Rb1 deletion suggest a complex function of pRb in the inner ear that is context dependent. This is particularly interesting as hair cells are generally considered uniform in terms of development and differentiation.
A large number of differentially expressed genes were identified in the pRb™/™ cochlea and utricle, suggesting they are ultimately regulated by the pRb pathway. One of the reasons for the involvement of many genes was likely due to the fact that we did not study the inner ear immediately after Rb1 deletion around E12, thus the changes we observed also included secondary and tertiary effects. As microarray measures total mRNA levels, and there are more hair cells in the pRb™/™ inner ear and more supporting cells in the pRb™/™ cochlea, it is likely that some of the differential expression observed by microarray was due to disproportionately higher hair cell and supporting cell numbers rather than higher expression on the cellular level. Conversely, it is also likely that the genes shown to be unchanged may be actually downregulated in the pRb™/™ inner ear. Furthermore, some of the changes observed were likely to come from surrounding cells in response to the cells with Rb1 deletion, such as Dkk3 in the GER. With these caveats, our in situ and immunostaining largely confirmed the general conclusions by microarray analysis on hair cells and supporting cells at the cellular levels, indicating a correlation between microarray analysis and expression within individual cells. The analysis provided a framework for detailed functional studies of the affected pathways.
Microarray analysis showed that the predominant event in the pRb™/™ inner ear is proliferation in the young pRb™/™ inner ear, manifested by activation of a large number of genes involved in the cell cycle. Those genes constitute a major part of shared genes and pathways between pRb™/™ cochlea and utricle, demonstrating they are the core pRb pathway genes in the inner ear. Among them, the pRb-E2F pathway is the most significantly enriched, correlating with the well-known functional roles of pRb-E2F in cell cycle control.Citation48,Citation49 A recent microarray study of human retinoblastoma retina identified enriched canonical pathwaysCitation50 that partially correlated with the shared pathways between pRb cochlea and utricle. The top enriched pathways in the pRb-mutant retina included G2/M DNA damage checkpoint regulation, role of BRCA1 in DNA damage response, mitotic role of Polo-like kinase, CHK in checkpoint control, ATM signaling and p53 signaling, all of which were most significantly enriched in the pRb cochlea and utricle. Thus, they constitute the common pathways of pRb in both retina and inner ear. In adult utricle with Rb1 deleted from early development (Pou4f3-Cre-pRb™/™), proliferation was no longer observed.Citation16 This correlated with far fewer cell cycle genes upregulated in the adult pRb™/™ utricle. Although the mechanism is unknown, it is clear that in adult utricle other mechanisms in cell cycle exit are likely to be activated that are sufficient to maintain the utricle in the quiescent status without Rb1.
It is striking that a majority of differentially expressed genes and their respective pathways were different between pRb™/™ cochlea and utricles, suggesting intrinsic differences between cochlear and utricular hair cells in response to Rb1 deletion. In the pRb™/™ cochlea, the most prominent pathways enriched were Wnt/β-catenin and Notch. For the Wnt/β-catenin pathway, the pRb™/™ cochlea showed a suppression of the pathway by the upregulation of the inhibitors and the suppression of β-catenin, in particular in the pRb™/™ cochlear hair cells (, D and F). Suppression of the Wnt/β-catenin pathway in the absence of pRb function is consistent with a recent study that showed E2F activity specifically inhibits β-catenin/Tcf mediated transcription.Citation51 Thus one possibility in the pRb™/™ cochlea is that Rb1 deletion releases E2F that acts to block Wnt/β-catenin pathway in hair cells. In addition, the upregulation of Wnt/β-catenin pathway inhibitors of Sox family members may add an additional layer of modulation of Wnt/β-catenin in the pRb™/™ hair cells. As the Wnt/β-catenin pathway is known to be involved in the specification of inner ear epithelium including the maintenance of sensory and non-sensory boundaries,Citation52,Citation53 our results suggest an important role of pRb in maintaining the Wnt/β-catenin pathway in the cochlea, particularly in hair cells.
In our model the Rb1 was deleted from early postmitotic hair cells during embryonic development. The clustering analysis in which pRb™/™ inner ear was clustered with younger control inner ear and the sustained expression of early genes in postnatal pRb™/™ suggested a delayed differentiation in the pRb™/™ inner ear (, and Sup. Fig. 1). We could not address the issue of dedifferentiation in the absence of pRb function, as it would require expression study of fully differentiated inner ear with acute Rb1 deletion.
In contrast to the pRb™/™ cochlea in which a number of hair cell genes were differentially expressed (Sup. Table 4, in the category of mechanoreceptor differentiation), there was no major change in the hair cell gene expression pattern in pRb™/™ utricle, indicating that utricular hair cells differentiate relatively normally. Additional cell cycle related pathway genes were upregulated in the pRb™/™ utricle and they formed the top enriched pathways in Chk checkpoint control, breast cancer and glioma signaling, as well as G2/M and Brca1 in DNA damage response. For instance, replication factor C (Rfc3, 5), replication protein A1 (Rpa1) and ataxia telangiectasia and Rad3 related (Atr), participated in DNA replication and were highly upregulated in the pRb™/™ utricle. As these genes are required for completion of recombination, their enhanced activities could result in normal progression of cell cycle of utricular hair cells.
Unlike pRb™/™ cochlea, the pRb utricles showed no evidence of major hair cell death. A large number of genes with functions in cell death were identified in both pRb™/™ cochlea and utricle, and it remains to be determined which of them contribute to apoptosis in pRb™/™ cochlear hair cells and survival in pRb™/™ utricular hair cells. Among the top enriched pathways, the canonical IGF1 pathway was identified as highly upregulated in pRb™/™ utricle but not in cochlea. The IGF1 pathway is well studied for its role in cell survival and involves activation of intracellular signaling of PI3k/Pdk1/Akt and Raf/Mek/Erk, which lead to phosphorylation and inactivation of proapoptotic genes including Bad, Caspase-9 and Fkhr.Citation54,Citation55 In the pRb™/™ utricle, a number of PI3k/Pdk1/Akt and Raf/Mek/Erk genes were upregulated, in contrast to the downregulation of Igf1 and Cyr61 in pRb™/™ cochlea. It is highly suggestive that the activation of the IGF1 pathway may play a role in hair cell survival in the utricle without Rb1, whereas the downregulation Igf1 and Cyr61 may lead to a reduced level of IGF1 signaling that impairs cell survival. This analysis provides a basis to study the IGF1 pathway in the survival of pRb™/™ hair cells.
Acute deletion of Rb1 failed to induce either proliferation or apoptosis in the adult mouse inner ear, in contrast to induction of both events at early postnatal ages, demonstrating an age-dependent effect of Rb1 deletion in cell cycle re-entry and cell death. Such an observation strongly suggests that compensatory mechanisms are involved in the maintenance of the quiescent status of adult mouse sensory epithelium. It is known that p21Cip1 and p19Ink4d are both highly expressed in postnatal hair cells, and deletion of p19Ink4d or double deletion of both genes led to attempted cell cycle re-entry and subsequent cell death.Citation12,Citation13 It is possible that the function of p21 and p19, or other negative cell growth genes in the postnatal hair cells, constitutes a compensatory mechanism in the adult inner ear to prevent Rb1 deletion-induced cell cycle re-entry and cell death.
The inability of adult inner ear cells to re-enter the cell cycle after Rb1 deletion, and apoptosis in proliferating pRb™/™ hair cells or supporting cellsCitation16–Citation18 pose two major challenges to utilize pRb reduction as a strategy for hair cell regeneration. To achieve proliferation in adult inner ear, one potential approach is to reprogram adult inner ear sensory epithelial cells by expression of early inner ear developmental genes or stem/progenitor cell genes, which could lead to reprogramming and dedifferentiation. Combining this with transient pRb blockade, it may be possible to induce fully mature inner ear sensory epithelial cells to re-enter the cell cycle, as Rb1 deletion enabled hair cell and supporting cell proliferation in the young inner ear. Further, by exploring the information regarding cell survival and death it is possible to design a strategy to rescue cell death by promoting survival and suppression of apoptosis pathways. The combination of cell cycle re-entry in adult inner ear and the survival of proliferating cells should lay a foundation for the regeneration of functional hair cells.
Materials and Methods
Mouse dissections and RNA microarray.
Creation of Pou4f3-Cre-pRbflox/flox conditional knockout mice and genotyping of the mice were described before.Citation16 P6 or 2-month control and Pou4f3-Cre-pRbflox/flox littermates were euthanized and the inner ear tissues were dissected.Citation16 Dissected utricles contained sensory epithelia (hair cells and supporting cells), stroma tissues and some residual nerve tissues, with the roof and non-sensory epithelial tissues removed. Dissected cochleas contained the organ of Corti, the greater epithelial ridge (GER) and the stria vascularis. Spiral ganglions were removed from the cochleas. For 2-month mice, 2 litters of a total of 4 Pou4f3-Cre-pRbflox/flox mice and 6 control mice were dissected. For P6, 3 Pou4f3-Cre-pRbflox/flox and 10 control mice were dissected. Total RNA was extracted from the pooled samples using Qiagen RNeasy Mini kit following the manufacturer's instructions. The purity of RNA was assessed with an Agilent Bioanalyzer 2100 (Agilent Technologies). Technical duplicates of the pooled RNA were used for microarray. Biotin labeled cRNA for microarray was prepared using the small sample preparation protocol II from Affymetrix. Hybridization to MOE430A and MOE430B Genechips® (Affymetrix) was done at MIT BioMicroCenter. All microarray data have been deposited in NCBI Gene Expression Omnibus Database (GEO; http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE25732.
Analysis of microarray data.
The initial analysis of raw data from hybridization was performed using MAS 5.0 from Affymetrix. Normalization and calculation of the expression levels and clustering of genes and samples were done in dChip.Citation56 All arrays were normalized to a baseline array with the median intensity using the “invariant set” method. PM/MM model was used to calculate the model based expression indexes (MBEI) or expression levels. Probe sets were first filtered by having “present” calls in at least 2 of 12 samples tested. Differentially expressed genes were defined as probe sets with log2 (fold change of expression levels) >0.6 or <−0.6 compared with controls and p value for one-tailed t-test ≤0.1. The low threshold cutoff was based on small variation between technical replicates so that we could include all the differentially expressed genes with detectable changes. Clustering of probe sets and samples was performed in dChip using the hierarchical clustering algorithm with correlation distance and centroid linkage method.Citation56
Annotations of genes were based on the NetAffx Annotation Release 30 (November 2009) from Affymetrix. When a probe set was not annotated with a gene name, the UniGene cluster number assigned to it was used for its identity. Analysis of Gene Ontology (GO) categories was done using DAVID 6.7 (david.abcc.ncifcrf.gov/).Citation19 Redundant probe sets with the same Entrez Gene ID were excluded for GO analysis. Default parameters on their website were used for functional annotation clustering. Clusters with enrichment score ≥1 were picked and the top GO “Biological Process” or “Molecular Function” categories with FDR ≤10% were presented. GO enrichment analysis by dChip generated similar results. For pathway analysis, data were analyzed through the use of Ingenuity Pathways Analysis (IPA, Ingenuity® Systems, www.ingenuity.com).
In situ hybridization and immunohistochemistry.
Frozen sections of Pou4f3-Cre-pRb™/™ and control inner ear tissues were prepared for immunolabeling or in situ hybridization using the protocols described before.Citation43 Antibodies used for immunofluorescence are: rabbit anti-Myo7a (1:2000, Proteus Biosciences), rabbit anti-β-catenin (1:2000, Sigma), mouse anti-phospho-β-catenin Y489 (1:1000, Developmental Studies Hybridoma Bank), mouse anti-Myo7a (1:2000, Developmental Studies Hybridoma Bank), mouse anti-Isl1 (1:100, Developmental Studies Hybridoma Bank) and goat anti-Sox2 (1:500, Santa Cruz).
Primers for probes used for in situ hybridization are listed in the table below. The cDNA clone for Cdkn2d (p19Ink4d) was bought from Open Biosystems (Clone Id: 4020283).
Tamoxifen-induced Rb1 deletion in adult cochlea culture and BrdU labeling.
ER-Cre mice (stock 004682, The Jackson Laboratory) were bred with pRbflox/flox mice to produce ER-Cre-pRbflox/flox mice. The expression pattern of ER-Cre was checked by breeding ER-Cre mice with Rosa26 reporter mice. Cre expression was determined by X-gal staining using a standard protocol.
To induce Rb1 deletion in adult mice, 4-hydroxytamoxifen (4OH-TM) (5 mg/40 g body weight) was injected into 3-month ER-Cre-pRbflox/flox mice daily for 3 days with BrdU (50 µg per gram body weight). The mice were sacrificed 5 days after the last injection. Wholemount cochlea in the injected and uninjected control mice were stained with antibodies against hair cell markers and BrdU.
Ethical Statements
All procedures were approved by the Institutional Animal Care and Use Committee of the Massachusetts Eye and Ear Infirmary.
Figures and Tables
Figure 1 Summary of microarray analysis. (A) A Venn diagram illustrating differentially expressed genes unique or shared among different tissues. (B) Among 232 shared genes (268 probe sets) between P6 pRb™/™ cochlea and utricle, a majority of them showed correlated expression by up or downregulation. (C) 68 genes (73 probe sets) were shared between P6 and adult pRb™/™ utricles. ∼50% of which showed inverse relationship by the directions of expression level changes. For instance, 33 genes showed upregulation in P6 but downregulation in adult pRb™/™ utricle. The numbers in (B and C) are slightly different from the union results in (A) due to a couple of genes with multiple probe sets on the microarray. In those rare cases the same gene was represented in different groups in the Venn diagram by different probe sets, therefore was counted more than once in (A). The y-axis in (B and C) showed the fold change of expression in a log of 2. Coch: cochlea; Ut; Utricle.
Figure 2 A highly-connected interactive network formed by top two function categories of differentially expressed genes shared between P6 pRb™/™ cochlea and utricle. IPA analysis showed that 100 genes formed an interactive network that was anchored at key genes Rb1 and E2f (arrows). The network contains other cell cycle genes with a majority of them being upregulated, an indication of active proliferation. For the list of genes, see the top two categories in Supplemental Table 2. Red genes upregulated. Green genes downregulated.
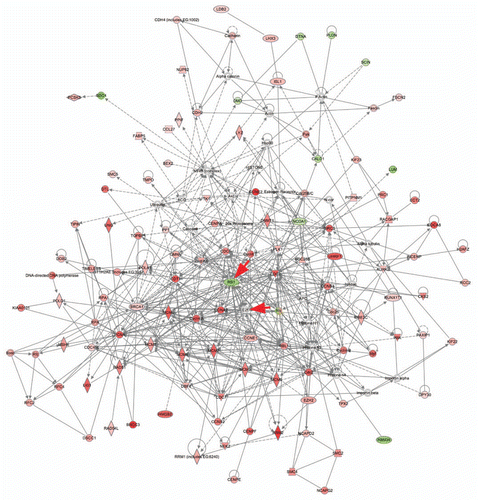
Figure 3 Confirmation of microarray analysis by in situ hybridization. (A–D) Anxa4 was highly upregulated in pRb™/™ utricular (B) and cochlear hair cells (D) compared to controls (A and C). (E–H) In comparison to control (E and G), Mlf1 was upregulated in pRb™/™ utricle (F) and cochlea (H), with more prominent upregulation in cochlear hair cells (IHC, OHC). (I–L) Cdkn2d (p19Ink4d) was significantly upregulated in pRb™/™ cochlear hair cells (L) compared to controls (K). In pRb™/™ utricle, more hair cells expressed Cdkn2a with an expression level similar to the control utricular hair cells (I and J). HC: hair cells; IHC: inner hair cells; OHC: outer hair cells. Scale bar: 20 µm.
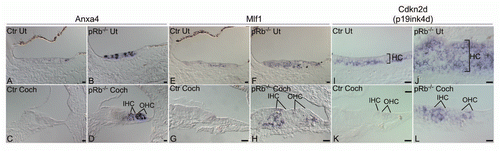
Figure 4 Divergent canonical pathways enriched by differentially expressed genes unique to the P6 pRb™/™ utricle or cochlea by IPA. The unique genes were defined as only differentially expressed in P6 pRb™/™ utricle or cochlea, respectively. The top ten canonical pathways in the pRb™/™ utricle were primarily involved in different aspects of the cell cycle, whereas the pRb™/™ cochlear canonical pathways were mainly involved in early inner ear development. For the list of genes see Supplemental Table 5.
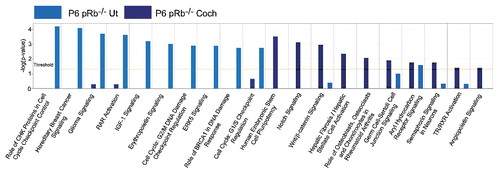
Figure 5 Differential expression of Wnt/β-catenin and Notch genes in the pRb™/™ inner ear. (A and B) Immunolabeling of a nuclear form of β-catenin (pCtnnb: phospho-β-catenin Y489, pY489) showed the absence of labeling in a majority of the pRb™/™ cochlear hair cells (B), whereas it was present in control hair cells (A). (C–F) Immunolabeling of β-catenin (Ctnnb) showed downregulation of β-catenin in the pRb™/™ cochlear hair cells (D and F) compared to control (C and E). (G and H) Dkk3, a Wnt/β-catenin signaling inhibitor, was highly upregulated in the greater epithelial ridge (GER) in the pRb™/™ cochlea (H). (I and J) Sox2, another Wnt/β-catenin pathway gene, was detected in the pRb™/™ cochlear hair cells (inset, J); whereas in control cochlear hair cells, Sox2 was completely absent. (K and L) Jag2, a Notch ligand, was upregulated in the pRb™/™ cochlear hair cells (L), whereas it was weakly detected in the control cochlear hair cells (K). IHC-inner hair cells; OHC-outer hair cells; SC-supporting cells; GER-greater epithelial ridge. Myo7a labels hair cells. Scale bar: 20 µm.
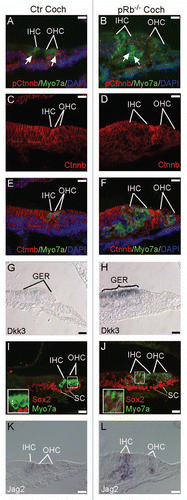
Figure 6 A diagram depicting the RAR pathway that was significantly downregulated in the pRb™/™ utricle. Suppression of the pathway was shown by downregulation of Aldh1a2 and Rbp4, two components necessary for production of retinoic acid, and upregulation of a number of genes (Akt, Pkc, Jun and Fos) whose functions were inhibited by retinoic acid-RAR-RXR complex. Red genes upregulated. Green genes downregulated.
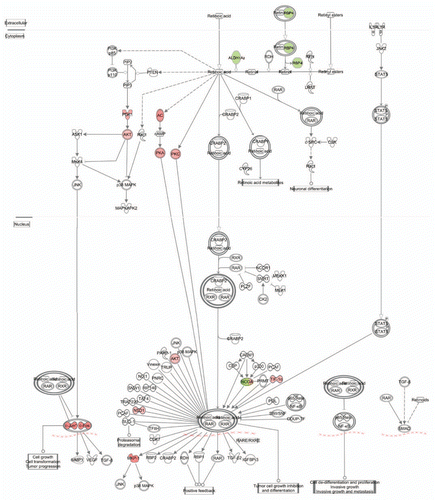
Figure 7 A diagram depicting the IGF1 pathway that was highly active in the pRb™/™ utricle. The canonical IGF1 pathway was enriched by upregulation of many IGF1 pathway genes in pRb™/™ utricle. Red genes upregulated. Green genes downregulated.
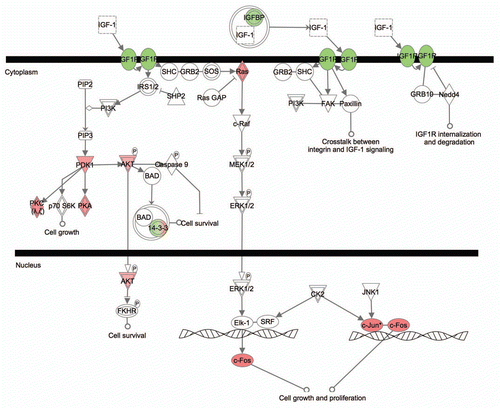
Figure 8 Clustering analysis of microarray data showing a delayed differentiation in the pRb™/™ inner ear. Sample clustering of pRb™/™ and normal developing mouse utricles, based on the differentially expressed P6 pRb™/™ utricular genes, showed that the P6 pRb™/™ utricles were closer to embryonic normal utricles (designated by E); whereas the P6 control utricles were clustered with the age appropriate postnatal groups (designated by P). Characteristics of the clustering were illustrated by upregulation of the early developing utricular genes (top bracket) and downregulation of the later genes (lower bracket) in P6 pRb™/™ utricle. Each column represents one sample. P6 pRb™/™ and control utricles were in purple boxes. The bar at the bottom illustrates relative expression level among samples from low (blue) to high (red).
Figure 9 Upregulation of early developing genes in the pRb™/™ inner ear. Two early inner ear genes, Atoh1 and Isl1, were upregulated in the P6 pRb™/™ cochlear hair cells. (A and B) In contrast to control (A), Atoh1 was upregulated in both IHC and OHC of the pRb™/™ cochlea (B). (C and D) No Isl1 was detected in the P6 control cochlear hair cells (C); whereas in the pRb™/™ cochlea Isl1 was prominently detected in a majority of hair cells (D, arrows to show Isl1-positive; arrowhead to show Isl1-negative hair cells). Scale Bar: 10 µm.
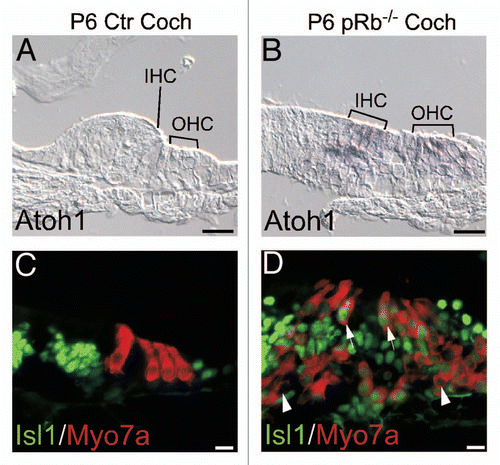
Figure 10 Acute Rb1 deletion does not result in proliferation or cell death in adult inner ear. (A and B) X-gal staining of adult inner ear, which were derived from a cross between ER-Cre and Rosa26 and injected with 4OH-TM, showed labeling in most adult cochlear cells (A) and utricular hair cells (HC) and supporting cells (SC) (B). (C and D) Adult ER-Cre-Rb1flox/flox mice injected with 4OH-TM showed normal number and distribution of cochlear hair cells (D), identical to that in 4OH-TM injected control adult ER-Cre-Rb1flox/+ mice (C), demonstrating that pRb blockade alone is not sufficient to induce proliferation or cell death. Blue staining is DAPI.
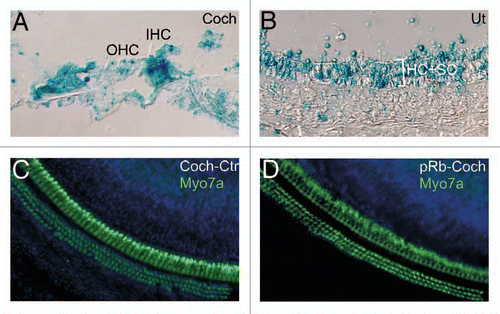
Table 1 Enriched GO categories by the shared P6 pRb−/− cochlear and utricular differentially expressed genes by DAVID analysis
Additional material
Download Zip (894.1 KB)Acknowledgements
The project was supported by R01 DC006908 (Z.Y.C.) and the Fredrick and Ines Yeatts Inner Ear Hair Cell Regeneration Fellowship (M.H. and M.P.).
References
- Chen ZY, Corey DP. An inner ear gene expression database. J Assoc Res Otolaryngol 2002; 3:140 - 148
- Chen ZY. Cell cycle, differentiation and regeneration: where to begin?. Cell Cycle 2006; 5:2609 - 2612
- Rocha-Sanchez SM, Beisel KW. Pocket proteins and cell cycle regulation in inner ear development. Int J Dev Biol 2007; 51:585 - 595
- Jorgensen JM, Mathiesen C. The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften 1988; 75:319 - 320
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science 1988; 240:1772 - 1774
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 1988; 240:1774 - 1776
- Marean GC, Burt JM, Beecher MD, Rubel EW. Hair cell regeneration in the European starling (Sturnus vulgaris): Recovery of pure-tone detection thresholds. Hear Res 1993; 71:125 - 136
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 1993; 259:1616 - 1619
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 1993; 259:1619 - 1622
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 1999; 126:1581 - 1590
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci USA 1999; 96:4084 - 4088
- Laine H, Doetzlhofer A, Mantela J, Ylikoski J, Laiho M, Roussel MF, et al. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J Neurosci 2007; 27:1434 - 1444
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, et al. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol 2003; 5:422 - 426
- Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 2005;
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 2005; 307:1114 - 1118
- Sage C, Huang M, Vollrath MA, Brown MC, Hinds PW, Corey DP, et al. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci USA 2006; 103:7345 - 7350
- Weber T, Corbett MK, Chow LM, Valentine MB, Baker SJ, Zuo J. Rapid cell cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proc Natl AcadSci USA 2008; 105:781 - 785
- Yu Y, Weber T, Yamashita T, Liu Z, Valentine MB, Cox BC, et al. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J Neurosci 2010; 30:5927 - 5936
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 2009; 4:44 - 57
- Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization and Integrated Discovery. Genome Biol 2003; 4:3
- Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: Unraveling the biology. Trends Biochem Sci 2004; 29:409 - 417
- Knudsen KE, Fribourg AF, Strobeck MW, Blanchard JM, Knudsen ES. Cyclin A is a functional target of retinoblastoma tumor suppressor protein-mediated cell cycle arrest. J Biol Chem 1999; 274:27632 - 27641
- Yoshida K, Inoue I. Expression of MCM10 and TopBP1 is regulated by cell proliferation and UV irradiation via the E2F transcription factor. Oncogene 2004; 23:6250 - 6260
- Inoue Y, Kitagawa M, Taya Y. Phosphorylation of pRB at Ser612 by Chk1/2 leads to a complex between pRB and E2F-1 after DNA damage. EMBO J 2007; 26:2083 - 2093
- Tokai N, Fujimoto-Nishiyama A, Toyoshima Y, Yonemura S, Tsukita S, Inoue J, et al. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J 1996; 15:457 - 467
- Tokai-Nishizumi N, Ohsugi M, Suzuki E, Yamamoto T. The chromokinesin Kid is required for maintenance of proper metaphase spindle size. Mol Biol Cell 2005; 16:5455 - 5463
- Puschendorf M, Terranova R, Boutsma E, Mao X, Isono K, Brykczynska U, et al. PRC1 and Suv39 h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat Genet 2008; 40:411 - 420
- Solovyan VT, Bezvenyuk ZA, Salminen A, Austin CA, Courtney MJ. The role of topoisomerase II in the excision of DNA loop domains during apoptosis. J Biol Chem 2002; 277:21458 - 21467
- Tomida J, Masuda Y, Hiroaki H, Ishikawa T, Song I, Tsurimoto T, et al. DNA damage-induced ubiquitylation of RFC2 subunit of replication factor C complex. J Biol Chem 2008; 283:9071 - 9079
- Wikenheiser-Brokamp KA. Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell Mol Life Sci 2006; 63:767 - 780
- Burkhart DL, Viatour P, Ho VM, Sage J. GFP reporter mice for the retinoblastoma-related cell cycle regulator p107. Cell Cycle 2008; 7:2544 - 2552
- Burkhart DL, Ngai LK, Roake CM, Viatour P, Thangavel C, Ho VM, et al. Regulation of RB transcription in vivo by RB family members. Mol Cell Biol 2010; 30:1729 - 1745
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci 2006; 7:837 - 849
- Ferres-Marco D, Gutierrez-Garcia I, Vallejo DM, Bolivar J, Gutierrez-Avino FJ, Dominguez M. Epigenetic silencers and Notch collaborate to promote malignant tumours by Rb silencing. Nature 2006; 439:430 - 436
- Campa VM, Gutierrez-Lanza R, Cerignoli F, Diaz-Trelles R, Nelson B, Tsuji T, et al. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol 2008; 183:129 - 141
- Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer cell 2010; 17:376 - 387
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol 2007; 9:883 - 892
- Goodman DS. Sporn MB, Roberts AB, Goodman DS. The Retinoids 1984; New York Academic Press 41 - 88
- Safi R, Kovacic A, Gaillard S, Murata Y, Simpson ER, McDonnell DP, et al. Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor gamma coactivator-1alpha on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer research 2005; 65:11762 - 11770
- Raz Y, Kelley MW. Retinoic acid signaling is necessary for the development of the organ of Corti. Dev Biol 1999; 213:180 - 193
- Kelley MW, Xu XM, Wagner MA, Warchol ME, Corwin JT. The developing organ of Corti contains retinoic acid and forms supernumerary hair cells in response to exogenous retinoic acid in culture. Development 1993; 119:1041 - 1053
- Yen A, Soong S. Retinoic acid-induced RB (retinoblastoma) hypophosphorylation enhanced by CGP 52411 (4,5-dianilinophthalimide), an EGF family tyrosine kinase receptor inhibitor. Eur J Cell Biol 1996; 69:327 - 334
- Huang M, Sage C, Li H, Xiang M, Heller S, Chen ZY. Diverse expression patterns of LIM-homeodomain transcription factors (LIM-HDs) in mammalian inner ear development. Dev Dyn 2008; 237:3305 - 3312
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol 2004; 477:412 - 421
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns 2003; 3:389 - 395
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 2002; 129:2495 - 2505
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 2002; 244:305 - 318
- van den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 2008; 9:713 - 724
- Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci 2004; 117:2173 - 2181
- Ganguly A, Shields CL. Differential gene expression profile of retinoblastoma compared to normal retina. Mol Vis 2010; 16:1292 - 1303
- Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, et al. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 2008; 455:552 - 556
- Stevens CB, Davies AL, Battista S, Lewis JH, Fekete DM. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol 2003; 261:149 - 164
- Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin upregulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J Biol Chem 2010; 285:392 - 400
- Vardatsikos G, Sahu A, Srivastava AK. The insulin-like growth factor family: molecular mechanisms, redox regulation and clinical implications. Antioxid Redox Signal 2009; 11:1165 - 1190
- Shelton JG, Steelman LS, White ER, McCubrey JA. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle 2004; 3:372 - 379
- Li C, Wong WH. Parmigiani G, Garrett ES, Irizarry RA, Zeger SL. DNA-Chip Analyzer (dChip). The analysis of gene expression data: methods and software 2003; New York Springer-Verlag 120 - 141